Abstract
In this chapter, the preparation, characterization, and applications of chlorosulphonated polyethylene (CSM) rubber based nano blends have been investigated. CSM rubber based nanoblend compounds have been prepared on a laboratory-size (300x600 mm) two-roll mixing mill maintained at 40±5 °C. Curing was performed at 160 °C up to the optimum cure time (t ), which was determined from the rheograph using a Monsanto R-100 model. The mechanical properties, namely tensile strength (TS), tensile modulus at 100 % and 300 % elongation (M100 and M300), elongation at break (Eb%) and hardness have been followed up as a function of nano fillers and irradiation dose (dose rate of 10 kGy h−1 and total absorbed dose of 100, 200, 300, and 400 kGy) as well as blend composition. Cross-linking density has been determined using swelling measurements. A scanning electron microscope has been used for morphological study. Thermal stability of samples has been determined using thermogravimetric (TG) measurements. Dynamical mechanical properties were studied on a mechanical analyzer. The assignment of the principal bands in chlorosulphonated polyethylene (CSM) rubber based nano blends has been carried out by FTIR ATR spectra. Elastomeric materials based on the chlorosulfonated polyethylene (CSM) rubber nano blend have a quite good crack resistance, wet grip, and weather resistance. On the other hand, they show a good resistance to interactions with alcohol’s as well as strong acids and bases. This type of material also exhibits excellent ultraviolet light and oxygen stability.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Blends of rubbers are of technological and commercial importance, since they allow the user to access properties of the final blended and vulcanized rubber that is not accessible from a single, commercially available rubber alone. These potentially improved properties include chemical, physical, and processing benefits. In reality, all blends show compositionally correlated changes in all of these properties compared to the blend components. The technology of rubber blends is largely focused on the choice of individual rubbers and the creation of the blends to achieve a set of final properties [1]. Miscible blends of rubbers that consist of a single rubber phase with microscopically uniform cross-linking and distribution of reinforcing agents reflect a compositionally weighted average of the microscopic properties of the constituents. Miscible blends are commonly used though they have been very rarely recognized. Analysis of such blends, particularly after vulcanization, is difficult. The current analytical techniques are only slightly more capable than the classical techniques of selective precipitation of the components of an unvulcanized rubber blend from solution [2]. Immiscible blends show additional, more complex changes due to a microscopically inhomogeneous phase structure of the two component rubbers. The two separate phases typically have differences in the retention of the fillers and plasticizers as well as vulcanization in the presence of the curative. Changing the properties of rubbers by uneven distribution of fillers and vulcanization is, however, the more common use of blends of immiscible rubbers [3].
Many reviews [4–6] are focused on immiscible blends of rubbers. Isobutylene-based rubbers include butyl rubber and halogenated butyl rubber, and due to their impermeability and resistance to heat and oxidation, these polymers find application in tire innerliners, innertubes, curing bladders and envelopes, and other specialty applications where air retention and resistance to heat and oxidation are desired [7]. Chlorosulfonated polyethylene (CSM) is an important rubber which has been frequently used in many applications such as sheeting cable and geomembrane due to its outstanding resistance to deterioration by heat, oils, ozone, and oxidation [8]. The blending together of isobutylene-based rubbers (IIR) with chlorosulfonated polyethylene (CSM) is intended to produce a vulcanizate with the best properties from each component, i.e., CSM’s impermeability and IIR and CIIR’s good strength properties and resistance to oil [9]. Chlorobutyl (CIIR) and bromobutyl (BIIR) rubbers are manufactured similarly to butyl rubber, but with an added subsequent halogenation step. Due to the nearly equivalent reactivity ratios of the monomers and low concentration of isoprene, the isoprenyl units in these polymers are randomly distributed along the polymer chain. Commercially, chlorobutyl rubber (CIIR) and bromobutyl rubber (BIIR) are the most important derivatives of butyl rubber. Their predominant application is in tire innerliners with the performance of the modern radial tire being impacted by the amount of halobutyl rubber used in the innerliner compound. Typical performance requirements of the tire innerliner are air retention, adhesion to tire carcass compound, resistance to fatigue and cracking, heat resistance, high tensile, and tear strength [10].
Silica has been used as non-black reinforcing filler in the rubber industry for a long time. Reinforced rubber blends are suitable materials for industrial practice [11]. Obviously, in such systems, both components (filler and rubber) have the reactive groups for the additional cross-linking reaction to take place [12]. Besides the so-called dispersion forces, there are a variety of other interactions between particles. A key one among them is hydrogen bonding, which tends to be a significant force in the case of fumed silica, where hydrogen bonding between surface silanol groups takes place. Many authors have investigated the role of filler networking in the elastic properties of elastomer composites [13]. The surface of hydrated or precipitated silica is highly polar and hydrophilic because of the presence of numerous silanol groups. The evaluation of the reinforcing effect is usually based on the determination of the processability of elastomers and the dynamic mechanical properties of cross-linked materials [14].
Among many other particulate fillers, the use of carbon black (CB) is very important in rubber technology for obtaining elastomers with good dynamic mechanical as well as ultimate properties. New materials are also considered as fillers, whether for more economical reasons or rather to impart some desirable characteristics [15, 16] of CB as one of the major inputs in tire industry, has some extraordinary features. The surface specific area of CB is considered to be one of the most critical parameters considered for achieving a high degree of reinforcement. This characteristic property supplies the necessary adsorption sites to the polymer chains for wetting. As polymer chains may also enter into the voids of the filler agglomerates and aggregates, the rheological properties (especially the viscosity) of the filled polymer melts are affected by both the so-called physical entrapment and the physical cross-links, created by a strong physisorption of the chains on the filler surface. These effects may also play an important role in the reinforcement of cross-linked elastomeric matrices. One of the most important parameters for the performance of a composite material is the degree of adhesion (phase bonding) at the matrix–filler interface [17]. In this study, fourteen different SBR/CSM formulations were produced by mechanical mixing and vulcanized. The mechanical properties such as the hardness, the modulus, the tensile strength, and the abrasion loss were investigated. On the other hand, some physical characteristics were investigated via the vulcanization test.
The main interest was related to the effect of biogenic silica (BSi) as a filler on the deformation mechanisms and mechanical properties of composites based on the polymer matrix. Diatomaceous comes as a biogenic silica (BSi) from the word diatom, which is the single-celled aquatic plant. Diatoms are living phytoplanktons which form an important part of marine and freshwater. The silicate shell, termed “frustule,” supports a fleshy body. The fossil beds are skeletons sunk to the bed of lake or sea after the body died and formed deposits there. Diatoms are found in a great variety of forms [18]. The skeletal remains of diatoms with submicron-sized hole are described as “indescribable particle shape” far from any simple classification [19]. The specific diatom morphology and chemical resistance could be the base of using this material as filler in composites. There is little in the literature on this subject. Adhesion between matrix and filler phases in composites is important in crack propagation and composite failure. The study of the deformation and failure process, their relation to the structure of composite, and the strength of interactions between the polymer matrix and filler phases may provide useful information for the development of the new composite materials with diatoms.
5.2 Preparation
The rubber-based nano-blend compounds were prepared using a laboratory mixing roll mill of dimensions 400 × 150 mm at a speed ratio of the rollers n 1/n 2 = 28/22, at a roller temperature of 40–50 °C. The time of the preparation of the blends was ca. 20 min. Curing was performed at 160 °C up to the optimum cure time (t c90), which was determined from the rheograph obtained using a Monsanto R-100 model. The addition of ingredients during mixing was carried following the same order and conditions of mixing. Two different types of rubbers were used IIR and CIIR blended with CSM at different ratios. IIR (Butyl 068), Mooney viscosity 51 (ML1+8 at 125 °C) with isoprene content of 1.15 mol% and CIIR (Chlorobutyl 1066), Mooney viscosity 38 (ML1+8 at 125 °C) with isoprene content of 1.95 mol% and halogen content of 1.26 mol% were supplied by Exxon Mobil, England. Their respective technical specifications are shown in Tables 5.1 and 5.2, and the compound formulations of the samples are given in Tables 5.3, 5.4, 5.5, and 5.6.
The compound formulation is given in Table 5.4.
5.3 Characterization
5.3.1 Cure Characteristics
The sheeted rubber compound was conditioned at 23 ± 2 °C for 24 h prior to cure assessment on a Monsanto moving die rheometer (model 100S, USA) at 160 °C. The compound formulations (Tables 5.3, 5.4, 5.5, and 5.6) are expressed in part per hundred parts of rubber (phr). All test specimens were compression-molded at 160 °C during the respective optimum cure time (t c90) determined from the Monsanto rheometer. The scorch time, t s2, is the time to 2 units of torque increase above minimum torque, and optimum cure time, t c90, is the time to 90 % of maximum torque development calculated from the following expression:
where M h is the maximum torque and M c90 is a optimum cure time determined from the cure traces generated at 160 ± 2 °C by oscillating disk rheometer curemeter at an angular displacement of ±3 and a frequency of 1.7 Hz [20]. The cure rate index (CRI) is the measure of rate of vulcanization based on the difference between optimum cure time of vulcanization t c90 and the scorch time t s2. It can be calculated from the relation:
5.3.2 Mechanical Properties
The sheets were cut, marked according to the time and temperature determined from the oscillating disk rheometer, and vulcanized in clean polished molds in a press at 160 °C. The vulcanized sheets were cut into dumbbell-shaped specimens (five replicates from each sample) for the evaluation of the mechanical properties using an electronic tensile testing machine (Zwick 1425, Germany) at a speed of 500 mm/min. Samples of at least 0.12 mm in thickness with flat surface were cut for hardness test. The measurement was carried out according to ASTM D 2240 using durometer of model 306L type. The tensile properties were determined using the tensile testing machine type Zwick 1445 according to ASTM D-412. The average value of the mechanical properties was calculated using at least three samples. A cross-head speed of 50 mm/min was used, and the tests were performed at 25 °C.
5.3.3 Hardness Measurements
Samples of at least 0.12 mm in thickness with flat surface were cut for hardness test. The measurement was carried out according to ASTM D 2240 using durometer of model 306L type. The unit of hardness is expressed in (Shore A).
5.3.4 Swelling Measurements and Cross-linking Density Determination
The investigated rubber composites were obtained as small sheets (1 g) weighed accurately and immersed in a 25 cm3 benzene solvent in closed simple bottles, at 25 °C. After 72 h, the samples were removed from the solvent and weighed in a glass-stopper bottle after removing the surface fluids by blotting with a filter paper. The volume swelling in percentage was calculated using the following equation [21]:
where R v and R w are the volume and mass ratio of rubber samples in the swollen gel; w and w 0 are weights of the sample before and after swelling, respectively; ρ r and ρ s are densities of the rubber and the solvent, respectively; and v 2 is the volume fraction of the sample in the swollen gel. The elastic modulus (E) values were obtained from dumbbell samples by stress–strain measurements [22] . The values of E were obtained from the initial slope of the curves in neo-Hooke’s stress–strain relation σ versus (α and α −2) in the equation below:
where α is the elongation ratio (L/L 0).
The E modulus is related to cross-linking density by the well-known equation from the kinetic theory of elasticity:
where the factor g < 1, n is the concentration of cross-linking density, R is the universal gas constant, and T is the absolute temperature.
where M c is the average molecular weight between two cross-links. The Flory–Huggins interaction parameter, w, was calculated for the rubber composites in a benzene solvent, according to the formula below:
where V s is the molar volume of the benzene solvent. Cross-linking density was determined from the swelling data. The samples were allowed to swell in the benzene solvent, and the equilibrium uptake was noted. The molecular weight between the cross-link M c was calculated using the following equation:
where M c is the molecular weight of the polymer between two cross-links; ρ r is the rubber composite density; V s is the molar volume of benzene solvent; V r is the volume fraction of rubber composites; and w is the filler–polymer interaction parameter. The Kraus model, used for the investigation of the filler–rubber interaction, shows how to quantitatively calculate the effect of particles that are either completely unbound or completely and permanently bound to the polymer. The Kraus equation is given in the equation [23]:
where V r0 and V rf are the volume fraction rubber in the solvent-unfilled and solvent-filled rubber composites; y is the volume fraction of the filler in the rubber composites; the parameter m is obtained from the graphic V r0/V rf versus Θ/1 − Θ and describes how the swelling of polymer matrix decreases when the filler content increases; and C is the constant characteristic for the filler, but it depends on the solvent.
5.3.5 Morphological Study Using a Scanning Electron Microscope
SEM images were obtained using a JEOL 5300 SEM operated at an acceleration voltage of 20 kV and equipped with energy-dispersive systems. All the studied samples were coated with a thin layer of gold in a nitrogen atmosphere.
5.3.6 Thermal Stability
Thermogravimetric (TG) measurements have been performed on the PerkinElmer TGS-2 instrument. Rubber samples were heated from 25 to 750 °C at a heating rate of 10 °C min−1 in the nitrogen atmosphere with the gas flow rate of 50 cm3 min−1. The mass of the samples ranged from 17 to 25 mg.
5.3.7 DSC Measurements of Rubber Blends
The thermal stability of pure CR/CSM rubber blend and CR/CSM rubber blends filled with nano- and micro-silica particles was investigated by non-isothermal thermogravimetric analysis (TG) using a PerkinElmer TGS-2 instrument. The measurements were conducted at heating rates of 10 °C min−1 in a dynamic nitrogen atmosphere (flow rate 25 cm3 min−1), in the temperature range from 25 to 750 °C. The average sample mass was about 8 mg.
5.3.8 Dynamic Mechanical Analysis (DMA)
Dynamic mechanical properties were studied on a mechanical analyzer Tritec 2000 (Triton Technology). Complex Young’s modulus E * = E′ + iE″ (E′ is a storage and E″ loss modulus) was measured in a single cantilever bending mode. Temperature dependencies of dynamic mechanical properties were measured at a heating rate of 2 °C/min in the temperature range from −50 to 150 °C at 5 frequencies (0.31; 1; 3.1; 10; and 31 Hz). Multifrequency mode was used to determine a dependence of E* on frequency (f) at frequency f = 1 Hz. Cross-linking density can be obtained using DMA by calculating M c, the (number) average molar mass of elastically effective chains (between cross-links), according to the following equation derived from the theory of rubber elasticity [24, 25]:
where E is the elastic modulus in the rubbery plateau (assimilated to the storage modulus E* measured at 1 Hz), R the gas constant, T the absolute temperature, and q the material density at T.
5.3.9 FTIR Analysis
Fourier transform infrared spectra (FTIR) were recorded on a Bruker IFS-66 spectrometer with an attenuated total reflection (ATR) attachment. The internal reflection element (IRE) chosen was a 45° KRS-5. Samples for measurements were approximately 0.3-mm-thick sheets, prepared by compression molding between two Teflon films at a temperature of 100 °C.
5.4 New Challenges
5.4.1 Nano- and Micro-silica-Filled CR/CSM Rubber Blends
The nano-silica-filled CR/CSM rubber blends have higher values for cure characteristics than micro-silica particle-sized filled compounds. Nano-silica type activated the vulcanizing process through the promotion of hydrogen sulfide formation and the rupture of S–N linkage when heated with sulfonamides in rubber either in the presence or in the absence of other compounding ingredients. The strong peak from SiO–C at 1070 and 1079 cm−1 in the IR spectrum of nano- and micro-silica-filled CR/CSM rubber blend suggested the presence of interaction with silica which leads to increased stability of the filled CR/CSM polymer blends. According to the Kraus equation, it can be observed that the nano-silica-filled CR/CSM rubber blend has higher Vr0/V rf values and better rubber filler interactions than that micro-silica-filled composites. The higher values of the glass transition temperatures for the nano- than the micro-filled cross-linked systems are indicated by DMA analysis. The density of the nano-silica-filled CR/CSM rubber blends was lower than that of the micro-silica-filled and was transparent at all filler concentration.
5.4.2 Cure Characteristics
The maximum and minimum torque (M h and M l ) and delta torque (∆M) at 160 °C (Table 5.7) increase with the increase of the nano- and micro-silica loading filler of the CR/CSM cross-linking systems. The difference between M h and M l could be used as an indirect indication of the cross-linking density of the rubber compound [26]. Both scorch time (t s2) and optimum curing time (t c90) are found to decrease noticeably with increasing silica loading. The nano-silica particle-sized filled rubber blends have higher values than micro-silica particle size for all cure characteristics.
5.4.3 IR Spectral Analysis
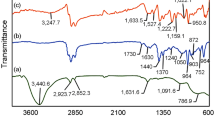
ATR spectra carried out on samples of pure CR/CSM rubber blend and of two composites with two different silica particle size contents are displayed in Fig. 5.1 (spectrum 1, 2, and 3). The assignment of the principal bands is presented in Table 5.8. Almost all bands [27] of the pure polymer blend especially those associated with the stretching bands of the CH2 groups are shifted to lower wave numbers in the filled polymer blend spectra. The bands located at 2943 and 2862 cm−1, respectively, assigned to the asymmetric and symmetric stretch of the methylene group, are found at 2943 and 2860 cm−1 for the nano-silica-filled CR/CSM composite and at 2926 and 2858 cm−1 for the micro-silica-filled CR/CSM composite sample. The absorption band at 1108 cm−1 in the cross-linked CR/CSM rubber blend is assigned to symmetric vibration of SO2 group.
The peak of –S–Cl which is attributed to the –SO2Cl group from CSM is absent, and the peak of –C–Cl from CR rubber is absent also. This suggests the cross-linking of the rubber blend macromolecules. Similar observations were made by Ahankaria et al. [28] with reference to the thermally induced self-cross-linking PVC/CR, as well as the PVC/CSM.
The absorption bands at 1655 and 1434 cm−1 in the cross-linked CR/CSM rubber blend are assigned, respectively, to symmetric and asymmetric bending vibration of C=C group. The weak absorption bands at 3536 and 3446 cm−1 which are attributed to hydrogen bands are strongly attenuated by reaction macromolecules and fillers. New absorption bands centered at 1070 and 1079 cm−1 could be assigned to the symmetric stretching modes of SiO–C group from silica filler, and moving symmetric and asymmetric bending vibration of C=C group from 1655–1651 to 1653 cm−1 and from 1434–1430 to 1445 cm−1 (Fig. 5.1, spectrum 2 and 3) suggests the interaction of the polymers matrix with the silica, also.
5.4.4 Mechanical Properties
Table 5.9 represents the effect of nano- and micro-silica loading on tensile properties (tensile strength, modulus, and elongation at break) and hardness of the rubber vulcanizates. The tensile strength increases with increasing nano- and micro-silica loading in CR/CSM rubber compound. Maximum value of tensile strength, for nano-silica-filled CR/CSM rubber compound, is by samples 4 and 7 with 35 phr nano- and micro-silica (15.8 and 8.5 MPa). The improvement in tensile properties for nano-silica-filled composites seems to be responsible for the improved dispersion of the nano-silica into the rubber matrix, leading to a higher bound rubber formation [29]. The values of 300 % modulus are increased from 6.5 to 7.1 MPa for nano-silica and 2.8 to 3 MPa for micro-silica-filled compounds, strongly attenuated by reaction macromolecules and fillers, respectively. The hardness values of micro-silica-filled compounds are the same and much lower than nano-silica-filled compounds as shown in Table 5.9.
Values of elongation at break are decreasing with increasing silica loading. The increase of hardness, modulus, and tensile strength and higher values for nano- than micro-silica composites can possibly be explained by the improvement of the degree of adhesion at interfaces between the nano-silica and the CR/CSM rubber blends.
5.4.5 Dynamic Mechanical Analysis
Dynamic mechanical analysis (DMA) is based on the differences in mechanical damping properties of unfilled and filled rubber vulcanizates [30]. Figure 5.2 and Table 5.10 show the elastic modulus, E 0, and tand determined from a dynamic mechanical analyzer under the conditions of 1 Hz and 0.1 % dynamic strain amplitude. The addition of filler to rubber matrix would lower the peak value of tand, designated as (tanδ max), in the plot of tanδ versus temperature. E 0 of blends increases significantly with the addition of silica, particularly at a temperature above the CR/CSM rubber blend glass transition temperature (T g). This result indicates a strong reinforcement caused by strong interaction between silica and CR/CSM rubber blend via silanol groups and chlorine atoms, respectively (Table 5.10) [31]. Values of glass transition temperature (T g) determined from the damping peak tanδ are summarized in Table 5.10. In theory, the shift in T g would be caused by silica–polymer interaction and/or a change in phase compatibility.
5.4.6 Cross-linking Density
The values of R W and R V (Table 5.11) decrease with increasing amount of both nano- and micro-silica.
The cross-linking density, V, increased with increasing silica concentration and was the largest for the sample containing nano- and micro-silica of 35 and 10 phr, respectively (samples 4 and 5). The largest chain molecular mass was obtained for micro-silica-filled compounds with amount of 35 phr (sample 7) and the smallest for the sample 4 with the highest amount of nano-silica. The swelling degree decrease can be explained by linkage combination between polymer chains and functional groups on the silica surface and by a possible increase in cross-linking bonds in the polymer matrix because of filler influence on the vulcanization reaction [32]. The cross-linking density (ν) of the nano- and micro-silica particle-sized filled CR/CSM rubber blend was calculated from the equilibrium storage modulus in the rubber region over the α-relaxation temperature according to the rubber elasticity theory. The correlations of some data for the modulus of elasticity (G) determined from stress–strain measurement and cross-linking density (ν) calculated from the storage modulus E 0 (at 70 °C, 1 Hz) or (V) determined by swelling are included in Table 5.10. Figure 5.3 shows the plot of V r0/V rf versus ϕ/(1 − ϕ) according to the Kraus equation [33]. It can be observed exhibit deviations from the proposed linear model, which are greater than 1.
As filler loading increases, the ratio of V r0/V rf decreased. The nano-silica-filled CR/CSM rubber blend has higher V r0/V rf values than micro-silica composites and show better polymer–filler interaction. Thermal stability of CR/CSM compounds filled with nano- and micro-silica particle size. The thermal stability of pure CR/CSM rubber blend and CR/CSM rubber blends filled with nano- and micro-silica particles has been discussed. The thermal decomposition of CR/CSM rubber blend and nano- and micro-silica particle-sized filled CR/CSM rubber blends occurs generally in two main stages (Fig. 5.4a, b; Table 5.12). The first step degradation occurs in the temperature region 250–300 °C, and the second-step degradation is about at 440 °C. The data for the initial decomposition temperature (IDT) (5 % mass loss) [34] and 10 and 30 % mass loss temperatures are given in Table 5.12 and Fig. 5.4a. It can be seen that according to the IDT nano-silica-filled CR/CSM compounds are more stable than unfilled and micro-silica-filled compounds. In the next step (at 10 % mass loss) with 20 phr of nano-silica and 30 phr of micro-silica particle-sized filled CR/CSM rubber have higher temperature values (262 and 259 °C) than other samples. At 30 % mass loss, the CR/CSM rubber blends filled with 20 phr of nano-silica and 30 phr of micro-silica particle size are the most stable also than other compounds. Figure 5.4a, b shows that unfilled CR/CSM rubber blend compounds are initially more stable than other samples. In the CR/CSM rubber blend compounds filled by nano- and micro-silica types (Table 5.12; Scheme 5.1), the degradation takes place in two stages. Up to 100 °C, there is no change and hence no mass loss. The first-step degradation occurs in the temperature region 250–350 °C and the mass loss in the range 21.15–27.93 and 21.15–28.76 % (depending on the type and amount of silica in CR/CSM rubber blends), most probably indicating the evaporation of oil (naphtalene). The second-step degradation starts at 440 °C and ends at 700 °C. The percent of mass loss at this region is in the range of 47.67–61.58 and 52.66–62.06 % for nano- and micro-silica-filled CR/CSM rubber blend indicating polymer degradation. The large flexible polysulfidic linkages undergo chain scission and convert into monosulfidic and disulfidic linkages. Synthetic rubber decomposes by random chain scission with intramolecular hydrogen transfer. Table 5.12 presents the DTG peak values of CR/CSM rubber compounds filled by nano- and micro-silica particle size. DTG peaks shift to higher temperature values indicating greater thermal stability of samples containing 20 phr nano and micro silica.
5.4.6.1 Morphology Microscopic Studies
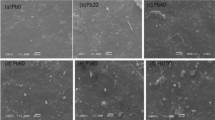
Scanning electron microscopic (SEM) studies of fractured surfaces were observed by scanning electron microscopy (SEM) in order to gain a better insight into the mechanism of failure of SiO2-reinforced NBR/CSM and CR/CSM cross-linked systems. The SEM microphotographs at 2000 magnification of the surfaces of CR/CSM polymer blends are shown in Fig. 5.5. The surface of the CR/CSM polymer blend with nano-SiO2 is more polished than that with micro-SiO2. In the microphotographs of micro-SiO2-filled CR/CSM blend a micro-crack of size about 50 μm can be seen.
5.4.7 SBR/CSM/ Carbon Black Rubber Composites
For SBR/CSM blends, the addition of filler leads to a significant enhancement in the physical properties if CSM is the major blend constituent. The blends containing CB N339 show higher hardness, tensile strength, and abrasion resistance when compared to the ones containing CB N772. The experimental data indicate that this can be caused by the improvement in the polymer–filler phase bonding which results from the interfacial interaction of functional groups of the polymer and the active sites of the filler surface leading to a higher apparent cross-linking density. As the concentration of CSM in the rubber blend increases, the cure characteristics, such as optimum cure time, scorch time, and induction time at 160 °C, increase for the blends filled with CB N339. The results for the curing behavior are consistent with the mechanical properties of the SBR/CSM blends.
5.4.8 Mechanical Properties
It was shown that for SBR/CSM blends, the addition of filler leads to a significant enhancement in the physical properties if CSM is the major blend constituent. The blends containing CB N339 show higher hardness, tensile strength, and abrasion resistance when compared to the ones containing CB N772. The experimental data indicate that this can be caused by the improvement in the polymer–filler phase bonding which results from the interfacial interaction of functional groups of the polymer and the active sites of the filler surface leading to a higher apparent cross-linking density. As the concentration of CSM in the rubber blend increases the cure characteristics, such as optimum cure time, scorch time and induction time at 160 °C increases for the blends filled with CB N339. The results for the curing behavior are consistent with the mechanical properties of the SBR/CSM blends. Figure 5.6 shows a comparison of the evolution of the hardness of SBR/CSM blends reinforced by the two types of highly structured carbon black (N339 and N772). It can be seen that the hardness of the blend increases in both systems when the content of CSM rubber increases. However, the increase of the hardness is much more pronounced in the case of the active filler N339 than in the one where N772 was used. Above 50 % of CSM in the blend, the hardness tends to reach a plateau value for N339, whereas it passes through a maximum in the blends filled with N772. The volume fraction of CB affects the mechanical properties of the polymer blends positively [35].
The relationship between the tensile strength, the modulus (M200) at 200 % elongation and the elongation at break (Eb) and the content of CSM in the blends is shown in Figs. 5.7, 5.8, 5.9, and 5.10. All the figures show increasing physical properties with the increasing CSM content in the blend. Again, the reinforcement by CB with smaller primary particle size becomes evident. If the modulus 200 is considered, it becomes evident that the targeted value for SBR/CSM/CB 339 passes a maximum value, whereas for SBR/CSM/N339, the modulus tends to a limiting value (Fig. 5.7).
The reinforcement by CB is shown evidently by the evolution of the tensile strength (Fig. 5.8). For SBR/CSM/CB N339, the values range from 6 to 39 MPa. In comparison with that, the effect of the more coarse black N772 increases the tensile strength from 6 to 18 MPa only. In both cases, the tensile strength increases approximately linearly with the content of CSM in the blend, indicating a preferential distribution of the CB into this polymer phase. If the elongation at break is considered (Fig. 5.9), it is shown from Fig. 5.8 that the values remain unchanged until 40–50 % of CSM in the blend is reached. After this threshold, the values increase strongly when the CSM content increases. In addition, a slight inversion of the reinforcing effects can be observed. The lower values (about 200 and 240 %) were obtained for systems reinforced by CB N339, and significantly higher values (260 and 350 %) were recorded for all systems reinforced by CB N772. As an overall picture, it seems that the values of tensile properties and elongation at break are synergistic above 50 % CSM leading to higher energies at break. The values gained for the abrasion loss are illustrated in Fig. 5.10. Despite the higher scatter of the experimental data, it can be shown that (i) the abrasion loss is higher for the compounds containing CB N772 than for the ones containing CB N339. However, as a systematic picture in both groups of compounds, the abrasion loss is passing through a maximum at 40–50% CSM underlining again the effect of the reinforcement in the range of high CSM concentrations. More details about the values of the specific mass are tabulated in Table 5.13. It can be seen from this table that the specific mass ranges from 1.082 to 1.312 g/cm3. The reason for this could be differences in the dispersion and distribution of the fillers in rubber blend.
Equilibrium swelling measurements were performed in order to evaluate the apparent cross-linking density in the blend compound, which contains the contribution of the chemical cross-links as well as the one of the strong physical attachments of the polymer to the filler. The values of the cross-links density calculated by using Eq. 5.14 are given in Fig. 5.11. A clear linear increase of the apparent cross-linking density with the CSM content in the blend was observed irrespective of the type of carbon black used. This indicates as the major contributor a strong interaction between the functional groups of CSM and the filler leading to strong physical cross-links and eventually also chemical ones which are not affected by the polar solvent used.
These results are to some extent supported by the observations of the curing characteristics. The maximum torque, the optimum cure time, and the scorch time change significantly with the blend composition. However, concerning the influence of the filler there were no much differences in the scorch and cure time of the blends filled with CB N339 and CB N772. The minimum torque values are for all the compounds in the same range, irrespective of the CSM content in the rubber blends. There was also no difference in the M l values of the materials filled with CB N339 and the ones filled with CB N772. However, concerning the M h values, there are two groups of values which show a certain influence by the type of the filler used. For the CB N339, the M h values increase significantly when the CSM content increases. This behavior is not observed for the group of compounds filled with CB N772. Differences occur between the M h values of the two series of compounds.
This difference can be caused by a more effective interfacial adhesion between CSM and the active CB. At the micro-level, physical and chemical interactions between the functional groups of CSM and the active sites of the high surface specific area CB can lead to a higher apparent cross-linking density. The formation of a greater number of interfacial cross-links may occur. As the concentration of CSM in the blend increases, the cure characteristics such as optimum cure time, scorch time, and induction time at 160 °C increase for the blends filled with CB N339. Therefore, the optimum cure time increases. Thus, the results are consistent with the ones from the mechanical behavior.
5.4.9 NR/CSM and NBR/CSM Rubber Blends Reinforced by Carbon Black
The influence of carbon black (with an average particle size of 40 nm) on NR/CSM and NBR/CSM rubber blends reinforced by carbon black was investigated. The mechanical properties of elastomer depend mainly on the carbon black loading. Tensile strength, modulus at 100 % elongation, and hardness were increased with increasing radiation dose, as well as carbon black loading, but the elongation at break values decreases with increasing radiation dose and carbon black content. The FTIR spectra of aged samples confirmed the formation of various oxidation products, i.e., alcohols, ethers and small amounts of lactones, anhydrides, esters, and carboxylic acids during radiation. Significant changes in spectra are induced by chain scission which was registered in the 1620–1450 cm−1 region. The formation of shorter polyene sequences and aromatic rings is assumed. The radiation doses lower than 100 kGy influenced only small changes in the rubber macromolecules structure if we consider the development of the bands in carbonyl and conjugated C=C bond regions. On the other hand, 200, 300, and 400 kGy doses caused a serious chemical modification of the prepared rubber blend samples.
5.4.10 Cure Characteristics
The obtained rheometer curves for curing of filled and unfilled rubber blend compounds are given in Fig. 5.12. The cure characteristics of NR/CSM and NBR/CSM rubber blends are summarized in Table 5.14 as the function of the carbon black content together with some other experimental data. The values for curing data depend mainly on the combination of network precursor. The maximum torque, which is a measure of the stiffness of the compound, is directly related to the modulus of the compounds.
The value of the cure time, t c90, is systematically decreased with increasing carbon black content for both rubber blend compounds. The NR/CSM rubber blends have considerably shorter t s2 and t c90 values than those of carbon black filler of 50 phr or more which resulted again in a noticeable increase in the rate of variation of M100 value with respect to the irradiation dose. These results may be attributed not only to the radiation-induced cross-linking, as well as different interphase linking, but also to the role played by the carbon black filler as a separate phase. In this respect and under a limited extension of 100 % elongation, the carbon black filler would be expected to exist predominantly in its “structure” state, particles aggregated solid and separate form [36]. The effective contribution to the M100 value of vulcanized composites from this solid phase of the filler would then be expected, when taking into consideration that the tensile modulus parameter is an additive in character. The obtained results are in accordance with the comparable results reported before for other rubber composites [37] (Figs. 5.13, 5.14, 5.15, and 5.16).
The variation of the values of elongation at break percent, Eb %, as a function of irradiation dose for unloaded and carbon black-loaded NR/CSM and NBR/CSM blends is depicted in Figs. 5.17 and 5.18, respectively. As expected, the values of Eb for both reinforced blends decrease with increasing irradiation dose. This systematic decrease in Eb values may be attributed to the induced cross-linking by gamma irradiation. On the other hand, the Eb values decreased with the increase in the carbon black filler loading. This reduction is due to stiffening of the matrix by carbon black filler particles [38]. In fact, with any further increase in filler loading, the molecular mobility decreased due to the formation of a physical bonding between the filler particles and the rubber chain. The decrease of Eb values for blends loaded with the increasing radiation dose may be explained as an additional bonding between the filler and the rubber due to the large number of free radicals formed under radiation. Therefore, an increased radical recombination is likely to generate strong chemical linkage with an increase of the irradiation dose.
The changes of hardness of loaded and unloaded NR/CSM and NBR/CSM rubber blends as a function of the radiation dose are illustrated in Figs. 5.19 and 5.20, respectively. Also, the data for loaded rubber without irradiation were introduced in the same figure for comparison. It may be observed that the values of hardness of the latter composition have increased effectively with increasing degree of loading with the filler. A similar behavior has been attained for the radiation-vulcanized composites when comparing its hardness values at the same irradiation dose. On the other hand, a comparatively limited increase has been attained in the value of hardness for one and the same composition, whether loaded or unloaded, with increasing irradiation dose from 100 to 400 kGy. These data indicate clearly that the contribution to hardness values from the radiation-induced cross-linking that has taken place in the totally amorphous rubber matrix of NBR/CSM blend is a limited one. On the other hand, the main contribution to the hardness value is affiliated with the occurrence of the filler in its aggregated solid and distinct phases, that is, its “structured” form as mentioned before.
5.4.11 Electron Scanning Microscopy
SEM micrographs of fractured surfaces for NR/CSM and NBR/CSM rubber blends filled with 60 phr are shown in Figs. 5.21 and 5.22, respectively. The formation of small micro-craters was observed in NBR/CSM rubber blends. A detachment of small micro-craters from the fractured surface is a result of poor polymer–filler adhesion. A comparatively good polymer–filler interaction with carbon black and NR/CSM blend increases the resistance to fracture [39]. Filler distribution in the blend affects the properties of the obtained elastomeric materials.
5.4.12 FTIR Spectra Measurements
Gamma radiation leads to significant changes in the FTIR spectra of carbon black-reinforced elastomers based on NR/CSM (Fig. 5.23a, b) and NBR/CSM (Fig. 5.24a, b) and rubber blend. The broad increase in absorption can be found in C=O, O–H, and C–O stretching vibration domains for both blends. Significant changes appeared in the region of conjugated double bonds, too. Several oxidation products contribute to band at the hydroxyl region, i.e., alcohols (band at 1028–1075 cm−1), ethers and small amounts of lactones, anhydrides, esters, and carboxylic acids. Due to the formation of oxidation products, the bands related to CH2, CH3, =C–H, and C=C vibrations decrease. CH2 deformation band at 1458 cm−1, CH3 asymmetric deformation at 1372 cm−1, and =C–H wagging at 831 cm−1 show a significant increase in the spectra of irradiation reinforced rubber blend.
A decrease in absorption at 1660 cm−1 is related to the loss of C=C bonds in 1,4-cis units. 1,4-cis units also absorb at 831 cm−1. Beside the oxidation of C=C bonds in 1,4-cis units, cis-trans isomerization can take place. The band at 2956 cm−1 related to methyl groups decreases, especially at the highest doses 200, 300, and 400 kGy. Researchers in the field elastomers based on polyisoprene, acrylonitrile, and chlorosulfonated polyethylene rubbers [40] did not report the appearance of new bands related to these processes in the conjugated double-bond region of radiated NR/CSM and NBR/CSM FTIR spectra. In thermally degraded NR/CSM and NBR/CSM rubber blends, there have been identified dipentene, two isomeric forms of dimethylvinylcyclohexene, isoprene, and some aromatic compounds that formed via the Diels–Alder reactions. Coran [41] reported that during thermal degradation of 1,4-cis-polyisoprene, the chains with conjugated C=C bonds are also formed. Changes corresponding to similar processes are also present in the region 1620–1450 cm −1 of the irradiated polyisoprene and acrylonitrile spectra, where no oxidation product absorbs. Polyenes and polyenals show several bands in the 1500–1650 cm−1 range. Benzene C=C stretching vibration bands are located between 1600 and 1450 cm−1; hydrogen bond can cause the reduction of the stretching vibration frequency. The subtraction spectra reveal the occurrence of the broad band with a significant increase in absorption at 1470 cm−1. This band can be assigned to aromates and shorter polyene structures. Absorption at 1470 cm−1 remains the same (the maximum is slightly shifted to higher wave numbers) for the highest doses, and absorption at 1461 cm–1 was lower in the case of the sample irradiated with the highest dose (Figs. 5.23 and 5.24). It implies that formed species are a subject of consecutive reactions. It can be concluded that radiation induces serious changes in NR/CSM and NBR/CSM rubber blends’ backbone chain scissions and various consecutive reactions take place. These changes are noticeable in the 1470–1450 cm−1 region that indicates the occurrence of the polyene sequences and aromatic rings. Contrary to the chain scission reactions [42], the formation of cross-links is difficult to detect by using IR spectroscopy due to the overlap of many bands in the region of C–C stretching vibrations. Doses exceeding 200, 300, and 400 kGy caused serious modification to the polymer structures.
The influence of carbon black (with an average particle size of 40 nm) on gamma radiation resistance of rubber blends was investigated. The mechanical properties of elastomer depend mainly on the carbon black loading. Tensile strength, modulus at 100 % elongation, and hardness were increased with increasing radiation dose, as well as carbon black loading, but the elongation at break values decreases with increasing radiation dose and carbon black content. The FTIR spectra of aged samples confirmed the formation of various oxidation products, i.e., alcohols, ethers and small amounts of lactones, anhydrides, esters, and carboxylic acids during radiation. Significant changes in spectra are induced by chain scission which was registered in the 1620–1450 cm−1 region. The formation of shorter polyene sequences and aromatic rings is assumed. The radiation doses lower than 100 kGy influenced only small changes in the rubber macromolecular structure if we consider the development of the bands in carbonyl and conjugated C=C bond regions. On the other hand, 200, 300, and 400 kGy doses caused a serious chemical modification to the prepared rubber blend samples.
5.4.13 CSM/IIR and CSM/CIIR Rubber Blend
The incorporation of CSM in CSM/IIR and CSM/CIIR blends increases the scorch time, t s2, and cure time, t c90. At a similar CSM content, the CSM/CIIR blend exhibits shorter scorch time t s2 and cure time t c90 than the CSM/IIR blend. The incorporation of CSM in the CSM/CIIR blend also increases the maximum torque (M h ) and torque difference (M h − M l ). However, for the CSM/IIR blend, the maximum torque and torque difference decrease with the incorporation of CSM, an observation which is attributed to the maldistribution of cross-link between the two rubber phases. M100 and M300 increase with increasing CSM content in both blends. The former increase is attributed to the enhancing effect of CSM, and the latter increase is associated with strain-induced crystallization of the respective rubbers. Tensile strength and elongation at break of the blends decrease gradually with CSM content, an observation which is ascribed to the decreasing effect of strain-induced crystallization of IIR and CIIR as CSM content is increased in the respective blends. The cross-linking density is found to slightly change with an increase in the concentration of the CSM rubber in CSM/CIIR and CSM/IIR rubber blends. Higher cross-linking density is obtained for CSM/CIIR rubber blends. The micrograph of CSM/CIIR exhibits that the composite’s surface is rough with many tear lines and branching indicates the higher tensile strength and a weak interaction between CSM and CIIR rubbers.
Figures 5.25 and 5.26 show the effect of CSM content on the scorch time t s2 and cure time, t c90, of the CSM/IIR and CSM/CIIR rubber blends. It can be seen that the t s2 and t c90 of these blends increase with increasing CSM content. CIIR and CSM are polar rubber. As the content of CSM in the blends is increased, the curative agent, i.e., sulfur, becomes more soluble in CSM and CIIR compared to IIR. Consequently, the curing rate of the blend increases with increasing content of CSM. Researches [43–45] reported that for blends of two rubbers with similar polarity, distribution of cross-links can be equal through phases. It can be seen also in Figs. 5.25 and 5.26 that at a similar CSM content, t s2 and t c90 of CSM/CIIR blend are shorter than those of CSM/IIR blend. The effect of CSM content on the maximum torque (M h ) is shown in Fig. 5.27. It can be seen that the M h increases with increasing CSM content in the CSM/CIIR blend. Figure 5.27 also shows that above 40 phr of CSM, M h of CSM/CIIR blends has higher values than CSM/IIR blend as a result of the miscibility presence between CSM and CIIR rubber. The relationship between the torque difference, ΔM = M h − M l (max. torque − min. torque), and content of CSM is shown in Fig. 5.24. It is known that the torque difference indirectly relates to the cross-linking density of the blends [46]. This figure clearly shows that CSM/CIIR blend is a more compatible blend than CSM/IIR blend. The decreasing torque difference for CSM/IIR indicates that a distribution of cross-link occurred which results in an over-cross-linked phase and a poorly cross-linked one.
Again at a similar CSM content, CSM/CIIR blend exhibits higher M h − M l than CSM/IIR blend. Markovic [47] reported that with normal sulfur-vulcanizing systems, the efficiency of CIIR vulcanization seemed to be improved. The isolated double bonds in CIIR inhibit the formation of intramolecular sulfide links, thus resulting in the increase of the cross-linking rate (Fig. 5.28).
5.4.14 Mechanical Properties
Figure 5.29 shows the variation of M100 with CSM content in the two CSM/IIR and CSM/CIIR rubber blends. It is obvious that as the percent of CSM increases, M100 also increases correspondingly.
This means that the incorporation of CSM in the blend would enhance cross-linking as M100 is a measure of degree of cross-linking. CSM/CIIR blend gives a higher M100 value than the CSM/IIR blends. This observation may be attributed to greater enhancement effect of CSM on CIIR. In fact, the cross-linking density is increased for both blends. As in the case of CSM/IIR blend, the lower M100 is attributed to the non-enhancement effect of CSM which is a polar polymer compared to IIR, a nonpolar rubber. Thus, the CSM/IIR blend has a lower cross-linking density than CSM/CIIR blends. The variation of M300 with CSM content is presented in Fig. 5.30. In this case, CSM/IIR blend consistently shows a higher M300 than CIIR blends.
The increase of M300 values indicates that CSM reinforces IIR and CIIR blends: The degree of reinforcement is greatest in IIR, followed by CIIR as the degree of crystallization decreases with increasing % of halogenated. Figure 5.11 shows the dependence of tensile strength of the two rubber blends on CSM content. Again, IIR blend consistently indicates a higher tensile strength than the CIIR blend as observed for M300. However, in this case, tensile strength decreases gradually with increasing CSM content for all the blends studied. This observation is associated with decreasing effect of strain-induced crystallization as CSM content is increased. As discussed earlier in M300, IIR exhibits the greatest strain-induced crystallization, followed by CIIR. This sequence of decreasing crystallization is reflected by the decreasing value of tensile strength for the respective single-component rubbers [48]. In the case of CSM, it has the lower tensile strength due to the non-crystallizable nature of the rubber. Figure 5.31 also shows that for CSM content greater than 60 phr, greater drop in tensile strength for the blends is observed. This finding may be ascribed to the dominant effect of CSM at higher loading of CSM.
The effect of CSM on elongation at break for CSM/IIR and CSM/CIIR rubber blends is shown in Fig. 5.32. The effect, in fact, is very similar to that of tensile strength; that is, elongation at break decreases gradually with increasing CSM content. Owing to the higher strength of IIR, its blend gives a much higher elongation at break compared to CIIR blend. For the single-component CSM, its elongation at break is the lowest among the rubbers studied due to the non-crystallizable property of CSM.
5.4.15 Swelling Properties
Some calculated data for the “relative” cross-linking density values, ν, obtained from the swelling measurements for rubber blends are given in Table 5.15. The cross-linking density is found to slightly change with an increase in the concentration of the CSM rubber in CSM/CIIR and CSM/IIR rubber blends. Higher cross-linking density is obtained for CSM/CIIR rubber blends; this is because of the restriction of swelling due to the similar (polar) nature of rubbers and formation of new cross-links according to free sulfur and residues of curatives react with rubbers, which causes an increase in Φ p and increases the cross-linking density. Thus, the observed tensile strength variation can be exactly correlated with the variation of ν from swelling studies. In swelling studies of these samples, cross-links are flexible to penetrate, but as the loading of CSM increases, they restrict the swelling. The swelling ratio, 1/Q, calculated using Eq. (5.1) is found to increase (Table 5.15).
5.4.16 Morphological Study
In Fig. 5.33a, b, the SEM photomicrograph of CSM/IIR and CSM/CIIR (60/40) rubber blends at 2000× magnification is shown. The micrograph of CSM/CIIR (Fig. 5.33b) exhibits that the composite’s surface is rough with many tear lines and branching.
This type of failure indicates the higher tensile strength and a weak interaction between CSM and CIIR rubbers.
5.5 Recent Advances on Chlorosulfonated Rubber Nano-blends
Thermostability and surface morphology of nano- and micro-filled NBR/CSM and CR/CSM rubber blends. Cross-linked NBR/CSM and CR/CSM rubber systems with a mass ratio of 50:50 (Table 5.5) have good weather resistance, color stability, high ozone resistance, and high extension values.
5.5.1 Thermal Stability
The thermal stability analysis was based on the temperature for certain weight losses, namely 10 and 30%, and the rate of weight loss changes as a function of temperature (first derivative of the dynamic TG curve) i.e., the initial and overall shapes. The first corresponds to the partial degradation of the sample, and the second is the result of complete thermal decomposition. The samples could be characterized by the position of the decomposition peaks and their size, the difference being the result of the different influence of their structure and the differences in the degradation processes. The data for the 10 and 30 % weight loss temperatures are given in Table 5.16.
From the values of the temperatures at 10 % weight loss and T end, it can be seen that the nanoparticle-sized SiO2-filled NBR/CSM cross-linked systems (303, 486 °C) are more stable than the micro-particle-sized SiO2-filled NBR/CSM cross-linked systems (296, 485 °C). In the CR/CSM cross-linked systems, the temperature values at 10 % weight loss show little difference in the thermostability between the micro-particle-sized SiO2 and the nanoparticle-sized SiO2-filled cross-linked systems. In the next step (30 % weight loss), the micro-filled cross-linked system had more stability than the nano-filled cross-linked system. Among the most important factors that can influence the initial TG behavior are the strength of the bonds in the main CR/CSM and NBR/CSM polymer chains and the bonds with SiO2. The single glass transition temperature for the NBR/CSM and CR/CSM cross-linked systems indicates that NBR, CSM, and CR are compatible. The higher values of the glass transition temperatures for the nano- than for the micro-filled cross-linked systems indicate that nano-filled cross-linking systems have greater number of SiO–C links than micro-filled cross-linking systems and more stability.
5.6 Industrial Values and Applications
In recent years, economic, technological, and other regulatory pressures have gradually narrowed the further development of new chemical varieties of polymers [49, 50]. A blend can offer a set of properties that may give it the potential of entering application areas not possible with either of the polymer comprising the blends. Among the authors investigating rubber blends, Baker [51] reported that replacement of polychloroprene by a 20/80 natural rubber/neoprene GRT blend could be accessible for many applications where neoprene GRT is used; Patra and Das [52] studied the flame retardancy and heat shrinkability of polyolefin/elastomer blends. The blending together of natural rubber (NR) and chlorosulfonated polyethylene rubber (CSM) is intended to produce a vulcanizate with the best properties from each component, i.e., the good strength properties of NR and the good weather resistance, color stability, and high extension values of CSM.
5.6.1 CSM/SMR 20 CV and CSM/Pergut S 40 Rubber Blends
The Mooney scorch time, t 5, of CSM/SMR 20 CV and CSM/Pergut S 40 blends decreases with increasing SMR20 CV and Pergut S 40 content in the blends. The reduction is more pronounced at lower temperatures, i.e., below 130 °C. For CSM/SMR 20 CV blend, the cure index shows a maximum at 20 phr of SMR 20 CV in the blend at lower vulcanization temperature, an observation which is attributed to the incompatibility between CSM and SMR 20 CV and the faster cure rates of the latter. In the case of CSM/Pergut S 40 blends, the cure rate virtually decreases with increasing Pergut S 40 content, as a result of the better compatibility between CSM and Pergut S 40, both of which are polar in nature. For temperature greater than 140 °C, the cure index for both blend systems exhibits less dependence on the blend ratio due to the availability of thermal energy to overcome the activation energy of vulcanization. Based on the morphological investigations, the fracture surfaces of CSM/SMR 20 CV (80:20) rubber blends are less homogeneous than those of CSM/Pergut S 40 (80:20).
5.6.1.1 Scorch Time
The variation of the Mooney scorch time, t 5, of the CSM/SMR 20 CV and CSM/Pergut S 40 blends with a blend ratio of SMR 20 CV and Pergut S 40 is shown in Figs. 5.34 and 5.35, respectively.
It can be seen that the t 5 of the blends decreases with increasing SMR 20 CV and Pergut S 40 content. CSM is a polar rubber. As the content of CSM in the blends decreases, the curing agents, i.e., sulfur, magnesium oxide, and tetramethylthiuram disulfide, become more soluble in the SMR 20 CV and Pergut S 40 than in the CSM. Consequently, the cure rate of the blend increases with decreasing content of CSM. According to Lewan [53], for blends with two rubbers differing in polarity, such as SMR 20 CV or Pergut S 40 with CMS, a distribution of cross-links can arise through preferential solubility of the curing agents and vulcanization intermediates. Both figures also indicate that the reduction of t 5 with a blend ratio of SMR 20 CV and Pergut S 40 is more pronounced at temperatures lower than 130 °C. At higher temperatures, i.e., higher than 140 °C, t 5 does not show a significant dependence on the blend ratio because enough thermal energy is available to overcome the activation energy of vulcanization. A comparison of t 5 for CSM/SMR 20 CV and CSM/Pergut S 40 blends at 120 °C is shown in Fig. 5.36. It can be seen that with a similar blend ratio, the t 5 of the CSM/SMR 20 CV blend is shorter than that of the CSM/Pergut S 40 rubber blend. According to Poh and Wong [54], more activated precursors to cross-links are formed which accelerate the vulcanization process.
5.6.1.2 Cure Index
The dependence of cure index of the CSM/SMR 20 CV blend on the blend ratio of SMR 20 CV for the various temperatures investigated in this study is shown in Fig. 5.37. For temperatures lower than 140 °C, the cure index is significantly dependent on the blend ratio of SMR 20 CV. It passes through a maximum at 20 phr of SMR 20 CV in the blend. This observation is attributed to the incompatibility between CSM (a polar rubber) and SMR 20 CV (a nonpolar rubber), the respective solubility parameters of which are 9.5 and 8.1.11 [55]. Also, owing to the higher unsaturation in SMR 20 CV, it cures faster than CSM, resulting in uneven vulcanization of the blend. For instance, at 120 °C, the cure index of CSM and that of SMR 20 CV are 7.2 and 8 min, respectively. As the SMR 20 CV content in the blend is increased from 0 to 20 phr, more sulfur reacts with the SMR20 CV rubber than with CSM. This means that less sulfur and magnesium oxide are available for the vulcanization of CSM, which is the dominant rubber component in the blend. However, beyond 20 phr of SMR 20 CV, the role of SMR 20 CV becomes more significant and results in a drop of the cure index of the blend. For the temperatures greater than 140 °C, the cure index shows less dependence on the blend ratio of SMR 20 CV, a phenomenon that is associated with the increase in cure rates for both rubbers resulting from an ample supply of thermal energy to overcome the activation of vulcanization for both rubbers, i.e., even vulcanization of the blend is obtained. In fact, above 150 °C, the cure index is almost independent of the blend ratio of SMR 20 CV.
The effect of blend ratio of Pergut S 40 on the cure index of CSM/Pergut S 40 blend is shown in Fig. 5.38. Generally, a gradual drop of cure index is observed as the phr of Pergut S 40 is increased, although a slight maximum is displayed in some cases. This finding is attributed to the better compatibility between CSM and Pergut S 40, both of which have a polar nature. The respective solubility parameters are 9.5 and 9.2.9 [56] which are closer than the corresponding values for CSM and SMR 20 CV, as discussed earlier. As in the case of the CSM/SMR 20 CV system (Fig. 5.38), the cure index of the blend was less dependent on the blend ratio of Pergut S 40 at higher vulcanization temperature, i.e., greater than 140 °C. Again, this is attributed to the availability of thermal energy to overcome the activation energy of vulcanization.
In order to compare the cure index of both blends, the cure index was plotted against the cure temperature for blends, containing either 20 phr SMR 20 CV or Pergut S 40 (Fig. 5.39). The cure index decreases with increasing temperature for both blends. The cross-linking of CSM takes place according to a completely different mechanism from those involved in the cross-linking of natural rubber i.e., the activation energy is different.
5.7 Conclusion
Blending of two or more different types of rubber is considered a useful technique for developing materials with properties superior to those of individual constituents or ones which demonstrate useful property combinations. The approach is important especially from a technical point of view. Elastomeric materials based on the chlorosulfonated polyethylene (CSM) rubber nano-blend have a quite good crack resistance, wet grip, and weather resistance. On the other hand, it shows a good resistance to interactions with alcohols as well as strong acids and bases. This type of material also exhibits excellent ultraviolet light and oxygen stability. In addition to applications for glovebox gloves, this rubber has additional uses such as in lining and sheath materials, coatings, and adhesives. CR/CSM rubber blend study illustrates the intrinsic potential of nano-silica particle size as reinforcing filler in elastomeric materials. The nano-silica-filled CR/CSM rubber blends have higher values for cure characteristics than micro-silica particle-sized filled compounds. Nano-silica type activated the vulcanizing process through the promotion of hydrogen sulfide formation and the rupture of S–N linkage when heated with sulfonamides in rubber either in the presence or in the absence of other compounding ingredients. The strong peak from SiO–C at 1070 and 1079 cm−1 in the IR spectrum of nano- and micro-silica-filled CR/CSM rubber blend suggested the presence of interaction with silica which leads to increased stability of the filled CR/CSM polymer blends. According to the Kraus equation, it can be observed that the nano-silica-filled CR/CSM rubber blend has higher V r0/V rf values and better rubber filler interactions than micro-silica-filled composites. The higher values of the glass transition temperatures for the nano- than the micro-filled cross-linked systems are indicated by DMA analysis. The density of the nano-silica-filled CR/CSM rubber blends was lower than that of the micro-silica-filled and was transparent at all filler concentration.
For SBR/CSM blends, the addition of filler leads to a significant enhancement in the physical properties if CSM is the major blend constituent. The blends containing CB N339 show higher hardness, tensile strength, and abrasion resistance when compared to the ones containing CB N772. The experimental data indicate that this can be caused by the improvement in the polymer–filler phase bonding which results from the interfacial interaction of functional groups of the polymer and the active sites of the filler surface leading to a higher apparent cross-linking density. As the concentration of CSM in the rubber blend increases, the cure characteristics, such as optimum cure time, scorch time, and induction time at 160 °C, increase for the blends filled with CB N339. The results for the curing behavior are consistent with the mechanical properties of the SBR/CSM blends.
The influence of carbon black (with an average particle size of 40 nm) on gamma radiation resistance of rubber blends was investigated. The mechanical properties of elastomer depend mainly on the carbon black loading. Tensile strength, modulus at 100 % elongation, and hardness were increased with increasing radiation dose, as well as carbon black loading, but the elongation at break values decrease with increasing radiation dose and carbon black content. The FTIR spectra of aged samples confirmed the formation of various oxidation products, i.e., alcohols, ethers and small amounts of lactones, anhydrides, esters, and carboxylic acids during radiation. Significant changes in spectra are induced by chain scission which was registered in the 1620–1450 cm−1 region. The formation of shorter polyene sequences and aromatic rings is assumed. The radiation doses lower than 100 kGy influenced only small changes in the rubber macromolecules structure if we consider the development of the bands in carbonyl and conjugated C=C bond regions. On the other hand, 200, 300, and 400 kGy doses caused a serious chemical modification of the prepared rubber blend samples.
The incorporation of CSM in CSM/IIR and CSM/CIIR blends increases the scorch, t s2, and cure time, t c90. At a similar CSM content, the CSM/CIIR blend exhibits shorter scorch time t s2 and cure time t c90 than the CSM/IIR blend. The incorporation of CSM in the CSM/CIIR blend also increases the maximum torque (M h ) and torque difference (M h − M l ). However, for the CSM/IIR blend, the maximum torque and torque difference decrease with the incorporation of CSM, an observation which is attributed to the maldistribution of cross-link between the two rubber phases. M100 and M300 increase with increasing CSM content in both blends. The former increase is attributed to the enhancing effect of CSM, and the latter increase is associated with strain-induced crystallization of the respective rubbers. Tensile strength and elongation at break of the blends decrease gradually with CSM content, an observation which is ascribed to the decreasing effect of strain-induced crystallization of IIR and CIIR as CSM content is increased in the respective blends. The cross-linking density is found to slightly change with an increase in the concentration of the CSM rubber in CSM/CIIR and CSM/IIR rubber blends. Higher cross-linking density is obtained for CSM/CIIR rubber blends. The micrograph of CSM/CIIR exhibits that the composite’s surface is rough with many tear lines and branching indicates the higher tensile strength and a weak interaction between CSM and CIIR rubbers.
Nano-silica filler interacts more with NBR/CSM and CR/CSM polymer matrix than micro-silica, which is the result of the greater contact area of the nano-filler, greater adsorption, and lower mobility of the polymer chains or the filler surface. The higher values of the glass transition temperatures for the nano- than the micro-filled cross-linked systems indicate that the nano-filled cross-linked systems have a larger number of SiO–C links than micro-filled cross-linked systems and hence increased stability. According to morphological investigation, can be concluded: (i) The surface of NBR/CSM polymer blends is more polished than that of CR/CSM polymer blends and without crack; (ii) the presence in both NBR/CSM and CR/CSM rubber blends with micro-particle size of SiO2 of rubber balls and some amount of fibrosity in the matrix, voids and crazes, as well as micro-cracks and crystallinity contribute toward failure of the macromolecular network structure. The increased number of cracks and voids in the CR/CSM polymer blends contribute to the earlier loss of physical and mechanical properties, compared to the NBR/CSM polymer blends.
The Mooney scorch time, t 5, of CSM/SMR 20 CV and CSM/Pergut S 40 blends decreases with increasing SMR20 CV and Pergut S 40 content in the blends. The reduction is more pronounced at lower temperatures, i.e., below 130 °C. For CSM/SMR 20 CV blend, the cure index shows a maximum at 20 phr of SMR 20 CV in the blend at lower vulcanization temperature, an observation which is attributed to the incompatibility between CSM and SMR 20 CV and the faster cure rates of the latter. In the case of CSM/Pergut S 40 blends, the cure rate virtually decreases with increasing Pergut S 40 content, as a result of the better compatibility between CSM and Pergut S 40, both of which are polar in nature. For temperature greater than 140 °C, the cure index for both blend systems exhibits less dependence on the blend ratio due to the availability of thermal energy to overcome the activation energy of vulcanization. Based on the morphological investigations, the fracture surfaces of CSM/SMR 20 CV (80:20) rubber blends are less homogeneous than those of CSM/Pergut S 40 (80:20).
References
Marković G, Samaržija-Jovanović S, Jovanović V, Marinović-Cincović M, Budinski-Simendić J (2009) Chem Ind Chem Eng Q 15(4):291–298
Omran AM, Youssef AM, Ahmed MM, Abdel-Bary EM (2010) Kautch Gummi Kunst 6:197–202
Wu S (1985) Polymer 26(12):1855–1863
Marković G, Samaržija-Jovanović S, Jovanović V, Marinović-Cincović M (2010) J Therm Anal Calorim 100:881–888
Essawy H, El-Nashar D (2004) Polym Testing 23(7):803–807
Marković G, Radovanović B, Marinović-Cincović M, Budinski-Simendić J (2006) Kautch Gummi Kunst 5:251–255
Voet A (1980) J Polym Sci 15:327–373
Flory PJ (1946) Ind Eng Chem 38(4):417–436
Roychoudhury A, De PP (1997) J Appl Polym Sci 63(13):1761–1768
Zhang L, Wang Y, Wang Y, Sui Y, Yu D (2000) J Appl Polym Sci 78(11):1873–1878
Mostafa A, Abouel-Kasem A, Bayoumi MR, El-Sebaie MG (2009) Mater Des 30:1561–1568
Liu Q, Zhang Y, Xu H (2008) Appl Clay Sci 42:232–237
Das A, Costa FR, Udo W, Heinrich G (2008) Eur Polym J. 44:3456–3465
Odlyhal M, Cohen NS, Foster GM, Aliev A, Verdonck E, Grandy D (2003) J Therm Anal Calorim 71:939–950
Furtado CRG, Leblanc JL, Nunes RCR (1999) Eur Polym J 35:1319
Yatsuyanagi F, Suzuki N, Ito M, Kaidou HE (2001) Polymer 42:9523
Akovali G, Ulkem I (1999) Polymer 40:7417
Mann DG, Evans KM (2007) Molecular genetics and the neglected art of diatomics. In: Brodie J, Lewis J (eds) Unravelling the algae—the past, present and future of algal systematics. CRC Press, Boca Raton (Florida)
Williams DM, Kociolek JP (2007) Eur J Phycol 42:313–319
Markovic GS, Veljkovic O, Marinovic-Cincovic MT, Jovanovic V, Samarzija-Jovanovic S, Budinski-Simendic JK (2013) Compos Part B Eng 45 br. 1, str. 178–184
Stojčeva-Radovanović B, Marković GS, Marinović-Cincović MT, Babić D , Nedeljković JM (2002) Hemijska industrija 56(10):415–421. doi:10.2298/HEMIND0210415S
Markovic GS, Marinovic-Cincovic MT, Jovanovic V, Samarzija-Jovanovic S, Budinski-Simendic J (2014) Chapter: Non-linear. Non-linear viscoelasticity of rubber composites and nanocomposites, pp 193–271
Da H. M. da Costa, R. C. R. Nunes, L. L. Y. Visconte, C. R. G. Furtado (2001) Kautschuk Gummi Kunststoffe 54: 5 242-249
Markovic GS, Marinovic-Cincovic MT, Jovanovic V, Samarzija-Jovanovic S, Budinski-Simendic J, Natural rubber materials: blends and IPNs (Chap. 1). Composites and nanocomposites, vol 1, pp 1–27
Markovic GS, Marinovic-Cincovic MT, Jovanovic V, Samarzija-Jovanovic S, Budinski-Simendic J, Composites and nanocomposites, pp 181–204
Janos M, Bela P (2008) J Ind Eng Chem 14:535–563
Das A, Stockelhuber KW, Jurk R, Saphiannikova M, Fritzsche J, Lorenz H et al (2008) Polymer 49:5276–5283
Ahankaria SS, Kamal KK (2008) Mater Sci Eng A 491:454–460
Fu SY, Xi-Qiao F, Bernd L, Yiu-Wing M (2008) Compos B 39:933–961
Leblanc JL (2002) Prog Polym Sci 27:627–687
Marković G, Marinović-Cincović M, Valentova H, Ilavsky M, Radovanović B, Budinski-Simendić (2005) Mater Sci Forum 494:475–481
Janowska G, Rybinski P (2004) J Therm Anal Calorim 78:839–847
Grauic N (1967) Encyclopedia of polymer science and technology, vol 4. Wiley, New York, p 159
Tarrıo-Saavedra J, Lopez-Beceiro J, Naya S, Artiaga R (2008) Polym Degrad Stab 93:2133–2137
Tanrattanakul V, Udomkichdecha W (2001) J Appl Polym Sci 82:650
López-Manchado MA, Valentín JL, Carretero J, Barroso F, Arroyo M (2007) Eur Polym J 43:4143–4150
Rattanasom N, Saowapark T, Deeprasertkul C (2007) Polym Testing 26:369–377
Chakraborthy SK, Setua DK, De SK (1982) Rubber Chem Technol 55:1286
Setua DK, De SK (1984) J Mater Sci 19:983–988
Coran AY, Hamed P, Goettler LA (1976) Rubber Chem Technol 49:1167–1181
Chakraborthy SK, Setua DK, De SK (1982) Rubber Chem Technol 55:1286
Arumugam N, Tamareselvy K, Venkata RK (1989) J Appl Polym Sci 37:2645–2659
Ding R, Leonov AI (1966) J Appl Polym Sci 61(3):455–463
Poh BT, Wong KW (1998) J Appl Polym Sci 69(7):1301–1305
de León R, Morales G, Acuña P, Soriano F (2010) Polym Eng Sci 50(2):373–383
Choi SS (2000) J Appl Polym Sci 75(11):1378–1384
Marković G, Marinović-Cincović M, Radovanović B, Budinski-Simendić J (2007) Chem Ind Chem Eng Q 13(4):186–191
Radovanović B, Marković G, Radovanović A (2008) Polym Compos 29:692–697
Ray S, Das CK (2001) Polym Plast Technol Eng 40
Zanzing DJ (1993) Rubber Chem Technol 66:456
Baker CSL (1974) Rubber Chem Technol 5:5
Patra PK, Das CK (1998) J Appl Polym Sci 68:597
Lewan MV (1998) Blends of natural rubber. Champman & Hall, London, p 52
Poh BT, Wong KW (1998) J Appl Polym Sci 69:1301
Bridson JA (1978) Rubber chemistry. Applied Science Publishers, London, p 83
Lewan MV (1998) Blends of natural rubber. Champman & Hall, London, p 52
Acknowledgments
The financial support for this study was granted by the Ministry of Science and Technological Development of the Republic of Serbia (Project nos. 45022, 45020, and 172056).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Marković, G., Marinović-Cincović, M., Jovanović, V., Samaržija-Jovanović, S., Budinski-Simendić, J. (2017). Chlorosulfonated Rubber-Based Nanoblends: Preparation, Characterization and Applications. In: Markovic, G., P. M., V. (eds) Rubber Nano Blends. Springer Series on Polymer and Composite Materials. Springer, Cham. https://doi.org/10.1007/978-3-319-48720-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-48720-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48718-2
Online ISBN: 978-3-319-48720-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)