Abstract
MHC genes are highly polymorphic as antigen presenting molecules for adaptive immune response in vertebrates. In this study, we evaluated the diversity of dog leukocyte antigen (DLA) class II genes among two Korean breeds, the Sapsaree and the Jindo, and three Chinese native breeds, Tibetan Mastiff, Pug, and Pekingese, and determined their genetic relationships. Sequence-based typing of DLA-DRB1 and DLA-DQB1 was performed in 138 dogs. We identified 27 alleles for DRB1 and 24 for DQB1, including five new alleles. DRB1*015:01 was shared among all five breeds and was a major allele for the Pug, Tibetan Mastiff, and Sapsaree. The observed heterozygosity for the five breeds ranged from 0.67 to 1.00 for DRB1 and from 0.80 to 1.00 for DQB1. A total of 40 DRB1-DQB1 haplotypes, including nine new haplotypes, were identified. Interestingly, most haplotypes (33/40, 82%) were specific to a single breed, and only seven were present in multiple breeds. Haplotype sharing analysis together with previously available data from 109 breeds revealed that compared to within region distances, Asian breeds were more distant than breeds from other regions. As a first report on the analysis of DLA genes of dog breeds in Korean peninsula, our results show that these breeds carry unique DLA class II haplotype lineages. Our results indicate that the diversity and breed specificity of DLA class II genes are much higher among East Asian breeds than among breeds from other regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The major histocompatibility complex (MHC) is a family of cell membrane proteins, consisting of classes I and II, that plays a critical role in the adaptive immune response of vertebrates (Zinkernagel and Doherty 1974; Maenaka and Jones 1999). MHC class I presents peptides generated from the breakdown of cytosolic antigens to CD8 + cytolytic T cells, whereas MHC class II, found in antigen-presenting cells that capture and internalize antigens in endosomes, presents peptides generated from these antigens to CD4 + helper T cells (Lafuente and Reche 2009). As a result of genetic diversity and the extreme polymorphism of the MHC loci, MHC molecules can bind a virtually unlimited range of nonself peptides that serve as T-cell epitopes, and even a single MHC molecule can bind a substantial number of peptides (Matsumura et al. 1992).
Previous studies have shown that diversifying and balancing selection at the MHC peptide-binding cleft is a major evolutionary force underlying the extreme polymorphism of MHC genes (Hughes and Nei 1988, 1989; Hughes and Hughes 1995). Currently, more than 7000 MHC alleles from 70 nonhuman species, including 420 alleles from canids, are registered in the Immuno Polymorphism Database (IPD) (https://www.ebi.ac.uk/ipd/mhc/). MHC polymorphisms have been reported to have effects on disease resistance and susceptibility, mate selection, and other traits and behaviors (Carrington et al. 1999; Milinski et al. 2005; Sommer 2005). MHC genes have also been used to evaluate the genetic fitness of animal populations (Oliver and Piertney 2012).
Canis lupus familiaris, the modern domestic dog, is believed to have been domesticated about 15,000 years ago, making it the earliest domesticated animal (Larson et al. 2012). Both archaeological and genetic evidence supports that the ancestors of modern domesticated dogs are gray wolves (Morell 1997), but the geographic origin is still debated. Studies using mitochondrial DNA (mtDNA) have proposed that modern dogs originated from East Asian gray wolves about 15,000 years ago (Savolainen et al. 2002). Shannon et al. (2015) concluded a central Asian origin based on comprehensive analyses using autosomal, mitochondrial, and Y chromosome data, whereas Wang et al. (2016) concluded a southeast Asian origin based on 58 canine whole genome sequencing (WGS) data. The Middle East has also been suggested as the main area of origin (Vonholdt et al. 2010). Interestingly, all of these studies have indicated an origin in Asia.
The extreme genetic diversity of MHC genes has been successfully used to determine the genetic diversity and population dynamics of mammals (Buhler and Sanchez-Mazas 2011; Niskanen et al. 2013; Le et al. 2020). Most recent information on polymorphisms in the canine MHC (also known as dog leukocyte antigen, DLA) is derived from studies of European breeds (Kennedy et al. 2002, 2007b) and has limited data available for Asian breeds (Niskanen et al. 2013). In this study, we analyzed the DLA class II genes DLA-DRB1 and DLA-DQB1 (DRB1 and DQB1 for short) in five East Asian dog breeds to understand phylogenetic relationships to each other based on MHC diversity. These included two Korean breeds, Sapsaree and Jindo, and three Chinese native breeds, Tibetan Mastiff, Pug, and Pekingese. This is the first study to examine the diversity of MHC genes in the Pekingese and any native dog breeds of the Korean Peninsula. Our study adds information on the global diversity of dog MHC genes and contributes to illuminating the genetic relationships among East Asian dog breeds and their immunogenetic relationships to breeds in other regions.
Materials and Methods
Extraction of Blood Samples and Preparation of Genomic DNA
Blood samples were collected from the foreleg veins of 138 dogs, including 94 Sapsarees with pedigree information, 13 Jindos, 9 Tibetan Mastiffs, 12 Pugs, and 10 Pekingese. The Sapsaree and Jindo are Korean native breeds, while the Tibetan Mastiff, Pug, and Pekingese originate from China (Lee et al. 2000; Swainston-Goodger 2006; Li et al. 2008; Gajaweera et al. 2019). To maximize genetic diversity, animals without direct pedigree relationships at least at the parental generation were used, except in the case of the Sapsarees. Jindo and Sapsaree blood samples were obtained from populations maintained, respectively, at Jindo Theme Park (Jindo County, South Korea) and the Sapsaree Institute (Kyungsan City, South Korea). Blood samples from Tibetan Mastiffs, Pugs, and Pekingese were obtained from local breeding centers in Jilin Province, China. Each sample was collected by a veterinarian according to a memorandum of understanding between the research team and breeding center. All blood samples were obtained in an ethical manner, following the guidelines for animal health and welfare of Konkuk University. The experimental protocols were approved and supervised by the Institutional Animal Care and Use Committee of Konkuk University. Genomic DNA were extracted from 1 mL of blood using the QIAamp DNA Blood Midi Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol.
Amplification and Direct Sequencing of DLA-DRB1 and DLA-DQB1
Primers for the second exons of DLA-DRB1 and DLA-DQB1 were used to amplify these locus-specific regions according to previously described methods (Soutter et al. 2015). Briefly, an ABI 9700 thermocycler (Applied Biosystems, Foster City, CA, USA) was used to carry out the polymerase chain reaction (PCR) in a 10-μL reaction volume containing 25 ng of genomic DNA, 10 pmol of the locus-specific primers (Table S1), 2.5 mM dNTPs, 1 × PCR buffer, and 0.5 U of Supertherm DNA polymerase (JMR Holdings, Kent, UK). To facilitate direct sequencing of the PCR products, the DRB1 reverse primer and the DQB1 forward primer both included the M13F universal primer sequence at the 5ʹ end (Table S1). The PCR profile consisted of an initial denaturation (5 min at 94 °C), 25 amplification cycles (30 s at 94 °C, 30 s at 50 °C, and 1 min at 72 °C), and a final extension (7 min at 72 °C). The amplicons for DRB1 (337 bp) and DQB1 (318 bp) were electrophoresed on a 1% agarose gel, stained with ethidium bromide, and visualized by UV light. Direct sequencing of the PCR products was performed as previously described (Park et al. 2010). The M13F universal primer was used for the sequencing reactions. In the case of a novel allele or unclear typing result, additional sequencing was carried out on the other strand using the appropriate locus-specific primer (DRB1 forward primer or DQB1 reverse primer).
Allele Identification
Reference sequences corresponding to 158 DLA-DRB1 alleles and 79 DLA-DQB1 alleles were obtained from DLA group entries in the IPD database (https://www.ebi.ac.uk/ipd/mhc/group/DLA/). Additional information on DLA class II alleles was obtained from the group curator of the Canine MHC Nomenclature Committee of the International Society for Animal Genetics (ISAG). DRB1 and DQB1 allele sequences were determined by aligning our sequence-based typing results with the annotated reference sequences from IPD-DLA database by using the multiple alignment tool of CLC Main Workbench 7 (CLC bio, Aarhus, Denmark). For homozygotes, typing results were recorded without further analysis. For heterozygotes, however, the typing results consisted of the combined sequences of two different alleles, necessitating deduction of the sequence of each individual allele by matching against the reference alleles. The results of allele assignment were confirmed using a MHC allele assignment tool, SOAPTyping (Zhang et al. 2020). BLAST searches of the NCBI nucleotide database (nr; https://www.ncbi.nlm.nih.gov/nucleotide/) were used to determine whether the alleles distinguished had been previously reported. For typing results involving putative new alleles or uninterpretable allele combinations, the amplicons were cloned using the TOPcloner TA cloning kit (Enzynomics, Daejeon, Korea) according to the manufacturer’s protocol. Subsequently, colony PCR was performed using independent colonies, and allele sequences were determined by direct sequencing as described above. At least eight colonies were sequenced for each sample, and the allele sequences were accepted when more than two identical sequences were observed from colonies of independent PCR. The sequences of new DRB1 and DQB1 alleles were submitted to NCBI, and accession numbers were obtained. New alleles were given official designations by the group curator of the ISAG Canine MHC Nomenclature Committee.
Phylogenetic Analysis and Haplotype Determination
The frequencies of alleles and genotypes and the level of locus heterozygosity were calculated using Arlequin software (ver. 3.5.2.2) (Excoffier and Lischer 2010). Allelic richness was calculated using FSTAT version 2.9.4 (Goudet 2003; Foulley and Ollivier 2006). Multiple sequence alignments and phylogenetic trees were constructed using MEGA-X 10.1.8 software with neighbor joining and a bootstrap value of 1000 (Kumar et al. 2018). Genetic distances were estimated using the Jukes-Cantor substitution model (Jukes and Cantor 1969). Determination of DRB1-DQB1 haplotypes was performed using a reference database of 156 haplotypes, obtained from previous studies involving 4186 dogs of 109 breeds and 175 wolves from northwest Canada (Angles et al. 2005; Kennedy et al. 2007a, b; Pedersen et al. 2011; Shiel et al. 2014; Soutter et al. 2015; Ziener et al. 2015; Gershony et al. 2019) (Table S2). For samples homozygous at both DRB1 and DQB1 (n = 21), haplotypes were recorded without further analysis. However, for samples in which only one of the two loci was homozygous (n = 13) or both loci were heterozygous (n = 104), two different allele combinations were distinguished based on comparison with known reference haplotypes. In addition, pedigree information was used for haplotype determination in the Sapsaree. Information regarding the origins of dog breeds was obtained from the American Kennel Club (www.akc.org) and Niskanen et al. (2013). Haplotype trees were constructed using 101 DRB1-DQB1 haplotypes from 19 dog breeds (> 50 animals per breed) and a wolf population from northwest Canada (Table S2). The presence or absence of each haplotype was scored from the haplotype data for each breed and used for to determine haplotype distances or no sharing among breeds. The haplotype discordance between breeds relative to the list of identified haplotypes from all breeds was estimated using DNAML, a program in the PHYLIP package (Felsenstein 2005), and visualized using Haplotype Viewer (Salzburger et al. 2011).
Results and Discussion
Characterization of DLA-DRB1 in 138 Dogs of Five East Asian Breeds, Including New Alleles
We performed sequence-based typing in 138 dogs from five East Asian breeds, including two Korean native breeds (Sapsaree and Jindo) and three Chinese breeds (Tibetan Mastiff, Pug, and Pekingese), and identified 27 distinct alleles of DLA-DRB1, with no failed samples (Table 1). Allelic diversity was highest in Jindos (12 alleles), followed by Sapsarees (9 alleles), Pugs (6 alleles), Pekingese (5 alleles), and Tibetan Mastiffs (5 alleles) (Table 2). We analyzed the phylogenetic relationships between the DRB1 alleles identified in this study (n = 27) and representative alleles for all previously reported DRB1 subgroups (n = 79) (Fig. S1). Although bootstrap support was low for many of the branches in the tree because of extreme polymorphisms among DRB1 alleles, the previously reported DRB1 alleles in the tree formed five major clusters, and all alleles identified in this study were clustered together with the major clusters, indicating that all major DRB1 subgroups were represented among the five breeds in the study. Four new alleles were identified, distributed among only three clusters.
The new alleles identified included DRB1*048:06 (accession no. MT639633) and *029:02 (MT639635), found in Sapsarees; *020:03 (MT639636), found in Jindos; and *092:02 (MT639634), found in Tibetan Mastiffs. Each sequence included intact code for DRB1 exon 2. These results demonstrate the high genetic diversity of DRB1 and the need for further MHC typing in less-studied breeds. The efforts to categorize the global diversity of MHC from diverse dog breeds should contribute to understanding the immunogenetic repertoires of different dog breeds and how variability at MHC affects individual fitness, population dynamics, and viability for dogs (Kennedy 2007).
Interestingly, in this study, 21 out of 27 DRB1 alleles (78%) were found in only a single breed in our panel (Table 1), showing the strong breed specificity of these sequences. Although this could be due partly to the small number of animals typed, which was ≤ 13 for all breeds except Sapsarees (n = 94), genetic distance among breeds or pure genetic drift in breed formation is more likely to be the cause of breed specificity (Wayne and Ostrander 2007). In contrast, DRB1*015:01 was found in all five breeds and was a major allele (frequency ranging from 7.7% to 66.7%) for Pugs, Tibetan Mastiffs, and Sapsarees. This suggest that alleles of the DRB1*015 subgroup might originate from the ancestral population common to Asian breeds. Interestingly, DRB1*015 alleles are also common among non-Asian breeds such as mongrel dogs in Brazil and various European dog breeds including German Shepherd, Yorkshire Terrier and Labrador (Kennedy et al. 2002, 2007b). This allele could have been selected across various dog breeds due to functional importance in immune defense against a common pathogen (Doherty and Zinkernagel 1975; Jeffery and Bangham 2000). MHC haplotypes are closely associated with disease resistance and susceptibility (Sinha et al. 2007).
The average observed heterozygosity (Ho) of DRB1 in the five East Asian breeds was 0.81, much higher than that previously reported for 27 North and East Aisa breeds (0.43; Kennedy et al. 2007b; Niskanen et al. 2013) (Table 2). However, the allelic richness of the previous study was larger (11.38) than this study (8.40), consistent with strong breed specificity of alleles in light of the larger number of breeds included in that study. Because observed heterozygosity should not be affected by the number of animals studied except for the effects of pure chance, observed heterozygosity should primarily reflect degree of genetic diversity despite small sample sizes. In Sapsarees, the most frequent alleles were DRB1*015:01 (33.0%), *023:01 (22.3%), and *015:03 (20.2%); as the Sapsaree sample size was large, this strongly suggests that these are the most frequent alleles for the breed overall, which may be due to a bottleneck effect resulting from the breed’s recent population expansion from a small number of individuals (Oliver and Piertney 2012; Ploshnitsa et al. 2012). The high frequency of DRB1*015:01 (67%) in Tibetan Mastiffs is also interesting, but more individuals must be analyzed to accurately evaluate the allelic diversity of the breed. It has been reported that the MHC region is associated with non-immune phenotypes in domestic animals in addition to its role in the adaptive immune respons, (Shiina et al. 2009). As our sampling strategy tried to exclude closely related individuals (as indicated by a close pedigree relationship), the high frequency of DRB1*015:01 in Tibetan Mastiffs is notable enough to warrant further investigation. Analysis of SNP array data has revealed several genes with selective signatures in Tibetan Mastiffs, including the EPAS1 gene, which is reported to influence high altitude adaptation (Li et al. 2014).
Characterization of DLA-DQB1 in 138 Dogs of Five East Asian Breeds, Including New Alleles
Dqb1 typing of five East Asian dog breeds resulted in the identification of 24 alleles (Table 3). One new allele, Dqb1*020:03:2 (accession no. Mt639637) was identified in a Tibetan Mastiff. We analyzed the phylogenetic relationships between the Dqb1 alleles identified in this study (N = 24) and reference alleles representing all Dqb1 subgroups (N = 37) (Fig. S2). The previously reported Dqb1 alleles in The tree formed four major clusters; as with Drb1, the alleles identified in this study were distributed among all these clusters, indicating that all major Dqb1 subgroups were represented among the five breeds in the study. The fact that only one new allele was identified is consistent with the lower genetic diversity of Dqb1 compared with that of Drb1.
Out of the 24 alleles identified, 12 (DQB1*001:01, *005:02, *008:01:1, *013:01, *013:05, *015:01, *019:01, *020:03:2, *026:01, *035:01, *048:02, and *054:01) were present in only a single breed, which is consistent with the results for DRB1 and suggests that each breed’s DQB1 gene pool is unique (Table 3). Although none of the identified alleles was found in all five breeds, the most widespread were DQB1*013:03 and *023:01, which were shared across four breeds and had relatively high frequencies. DQB1*013:03 was the most abundant allele in Jindos and Tibetan Mastiffs. The allele with the highest frequency was DQB1*005:01 (15.6% of total DQB1 alleles, 22.3% frequency in Sapsarees). The major alleles for DQB1 thus appear to be less predominant than those for DRB1. The genetic diversity ofDQB1 was highest in Tibetan Mastiffs (12 alleles), followed by Sapsarees (10 alleles), Jindos (9 alleles), Pugs (7 alleles), and Pekingese (5 alleles) (Table 4).
Extreme Diversity of DLA Class II Genes in Jindos and Tibetan Mastiffs
The diversity of MHC genes is important for population fitness and can be described as the level of heterozygosity in the population (Oliver and Piertney 2012). For the five breeds examined in this study, the level of observed heterozygosity (Ho) ranged from 0.67 to 1.00 (mean 0.81) for DLA-DRB1 and from 0.80 to 1.00 (mean 0.89) for DLA-DQB1 (Tables 2 and 4). These values were significantly higher than those determined in a previous study of the DRB1 gene, which reported a mean Ho of 0.67 for European breeds and a Ho range of 0.37–0.44 among 128 Asian breeds (Niskanen et al. 2013). DRB1 heterozygosity in the gray wolf (Canis lupus), the wild ancestor of the dog, ranges from 0.62 to 0.87 (Hedrick et al. 2000; Seddon and Ellegren 2004), which is closer to the results of this study than to those of the previous study.
The complete heterozygosity of DQB1 in Jindos and DRB1 in Tibetan Mastiffs is interesting and indicates that they maintain extreme diversity in DLA class II genes (Tables 2 and 4). Jindos, in particular, showed extreme heterozygosity in both DRB1 and DQB1. Differences in DLA heterozygosity values between our study and previous ones could be due to differences in the constitution of the breeds examined (Niskanen et al. 2013). Only five East Asian breeds were included in this study, and they might have had a shorter history of intensive systematic breeding than the breeds in previous studies. Interbreed comparison of the heterozygosity ratio between MHC and non-MHC genes may help illuminate this issue.
Identification of 40 DRB1-DQB1 Haplotypes, Including 11 New Haplotypes
We compared the DRB1-DQB1 haplotypes from all samples, except those containing new alleles, with previously reported haplotypes. Haplotypes not included among the known ones were identified as new haplotypes. We identified a total of 40 haplotypes, including 11 new ones (DRB1*048:06-DQB1*023:01, 029:02–004:01, 029:01–049:01, 015:01–013:01, 015:01–013:02, 049:02–019:01, 092:02–013:05, 015:01–020:03:2, 017:03–013:03, 020:03–013:03, and 004:02–005:01) (Table 5).
Thirty-three haplotypes (82.5%) were specific to a single breed, while only seven were present among multiple breeds, indicating that each breed has its own unique lineage of DLA class II genes. A previous study suggested that most or all the alleles found in the current dog population were already present among the wolf ancestors, considering the non-synonymous substitution rate of 1.18*10–8 (Niskanen et al. 2013). These results are similar to those for pigs, a well-studied livestock species that likewise displays many breed-specific haplotypes (Le et al. 2012).
Haplotype diversity by the number of haplotypes identified from each breed was greatest in Tibetan Mastiffs (13 haplotypes), followed by Sapsarees (12 haplotypes), Jindos (12 haplotypes), Pugs (8 haplotypes), and Pekingese (5 haplotypes). As might be expected from the DRB1 typing results, haplotypes containing the DRB1*015 allele group (DRB1*015:01, *015:02, and *015:03) were found in all five breeds (Table 5). Haplotype DRB1*015:01-DQB1*003:01 was also found in the wolf population (Galaverni et al. 2013). Interestingly, the haplotypes DRB1*003:02-DQB1*008:02 and DRB1*029:01-DQB1*049:01 were shared by both Korean breeds. Seven haplotypes (DRB1*015:01-DQB1*048:02, 015:03–048:02, 023:01–005:01, 075:01–013:03, 048:06–023:01, 011:01–013:02, and 029:02–004:01) were specific to the Sapsaree.
High Diversity of DLA Class II Haplotypes among Asian Dog Breeds
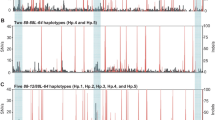
Data were obtained from previous studies of canine DRB1-DQB1 haplotypes that examined > 50 individuals per breed (Table S2) and combined and analyzed to determine the extent of haplotype sharing among 19 dog breeds (12 European, 3 Asian, 3 American, and 1 African) and a wolf population from northwest Canada (Fig. 1). It showed the contribution of each haplotype to 19 selected dog breeds and wolves. Thirty-three percent (34 out of 101) of haplotypes were shared among different breeds. DRB1*006:01-DQB1*007:01 was the most shared haplotype, occurring in 14 breeds. Haplotypes DRB1*006:01-DQB1*007:01 and DRB1*009:01-DQB1*008:01:1 were shared with the wolf. As expected, the wolves showed the greatest number of unique haplotypes (n = 17).
Occurrence of specific DRB1-DQB1 haplotypes among 19 selected dog breeds and wolves, based on available haplotype information. Each breed is represented by a different color. Breeds with more than 50 available DRB1-DQB1 haplotype samples were used to construct the diagram. DRB1-DQB1 haplotype numbers are tentative and given only to facilitate data presentation. Detailed haplotype data are given in Table S2. The new haplotypes DRB1*048:06-DQB1*023:01 (Hap 54), DRB1*049:02-DQB1*004:01 (Hap 55), and DRB1*029:01-DQB1*049:01 (Hap 95) are indicated by an asterisk (*) after their names
All European breeds except the Beagle, together with the sole African breed (Rhodesian Ridgeback), clustered around the center of the tree (Fig. 2), indicating that they shared a higher proportion of DLA haplotypes with one another than with the remaining breeds. This suggests the occurrence of crosses among their early ancestors. The low degree of DLA haplotype sharing between Beagles and other European breeds could be due to the haplotype difference between laboratory and pet Beagles (Soutter et al. 2015). Mongrel Brazilian dogs (mixed breeds from South America) also showed a large genetic distance from the rest.
Tree illustrating dog leukocyte antigen class II haplotype sharing (DRB1-DQB1) among 19 dog breeds. The diagram was plotted using 101 DRB1-DQB1 haplotypes from 12 European breeds, three Asian breeds, three American breeds, and one African breed. The numbers within circular terminal nodes correspond to particular breeds, listed with their sample sizes to the left of the tree. The regional origins of the breeds are indicated by the colors of the terminal nodes (gray, blue, green, and red for Europe, America, Africa, and Asia, respectively). A gray wolf population in northwest Canada (black terminal node) was used as an outgroup. Dots on the branches indicate intermediate states corresponding to one haplotype difference. For example, 17 haplotypes were discordant in the total list of identified haplotypes (n = 101) between Sapsaree (node 1) and Newfoundland (node 15). Haplotype distance was calculated using the DNAML program, part of the PHYLIP package
Among three Asian breeds (Pug, Sapsaree, and American Akita), the Pug showed a closer relationship to European breeds than to the other two in DRB1-DQB1 haplotype sharing, suggesting genetic exchanges between Europe and China in the history of the modern Pug, possibly due to the popularity of the breed in Europe. Previous studies using SNPs and microsatellites showed that Korean Sapsaree and Jindo breeds are most closely related to each other and form a part of larger clusters with Chinese and Japanese breeds (Kim 2001; Gajaweera et al. 2019). Considering their close regional origin and established genetic proximity, the presence of a large diversity of DLA class II alleles among East Asian dog breeds despite small sample sizes is interesting. This may be explained by dog domestication from a large population of wolves in East Asia (Niskanen et al. 2013).
In this study, we performed sequence-based typing of two major DLA class II genes, DRB1 and DQB1, for a population of Korean Sapsarees and four other East Asian breeds. We identified five new alleles and showed that the genetic diversity and breed specificity of DLA class II genes are much higher in Asian breeds than in those from other regions of the world. Because three of the new alleles, DRB1*092:02, DRB1*029:02, and DQB1*020:03:2, were identified from only a single individual, their results were confirmed by multiple typing and subsequent cloning. Although our study was limited to DLA class II genes, our results demonstrate a wide diversity of MHC genes in East Asian dog breeds and illuminate these breeds’ immunogenetics and population genetics.
Availability of Data and Material
All genetic data used in this paper are accessible through the registered accession number, and all other information is specified in the paper.
References
Angles JM, Famula TR, Pedersen NC (2005) Uveodermatologic (VKH-like) syndrome in American Akita dogs is associated with an increased frequency of DQA1*00201. Tissue Antigens 66(6):656-65
Buhler S, Sanchez-Mazas A (2011) HLA DNA sequence variation among human populations: molecular signatures of demographic and selective events. PLoS One 6(2):e14643
Carrington M, Nelson GW, Martin MP, et al. (1999) HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283(5408):1748-52
Doherty PC, Zinkernagel RM (1975) Enhanced immunological surveillance in mice heterozygous at the H-2 gene complex. Nature 256(5512):50-2
Excoffier L, Lischer HE (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10(3):564-7
Felsenstein J (2005) PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle
Foulley J-L, Ollivier L (2006) Estimating allelic richness and its diversity. Livestock Science 101(1-3):150-158
Gajaweera C, Kang JM, Lee DH, et al. (2019) Genetic diversity and population structure of the Sapsaree, a native Korean dog breed. BMC Genet 20(1):66
Galaverni M, Caniglia R, Fabbri E, Lapalombella S, Randi E (2013) MHC Variability in an Isolated Wolf Population in Italy. J Hered 104(5):601-612
Gershony LC, Belanger JM, Short AD, et al. (2019) DLA class II risk haplotypes for autoimmune diseases in the bearded collie offer insight to autoimmunity signatures across dog breeds. Canine Genet Epidemiol 6(1):2
Goudet J (2003) FSTAT version 2. 9. 4: a program to estimate and test population genetics parameters. Updated from Goudet [1995].
Hedrick PW, Lee RN, Parker KM (2000) Major histocompatibility complex (MHC) variation in the endangered Mexican wolf and related canids. Heredity (Edinb) 85(Pt 6):617-24
Hughes AL, Hughes MK (1995) Natural selection on the peptide-binding regions of major histocompatibility complex molecules. Immunogenetics 42(4):233-43
Hughes AL, Nei M (1988) Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature 335(6186):167-70
Hughes AL, Nei M (1989) Nucleotide substitution at major histocompatibility complex class II loci: evidence for overdominant selection. Proc Natl Acad Sci U S A 86(3):958-62
Jeffery KJM, Bangham CRM (2000) Do infectious diseases drive MHC diversity? Microb and Infection 2(11):1335-1341
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21-132
Kennedy LJ (2007) 14th International HLA and Immunogenetics Workshop: report on joint study on canine DLA diversity. Tissue Antigens 69 Suppl 1(s1):269–71
Kennedy LJ, Angles JM, Barnes A, et al. (2007a) DLA-DRB1, DQA1, and DQB1 alleles and haplotypes in North American Gray Wolves. J Hered 98(5):491-9
Kennedy LJ, Barnes A, Happ GM, et al. (2002) Extensive interbreed, but minimal intrabreed, variation of DLA class II alleles and haplotypes in dogs. Tissue Antigens 59(3):194-204
Kennedy LJ, Barnes A, Short A, et al. (2007b) Canine DLA diversity: 1. New alleles and haplotypes. Tissue Antigens 69 Suppl 1(s1):272–88
Kim KS (2001) Genetic Variability in East Asian Dogs Using Microsatellite Loci Analysis. J Hered 92(5):398-403
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547-1549
Lafuente EM, Reche PA (2009) Prediction of MHC-peptide binding: a systematic and comprehensive overview. Curr Pharm Des 15(28):3209-20
Larson G, Karlsson EK, Perri A, et al. (2012) Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc Natl Acad Sci U S A 109(23):8878-83
Le MT, Choi H, Choi MK, et al. (2012) Comprehensive and high-resolution typing of swine leukocyte antigen DQA from genomic DNA and determination of 25 new SLA class II haplotypes. Tissue Antigens 80(6):528-35
Le MT, Choi H, Lee H, et al. (2020) SLA-1 genetic diversity in pigs: extensive analysis of copy number variation, heterozygosity, expression, and breed specificity. Sci Rep 10(1):743
Lee CG, Lee JI, Lee CY, Sun SS (2000) A review of the Jindo, Korean native dog - review. Asian-australas J Anim Sci 13(3):381-389
Li Q, Liu Z, Li Y, et al. (2008) Origin and phylogenetic analysis of Tibetan Mastiff based on the mitochondrial DNA sequence. J Genet Genomics 35(6):335-40
Li Y, Wu DD, Boyko AR, et al. (2014) Population variation revealed high-altitude adaptation of Tibetan mastiffs. Mol Biol Evol 31(5):1200-5
Maenaka K, Jones EY (1999) MHC superfamily structure and the immune system. Curr Opin Struct Biol 9(6):745-53
Matsumura M, Fremont DH, Peterson PA, Wilson IA (1992) Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science 257(5072):927-34
Milinski M, Griffiths S, Wegner KM, Reusch TB, Haas-Assenbaum A, Boehm T (2005) Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc Natl Acad Sci U S A 102(12):4414-8
Morell V (1997) The origin of dogs: running with the wolves. Science 276(5319):1647-8
Niskanen AK, Hagstrom E, Lohi H, et al. (2013) MHC variability supports dog domestication from a large number of wolves: high diversity in Asia. Heredity (Edinb) 110(1):80-5
Oliver MK, Piertney SB (2012) Selection maintains MHC diversity through a natural population bottleneck. Mol Biol Evol 29(7):1713-20
Park K, Choi H, Thong LM, et al. (2010) Simple and comprehensive SLA-DQB1 genotyping using genomic PCR and direct sequencing. Tissue Antigens 76(4):301-10
Pedersen N, Liu H, Millon L, Greer K (2011) Dog leukocyte antigen class II-associated genetic risk testing for immune disorders of dogs: simplified approaches using Pug dog necrotizing meningoencephalitis as a model. J Vet Diagn Invest 23(1):68-76
Ploshnitsa AI, Goltsman ME, Macdonald DW, Kennedy LJ, Sommer S (2012) Impact of historical founder effects and a recent bottleneck on MHC variability in Commander Arctic foxes (Vulpes lagopus). Ecol Evol 2(1):165-80
Salzburger W, Ewing GB, Von Haeseler A (2011) The performance of phylogenetic algorithms in estimating haplotype genealogies with migration. Mol Ecol 20(9):1952-63
Savolainen P, Zhang YP, Luo J, Lundeberg J, Leitner T (2002) Genetic evidence for an East Asian origin of domestic dogs. Science 298(5598):1610-3
Seddon JM, Ellegren H (2004) A temporal analysis shows major histocompatibility complex loci in the Scandinavian wolf population are consistent with neutral evolution. Proc Biol Sci 271(1554):2283-91
Shannon LM, Boyko RH, Castelhano M, et al. (2015) Genetic structure in village dogs reveals a Central Asian domestication origin. Proc Nat Acad Sci U S A 112(44):13639-13644
Shiel RE, Kennedy LJ, Nolan CM, Mooney CT, Callanan JJ (2014) Major histocompatibility complex class II alleles and haplotypes associated with non-suppurative meningoencephalitis in greyhounds. Tissue Antigens 84(3):271-276
Shiina T, Hosomichi K, Inoko H, Kulski JK (2009) The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet 54(1):15-39
Sinha P, Snyder JA, Kim EY, Moudgil KD (2007) The major histocompatibility complex haplotypes dictate and the background genes fine-tune the dominant versus the cryptic response profile of a T-cell determinant within a native antigen: relevance to disease susceptibility and vaccination. Scand J Immunol 65(2):158-65
Sommer S (2005) The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool 2(1):16
Soutter F, Kennedy LJ, Ollier WE, Solano-Gallego L, Catchpole B (2015) Restricted dog leucocyte antigen (DLA) class II haplotypes and genotypes in Beagles. Vet J 203(3):345-7
Swainston-Goodger W (2006) The Pug-Dog - Its History and Origin. Vintage Dog Books
Vonholdt BM, Pollinger JP, Lohmueller KE, et al. (2010) Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464(7290):898-902
Wang GD, Zhai W, Yang HC, et al. (2016) Out of southern East Asia: the natural history of domestic dogs across the world. Cell Res 26(1):21-33
Wayne RK, Ostrander EA (2007) Lessons learned from the dog genome. Trends Genet 23(11):557-67
Zhang Y, Chen Y, Xu H, et al. (2020) SOAPTyping: an open-source and cross-platform tool for sequence-based typing for HLA class I and II alleles. BMC Bioinformatics 21(1):295
Ziener ML, Dahlgren S, Thoresen SI, Lingaas F (2015) Genetics and epidemiology of hypothyroidism and symmetrical onychomadesis in the Gordon Setter and the English Setter. Canine Genet Epidemiol 2(1):12
Zinkernagel RM, Doherty PC (1974) Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 248(5450):701-702
Acknowledgements
The authors thank Jindo Theme Park (Jindo County, South Korea) and the Sapsaree Institute (Kyungsan City, South Korea), for kindly providing samples of Jindo and Sapsaree dogs.
Funding
This work was supported by the Cooperative Research Program of the Center for Companion Animal Research (Project No. PJ01456801), Rural Development Administration, Republic of Korea and by Konkuk University Researcher Fund in 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This research does not involve any human participants, their data or biological material.
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kang, M., Ahn, B., Youk, S. et al. High Allelic Diversity of Dog Leukocyte Antigen Class II in East Asian Dogs: Identification of New Alleles and Haplotypes. J Mammal Evol 28, 773–784 (2021). https://doi.org/10.1007/s10914-021-09560-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-021-09560-x