Abstract
Long necks have evolved independently in several different taxa, but the processes underlying the evolution of this trait are not yet fully understood. In this study, we examined the skeletal mechanism underlying the neck elongation in the tribe Antilopini (Bovidae, Artiodactyla). We calculated the growth patterns of the cervical vertebrae in the gerenuk (Litocranius walleri), which possesses the longest neck in this tribe, and compared it with those in two related species. The growth rates of the vertebrae were not significantly different between species, suggesting that the long neck of the gerenuk has resulted from the elongation of the cervical vertebrae during the fetal or juvenile stage. The morphology of the cervical vertebrae of gerenuks differed from that of the closely related, relatively long-necked dama gazelle (Nanger dama), with protrusions occurring on the dorsal surface of the ventral arch of the atlas. This implies that gerenuks possess a well-developed transverse ligament of the atlas that functions to hold the dens of the axis against the atlas. We also found that the atlas lies in close proximity to the neural spine of the axis in the gerenuk, suggesting that hyperextension of the atlantoaxial joint is osteologically limited in this species. While foraging on high foliage, gerenuks flex and extend their necks freely in a bipedal posture without moving their entire body. These morphological characteristics peculiar to the gerenuk enhance the rigidity of the atlantoaxial joint and decrease the risk of subluxation of the joint during this unique foraging behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A long and flexible neck has evolved independently in a wide range of extant and extinct taxa (Wilkinson and Ruxton 2012). This adaptation allows animals to reach high foliage and to look around surrounding scenery, including behind, without needing to move their entire body (Darwin 1871; Cameron and du Toit 2007; Christian 2010; Taylor et al. 2011). Furthermore, in some species, the long neck contributes to thermoregulation and dominance during male competition (Simmons and Scheepers 1996; Senter 2007; Ward et al. 2008). There have been many discussions about the evolutionary pressures underlying neck elongation in these species (Parrish 2006; Dzemski and Christian 2007; Sander and Clauss 2008; Mitchell et al. 2009; Stevens 2013), but mechanisms behind elongation of the cervical series are little understood.
Neck length is determined by two skeletal factors: the number and individual lengths of the cervical vertebra (Taylor and Wedel 2013a). In birds and other reptiles, neck elongation arises from both of these factors, which complicates our understanding of neck elongation in these taxa. However, in mammals, the number of cervical vertebrae is almost always fixed at seven (Narita and Kuratani 2005), which facilitates the detection of scaling patterns of the cervical skeleton that are related to neck elongation. Thus, an understanding of this scaling pattern in a long-necked mammal may provide new insight into the evolution of this adaptation.
The long neck of the giraffe (Giraffa camelopardalis) has been the most commonly used to investigate the evolution of neck elongation in mammals. Previous studies have calculated the scaling pattern of the cervical vertebrae in giraffe, revealing that their growth rates are higher than those of other vertebral regions in giraffe and the cervical vertebrae in other artiodactyls (Badlangana et al. 2009; van Sittert et al. 2010). These findings have been interpreted in the context of hypotheses concerning the evolution of long necks in a broader sense, as well as the origin and evolution of the neck of giraffe. However, the vertebral column of giraffe represents a unique musculoskeletal adaptation, with the seventh cervical vertebra (C7) being morphologically distinct from that of other artiodactyls (Lankester 1908), and the first thoracic vertebra (T1) showing morphological and functional similarities to C7 of okapi (Okapia johnstoni) (Solounias 1999; Gunji and Endo 2016). This morphological shift in the cervicothoracic boundary, which has only been confirmed in the extant giraffe to date, also affects the growth rates of C7 and T1 in giraffe (Badlangana et al. 2009), and so the scaling pattern of its cervical vertebrae may not apply to other long-necked mammals. Furthermore, the Giraffidae now consists of only two extant species– the giraffe and okapi– which have markedly different neck lengths and body proportions, making it difficult to use the family Giraffidae to generalize about neck elongation in mammals.
The Antilopini (Bovidae, Artiodactyla) is a diverse clade of mostly African antelopes (Bärmann et al. 2013), including the gerenuk (Litocranius walleri) whose necks are among the longest for their body size of any artiodactyls (Wilkinson and Ruxton 2012; Fig. 1). As gerenuks are primarily browsers (Leuthold 1978; Kingdon 2004) and do not use their neck as a weapon, neck elongation in the gerenuk is considered an adaptation for reaching high foliage (Wilkinson and Ruxton 2012). Antilopini also includes the saiga (Saiga tatarica), which is a short-necked antelope that inhabits the Eurasian steppe zone. Moreover, the Antilopini contains various species with an ordinary neck showing intermediate length. Thus, this group enables us to compare the cervical skeleton between closely related species with different neck lengths, providing the ability to gain an understanding of the relationship between the scaling pattern of the cervical skeleton and the diversification of neck length.
Here, we evaluated the growth pattern of each cervical vertebra in the gerenuk, which possesses the longest neck in the Antilopini, and compared it with closely related species to examine the mechanism behind its neck elongation. We also compared the intracervical skeletal proportion between species to understand how the cervical skeleton has been structurally modified during the evolution of neck elongation. Finally, we compared the morphological characteristics of the cervical vertebrae of the gerenuk with those of the related species and discussed the kinematic function of its neck.
Materials and Methods
Materials
We examined the vertebral columns of 72 specimens (ten genera, 14 species) of Antilopini species stored at the American Museum of Natural History (AMNH). These specimens included eight gerenuks, ranging from juvenile to a large mature adult. Information about these specimens is provided in Table 1 (see Appendix for the collection numbers of examined specimens).
Measurement
In artiodactyls, the centrum of each vertebra is convex cranially and concave caudally. Therefore, we defined the ‘functional vertebral length’ as the straight-line distance between the most protruded point on the cranial extremity of the centrum and the deepest point on the caudal extremity of the centrum. To measure the functional vertebral length, we modified the caliper by following the method proposed by Taylor and Wedel (2013b). This involved gluing a bolt onto the inside of one jaw of the caliper and recalibrating the caliper so that it read zero when the bolt was in contact with the other jaw (Fig. 2).
Modified caliper for measuring the functional vertebral length. This was made by following the method proposed by Taylor and Wedel (2013b). The bolt was glued onto the left jaw of the caliper so that it lay perpendicular to the other jaw
We measured the length of each vertebra from the atlas to the sacrum. As the atlas does not possess a centrum, its length was measured medially along the ventral arch using an unmodified caliper, whereas the functional lengths of the vertebrae between the axis and the last lumbar vertebra were measured using the modified caliper. The odontoid process of the axis, which is an embryological derivation of the centrum of the atlas (Liem et al. 2001), was included in the measurement of the functional length of the axis. During measurements, the vertebra was placed between the jaws of the modified caliper, with the unmodified jaw contacting the most projecting point on the cranial extremity of the centrum and the bolt touching the deepest point on the caudal extremity. The length of the sacrum was measured parallel to the midsagittal plane of the vertebral complex that consists of the fused sacral vertebrae. All measurements are provided in the Electronic Supplementary Material.
Data Analysis
To remove any size effects, we corrected all data by trunk length, which was estimated by combining the lengths of the vertebrae from the first thoracic vertebra to the sacrum. We estimated the growth pattern of the cervical vertebrae in gerenuks by calculating the ordinary least-squares regression line between the length of each cervical vertebra and trunk length. Additionally, we assessed the growth pattern of the cervical vertebrae in blackbuck (Antilope cervicapra) and oribi (Ourebia ourebi) with the same method. To evaluate whether the regression line in the gerenuks differed significantly from those in blackbuck and oribi, we used the analysis of covariance (ANCOVA) and tested the difference in slope and intercept of the regression lines (McDonald 2014).
We also calculated the ratio of the length of each cervical vertebra to the total neck length to elucidate the intracervical proportion in each species. Following Badlangana et al. (2009), we defined the total neck length as the sum of the functional lengths of the vertebrae between the C2 and C7. We then used the Tukey-Kramer test to compare the ratio of each cervical vertebra in gerenuk with the other Antilopini species.
Morphological Description
We described and compared the morphological characteristics of the cervical vertebrae in gerenuk, dama gazelle (Nanger dama), and Thomson’s gazelle (Eudorcas thomsonii). The dama gazelle is distinguished from other gazelles by the large body, long legs, and a relatively long and slender neck (Barbosa and Espeso 2006; Wilkinson and Ruxton 2012). The Thomson’s gazelle, the most common gazelle in East Africa, was utilized as a comparison species with an ordinary neck length. The detailed information about the specimens examined in the morphological comparison is provided in Table 2. The growth stage of the specimens was divided into three stages by degree of ossification of the epiphyseal plate in the long bones: juvenile, sub-adult, and adult (Hildebrand and Goslow 2001). In this paper, the juvenile specimens were defined as those possessing the epiphyseal plate separated from its diaphysis. The sub-adult specimens were defined as those in which the epiphyseal plate fused to the diaphysis and exhibiting an obvious suture line. The adult specimens were defined as those with a completely fused epiphyseal plate with no visible suture line.
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Results
Relationship between Each Cervical Vertebral Length and Trunk Length
The length of each cervical vertebra was correlated with the trunk length, in all three species: gerenuk, blackbuck, and oribi (Table 3, Fig. 3). However, all of the cervical vertebrae in gerenuk were longer than the length that would be expected based on the regression line for the other two species.
Scaling pattern of the vertebral length of C2. The open triangles indicate gerenuk (Litocranius walleri) data. The black circles indicate the data of blackbuck (Antilope cervicapra), and the open circles indicate the data of oribi (Ourebia ourebi). The grey circles indicate the data of the other 11 Antilopini species examined. The regression line between trunk length and vertebral length of C2 is denoted by a solid line for gerenuks (y = 0.11× + 17.3, adjusted r2 = 0.98, p < 0.01), a dashed line for blackbucks (y = 0.083× + 12.8, adjusted r2 = 0.30, p = 0.017), and a dotted line for oribis (y = 0.11× − 7.0, adjusted r2 = 0.46, p < 0.01). Note that the slope of the regression line has no significant difference between gerenuk and the other two species, respectively. This suggests that the elongation of the vertebra in the gerenuk arises from the elongation of the vertebral body at an early developmental stage, rather than from the accelerated growth rate of the vertebrae after birth
According to the ANCOVA results, there was no significant difference in the slopes of the regression lines between the gerenuk and the two comparison species, respectively; whereas, the intercepts were significantly greater in gerenuk (Table 4, Fig. 3).
Intracervical Proportion
The ratio of the length of each cervical vertebra to the total neck length was highest in the axis, in every species examined in this study (Fig. 4). This ratio then decreased progressively in a caudal direction from C2 to C7. The Tukey-Kramer test demonstrated that there was no significant difference in the intracervical proportion between gerenuk and the other Antilopini species (Turkey-Kramer test; C2, p = 0.19; C3, p = 0.37; C4, p = 0.34; C5, p = 0.46; C6, p = 0.19; C7, p = 0.64).
Ratio of the length of each cervical vertebra to the total neck length. The boxplots show the ratios for each vertebra in the 13 Antilopini species examined, not including gerenuk (Litocranius walleri). The median value for each species was used in this analysis. The open circles indicate the outliers. Arrows indicate the median value of the ratio for each vertebra in the gerenuk. Regardless of the length of the neck, the intracervical proportion showed a trend common in the Antilopini
Vertebral Morphology
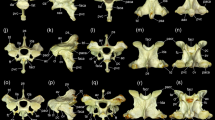
Remarkable caudal protrusions occurred on the wings of the atlas in both the gerenuk and dama gazelle (Fig. 5a, c). The wings of the atlas were not as laterally extended in these species as in Thomson’s gazelle (Fig. 5). The caudal articular facet of the atlas exhibited a flat surface in the dama gazelle and Thomson’s gazelle (Fig. 5c, d). This morphological characteristic was also observed in all female gerenuks and juvenile male gerenuks. However, in the adult and subadult male gerenuks, the right and left ventrolateral edges of the articular facets were slightly protruded and curved caudally, so that they enclosed the cranial articular process of the axis (Fig. 5a, b). Moreover, these specimens also possessed a circular concave region at the caudal end of the atlas surrounding the vertebral foramen (Fig. 6a), in the ventral region of which fitted the dens of the axis. The most notable characteristic of the atlas of the gerenuk was a pair of protrusions on the dorsal surface of the ventral arch (Fig. 7a–d), which extended in a caudal direction. These protrusions were confirmed in all adult and subadult specimens of both male and female gerenuks, whereas there was no clear protrusion in the juvenile specimens. The protrusions were more noticeable in the adult males than in adult females (Fig. 7b, d). We did not observe such protrusions in two comparison species, even though all specimens were adult or subadult (Fig. 7e, f).
Dorsal view of the atlas in a, an adult male gerenuk (Litocranius walleri; AMNH M-81170); b, the same adult male gerenuk showing a close-up of the atlantoaxial joint; c, an adult male dama gazelle (Nanger dama; AMNH M-80091); and d, an adult male Thomson’s gazelle (Eudorcas thomsonii; AMNH M-82058). w, wing of the atlas; cw, caudal protrusion of the wing of the atlas; 1, the curvature of the caudal articular facet. Scale bars indicate 2 cm. Note that the ventrolateral edges of the articular facets in the male gerenuk were slightly protruded and curved caudally, and enclosed the cranial articular process of the axis
Caudal view of the atlas in a, an adult male gerenuk (Litocranius walleri; AMNH M-81170); b, an adult male dama gazelle (Nanger dama; AMNH M-80091); and c, an adult male Thomson’s gazelle (Eudorcas thomsonii; AMNH M-82058). caf, caudal articular facet; vt, ventral tubercle; 1, curvature of the ventrolateral edge of the caudal articular facet in the male gerenuk; 2, circular concave region on the caudal end of the atlas surrounding the vertebral foramen in the male gerenuk. Scale bars indicate 2 cm
Cranial view of the atlas in a, an adult male gerenuk (Litocranius walleri; AMNH M-81170); b, the same adult male gerenuk showing a close-up of the protrusion located on the dorsal surface of the ventral arch of the atlas; c, an adult female gerenuk (Litocranius walleri; AMNH M-187829); d, the same adult female gerenuk showing a close up of the protrusion; e, an adult male dama gazelle (Nanger dama; AMNH M-80091); and f, an adult male Thomson’s gazelle (Eudorcas thomsonii; AMNH M-82058). pr, protrusion. Scale bars indicate 2 cm. Note that both male and female gerenuks possess a pair of the protrusions occurring on the dorsal surface of the ventral arch of the atlas. The protrusions were more remarkable in the male than the female
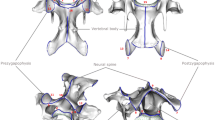
The posterior articular processes of the axis of the gerenuks were short and hardly protruded from the centrum (Fig. 8). The cranial part of the neural spine was quite close to the dorsal arch of the atlas when the articular surfaces of the atlas and axis were fully overlapped (Fig. 8a).
Left lateral view of the atlas-axis complex in a, an adult male gerenuk (Litocranius walleri; AMNH M-81170); b, an adult male dama gazelle (Nanger dama; AMNH M-80091); and c, an adult male Thomson’s gazelle (Eudorcas thomsonii; AMNH M-82058). ns, neural spine; pap, posterior articular process; tp, transverse process. Scale bars indicate 5 cm. Note that the neural spine of the axis lies in close proximity to the dorsal arch of the atlas, in the gerenuk (1)
In the vertebrae between the C2 and C5, the caudal end of the vertebral arch of the cervical vertebrae was concave at the midline region between the right and left articular processes in dama gazelles and Thomson’s gazelles (Fig. 9c, d). However, in male gerenuks, the caudal end of the vertebral arch marked a straight line from the right articular process to the left articular process, without any concavity (Fig. 9a). This characteristic was confirmed in all male specimens from juvenile to adult. In female gerenuks, regardless of the growth stages, the caudal midline area of the vertebral arch protruded from the line connecting the caudal ends of the right and left articular processes (Fig. 9b). When the articular facets of the two adjacent vertebrae overlapped one another, the caudal end of the vertebral arch was located in close proximity to the cranial part of the vertebral arch of the adjacent vertebra in both male and female gerenuks (Fig. 9a, b). By contrast, in dama and Thomson’s gazelles, there was a notch in the region between the caudal end of the vertebral arch of a vertebra and the cranial part of the vertebral arch of the adjacent vertebra (Fig. 9c, d). The transverse processes of the vertebrae between C3 and C5 were not as laterally protruded in gerenuks as in dama gazelles and Thomson’s gazelles (Fig. 9). Furthermore, the processes were located in the central area of the vertebrae in dama and Thomson’s gazelles, but in the caudal region in gerenuks.
Dorsal view of C3 and C4 in a, an adult male gerenuk (Litocranius walleri; AMNH M-81170); b, an adult female gerenuk (AMNH M-187829); c, an adult male dama gazelle (Nanger dama; AMNH M-80091); and d, an adult male Thomson’s gazelle (Eudorcas thomsonii; AMNH M-82058). pap, posterior articular process; tp, transverse process; 1, proximity between the caudal end of the vertebral arch of C3 and the cranial edge of the neural spine of C4. Scale bars indicate 2 cm. Both in male and female gerenuks, there is no notch at the midline region of the two adjacent vertebrae; whereas, the notch was observed in the two comparison species
In C6 and C7, the vertebral laminae were more deeply concave in the gerenuks than in the dama gazelles and Thomson’s gazelles (Figs. 10 and 11). In addition, the neural spine of C7 was wider in the craniocaudal direction in the gerenuks compared with the dama and Thomson’s gazelles (Fig. 11). Unlike the vertebrae from C2 to C5, there was a notch between the caudal end of the vertebral arch of a vertebra and the cranial part of the vertebral arch of the adjacent vertebra in the C5/C6 and C6/C7 joints in the gerenuks. We confirmed the notch in the C5/C6 and C6/C7 joints also in the dama and Thomson’s gazelles.
Left lateral view of C6 in a, an adult male gerenuk (Litocranius walleri; AMNH M-81170); b, an adult male dama gazelle (Nanger dama; AMNH M-80091); and c, an adult male Thomson’s gazelle (Eudorcas thomsonii; AMNH M-82058). ns, neural spine; tp, transverse process; vl, ventral lamella; 1, deep concave region in the lamina of the vertebra. Scale bars indicate 2 cm
Left lateral view of C7 in a, an adult male gerenuk (Litocranius walleri; AMNH M-81170); b, an adult male dama gazelle (Nanger dama; AMNH M-80091); and c, an adult male Thomson’s gazelle (Eudorcas thomsonii; AMNH M-82058). ns, neural spine; tp, transverse process; 1, deep concave region in the lamina of the vertebra. Scale bars indicate 2 cm
Discussion
Scaling Pattern of the Cervical Vertebrae in Gerenuks
Our analysis revealed that the length of each cervical vertebra was correlated with trunk length, and that the slope of the regression line for gerenuks was parallel to that for the blackbucks and oribis (Table 4). This indicates that each cervical vertebra of the gerenuk is already elongated by at least the juvenile stage, that the neck grows isometrically relative to other vertebrae after birth, and that the growth rates of the vertebrae are equal to those of the other species. A recent study reported a similar pattern in the diversification of the length of the limb bone in Anolis lizards, whereby the growth rate of the limb bones was equal in long-limbed and short-limbed species (Sanger et al. 2012). In these lizards, it was demonstrated that the elongation of the limb bone arose from an increase in the size of the embryonic limb template before the formation of the cartilaginous anlagen (Sanger et al. 2012). By contrast, in mice, diversification in the length of the limb bone resulted from differences in growth rate between three and five weeks of age (Sanger et al. 2011). In this case, a variation during early development is then overridden by any variation that is generated later in life (Sanger et al. 2011). In the present study, we did not examine the growth pattern of the vertebrae of gerenuks at the fetal and neonatal stages, and so are unable to discuss the detailed developmental mechanism that promotes neck elongation. Therefore, further study is required to verify the hypothesis that the diversification of neck length in the Antilopini results from differences in the size of each vertebra at an early developmental stage.
Previous studies have demonstrated that neck elongation in giraffe results from the high growth rate of the cervical vertebrae after birth as well as the elongation of the vertebrae at an earlier developmental stage (Badlangana et al. 2009; van Sittert et al. 2010). Additionally, Badlangana et al. (2009) suggested that the long neck in the camelids arose from the elongation of the vertebrae at an early developmental stage, not from an accelerated growth rate of the vertebrae after birth. They found that the mechanism of evolving a longer neck was dissimilar between giraffe and camelids, and predicted that the gerenuk would show yet another independent skeletal pattern in association with the lengthening of the neck. However, the present study demonstrates that the long neck of the gerenuk results from the difference of the vertebral size at an early developmental stage, as with the previous result in the camelids. Our study emphasizes anew the uniqueness of the skeletal mechanism of evolving a longer neck in the giraffe.
The intracervical proportion was not significantly different between gerenuk and the other Antilopini species examined, despite the individual cervical vertebrae being much longer in the gerenuk. This indicates that the long neck of gerenuks is not achieved by the elongation of a subset of cervical vertebrae, but rather results from the elongation of all of the cervical vertebrae at an equal rate. Thus, the gerenuk has evolved a long neck under a possible phylogenetic constraint that relates to a scaling law of the cervical vertebrae. The observed invariance in the intracervical proportion also suggests that the long neck of gerenuks has evolved without any significant modification to the relative position of the muscular, nervous, and vascular structures in the neck.
Morphological Adaptations of the Cervical Vertebrae in the Gerenuk
We found few common characteristics of the cervical vertebrae between gerenuk and dama gazelle, although dama gazelle was regarded as a species with a relatively long neck. Furthermore, the morphology of the cervical vertebrae in gerenuk differed considerably from that in both dama gazelle and Thomson’s gazelle, indicating that there is little derivative morphology of the cervical vertebrae relating to neck elongation.
In gerenuks especially, the atlantoaxial complex exhibited various significantly specialized morphological characteristics. Among these, we found a pair of protrusions on the dorsal surface of the ventral arch of the atlas in gerenuk (Fig. 7a–d), which have never previously been observed in the other Antilopini species or other long-necked species such as camels and giraffes. However, transverse ligaments of the atlas have been observed in humans, carnivorans, and pigs at the same location as the protrusions observed in gerenuk (Evans 1993; König and Liebich 2006; Kupczynska et al. 2013; Standring 2015). The transverse ligament of the atlas is a strong ligament that connects one side of the ventral arch of the atlas to the other side via the dorsal surface of the dens of the axis. It plays a role in holding the dens of the axis against the body of the atlas and in limiting anterior gliding of the atlas during neck flexion (Fielding et al. 1974; Evans 1993). The protrusions that were observed on the dorsal surface of the ventral arch of the atlas in gerenuks are suggestive that this species possesses a well-developed transverse ligament of the atlas.
Additionally, we confirmed that the cranial part of the neural spine of the axis lies in close proximity to the dorsal arch of the atlas (Fig. 8a). In the vertebrae from C2 to C5 in gerenuks, we described a closeness between the caudal end of the vertebral arch of the vertebra and the cranial part of the vertebral arch of the posterior adjacent vertebra (Fig. 9a, b). These structures could be regarded as an osteological stop that prevents hyperextension of the vertebral joints.
Gerenuks exhibit a unique foraging behavior: they often stand up on their hind legs, supporting their body with their forefeet on the tree, and then pluck leaves and shoots using their giraffe-like long upper lip (Schomber 1966). During foraging, they flex and extend their neck freely in a bipedal posture with their entire bodies stationary, which allows them to harvest leaves and shoots over a wide range. The well-developed transverse ligament of the atlas will enhance the stability of the atlantoaxial joint and reduce the risk of subluxation of the joint when the gerenuk protracts its head and neck to browse foliage at the same height as its head (Fig. 12a). Moreover, the osteological stops observed in the vertebrae from the atlas to C5 contribute to enhancing the rigidity of the neck and will prevent hyperextension of the vertebral joints. Especially, the osteological stop caused by the neural spine of the axis should play a key role in preventing subluxation of the atlantoaxial joint when the gerenuk leans its head dorsally to browse leaves and shoots at a higher level than its head (Fig. 12b). Subluxation of the atlantoaxial joint has been reported in dogs and cattle, and results in serious damage to a vital part of the spinal cord (Geary et al. 1967; White et al. 1978). Therefore, the structural limitation of mobility of the atlantoaxial joint is of significant importance for the unique foraging behavior of the gerenuk.
Diagram of the atlantoaxial joint when a, the gerenuk (Litocranius walleri) flexes and protracts its neck and head to harvest foliage at the same level as its head; and b, the gerenuk extends its neck and leans its head dorsally to harvest foliage at a higher level than its head. tl, transverse ligament of the atlas; d, dens of the axis; ns, neural spine of the axis; 1, transverse ligament of the atlas fastening the atlas to the dens of the axis; 2, neural spine of the axis supporting the atlas during hyperextension of the neck
We also observed that the protrusions on the dorsal surface of the ventral arch of the atlas were larger in males than females, suggesting that male gerenuks may possess a more developed transverse ligament of the atlas than females. Furthermore, the caudal articular surface of the atlas in male gerenuks enclosed the cranial articular process of the axis (Fig. 5a, b). A curvature of the caudal articular surface of the atlas would enhance the stability of the atlantoaxial joint and facilitate rotational movement of the atlas. Male gerenuks have a circular concave region, regarded as the articular fovea for the dens of the axis, on the caudal end of the atlas surrounding the vertebral foramen (Fig. 6a). All of these structures, which were not observed in female gerenuks, would contribute to increasing the stability of the atlantoaxial joint and preventing subluxation of the joint. Male gerenuks possess large and heavy horns, and competes against other males via collision of the horns and violent nods of the head (Schomber 1966; Kingdon 1988). Thus, the atlantooccipital and atlantoaxial joints of males would be subjected to powerful external forces during fighting behavior. In addition, the heavy horns would impose a strain on the atlantoaxial joint during neck flexion and extension during foraging. Consequently, males would require greater stability of the atlantoaxial joint than females. The observed structural peculiarities in male gerenuks would fulfill these functional demands associated with their fighting and foraging behaviors.
References
Badlangana NL, Adams JW, Manger PR (2009) The giraffe (Giraffa camelopardalis) cervical vertebral column: a heuristic example in understanding evolutionary processes? Zool J Linn Soc 155:736–757
Barbosa A, Espeso G (2006) International Studbook, Gazella dama mhorr. Consejo Superior de Investigaciones Científicas, Madrid
Bärmann EV, Rössner GE, Wörheide G (2013) A revised phylogeny of Antilopini (Bovidae, Artiodactyla) using combined mitochondrial and nuclear genes. Mol Phylogenet Evol 67:484–493
Cameron EZ, du Toit JT (2007) Winning by a neck: tall giraffes avoid competing with shorter browsers. Am Nat 169:130–135
Christian A (2010) Some sauropods raised their necks –evidence for high browsing in Euhelopus zdanskyi. Biol Lett 6:823–825
Darwin C (1871) The Descent of Man and Selection in Relation to Sex. John Murray, London
Dzemski G, Christian A (2007) Flexibility along the neck of the ostrich (Struthio camelus) and consequences for the reconstruction of dinosaurs with extreme neck length. J Morphol 268:701–714
Evans HE (1993) Miller’s Anatomy of the Dog, 3rd ed. WB Saunders, Philadelphia
Fielding JW, Cochran GVB, Lansing JF, Hohl M (1974) Tears of the transverse ligament of the atlas. J Bone Joint Surg Am 56:1683–1691
Geary JG, Oliver JE, Hoerlein BF (1967) Atlanto axial subluxation in the canine. J Small Anim Pract 8:577–582
Gunji M, Endo H (2016) Functional cervicothoracic boundary modified by anatomical shifts in the neck of giraffes. Roy Soc Open Sci 3:150604
Hildebrand M, Goslow G (2001) Analysis of Vertebrate Structure, 5th edn. John Wiley & Sons, Inc., New York
Kingdon J (1988) East African mammals: An Atlas of Evolution in Africa: Part D. University Chicago Press, Chicago
Kingdon J (2004) The Kingdon Pocket Guide to African Mammals. A&C Black, London
König HE, Liebich HG (2006) Veterinary Anatomy of Domestic Mammals: Textbook and Colour Atlas. Schattauer Verlag, Stuttgart
Kupczynska M, Barszcz K, Janczyk P, Wasowicz M, Czubaj N (2013) Morphology of the transverse ligament of the atlas and the alar ligaments in the silver fox (Vulpes vulpes var). BMC Vet Res 9:64
Lankester R (1908) On certain points in the structure of the cervical vertebrae of the okapi and the giraffe. Proc Zool Soc Lond 1908:320–334
Leuthold W (1978) On the ecology of the gerenuk Litocranius walleri. J Anim Ecol 47:561–580
Liem KF, Bemis WE, Walker WF Jr, Grande L (2001) Functional Anatomy of the Vertebrates: An Evolutionary Perspective, 3rd edn. Harcourt, San Diego
McDonald JH (2014) Handbook of Biological Statistics, 3rd edn. Sparky House Publishing, Baltimore
Mitchell G, van Sittert S, Skinner JD (2009) Sexual selection is not the origin of long necks in giraffes. J Zool 278:281–286
Narita Y, Kuratani S (2005) Evolution of the vertebral formulae in mammals: a perspective on developmental constraints. J Exp Zool 304:91–106
Parrish JM (2006) The origins of high browsing and the effects of phylogeny and scaling on neck length in sauropodomorphs. In: Carrano MT, Gaudin TJ, Blob RW, Wible JR (eds) Amniote Paleobiology: Phylogenetic and Functional Perspectives on the Evolution of Mammals, Birds, and Reptiles. University of Chicago Press, Chicago, pp 201–224
Sander PM, Clauss M (2008) Sauropod gigantism. Science 322:200–201
Sanger TJ, Norgard EA, Pletscher LS, Bevilacqua M, Brooks VR, Sandell LM, Cheverud JM (2011) Developmental and genetic origins of murine long bone length variation. J Exp Zool B (Mol Dev Evol) 316B:146–161
Sanger TJ, Revell LJ, Gibson-Brown JJ, Losos JB (2012) Repeated modification of early limb morphogenesis programmes underlies the convergence of relative limb length in Anolis lizards. Proc R Soc B 279:739–748
Schomber HW (1966) Giraffengazelle und Lamagazelle. A. Ziemsen Verlag, Wittenberg Lutherstadt
Senter P (2007) Necks for sex: sexual selection as an explanation for sauropod dinosaur neck elongation. J Zool 271:45–53
Simmons RE, Scheepers L (1996) Winning by a neck: sexual selection in the evolution of giraffe. Am Nat 148:771–786
van Sittert SJ, Skinner JD, Mitchell G (2010) From fetus to adult – an allometric analysis of the giraffe vertebral column. J Exp Zool (Mol Dev Evol) 314B:469–479
Solounias N (1999) The remarkable anatomy of the giraffe’s neck. J Zool 247:257–268
Standring S (2015) Gray’s Anatomy, 41th edn. Churchill Livingstone, London
Stevens KA (2013) The articulation of sauropod necks: methodology and mythology. PLoS One 8:e78572
Taylor MP, Hone DWE, Wedel MJ, Naish D (2011) The long necks of sauropods did not evolve primarily through sexual selection. J Zool 285:150–161
Taylor MP, Wedel MJ (2013a) Why sauropods had long necks; and why giraffes have short necks. PeerJ 1:e36
Taylor MP, Wedel MJ (2013b) The effect of intervertebral cartilage on neutral posture and range of motion in the necks of sauropod dinosaurs. PLoS One 8:e78214
Ward J, McCafferty DJ, Houston DC, Ruxton GD (2008) Why do vultures have bald heads? The role of postural adjustment and bare skin areas in thermoregulation. J Therm Biol 33:168–173
White ME, Pennock PW, Seiler RJ (1978) Atlanto-axial subluxation in five young cattle. Can Vet J 19:79–82
Wilkinson DM, Ruxton GD (2012) Understanding selection for long necks in different taxa. Biol Rev 87:616–630
Acknowledgements
We are grateful to Ms. Eleanor Hoeger and Ms. Eileen Westwig (AMNH) for access to skeletal specimens under their care. We feel strong gratitude toward Dr. Nikos Solounias (New York Institute of Technology) for helping our investigation at AMNH. We thank Dr. Shin-ichiro Kawada (National Museum of Nature and Science, Tokyo) for allowing us to access the taxidermied specimens of the gerenuk. We thank Dr. Yasuhisa Nakajima (The University of Tokyo) for helpful discussions. We thank Dr. Eric Snively (University of Wisconsin) for improving our English.
This study was supported by JSPS fellowship for young scientists (Research project no. 14 J04974).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(XLSX 57 kb)
Appendix
Appendix
Species | collection no. | |
|---|---|---|
Chinkara gazelle | Gazella bennettii | M-54506 |
Rhimgazelle | G. leptoceros | M-238347 |
Goitored gazelle | G. subgutturosa | M-54896, M-57350 |
Blackbuck | Antilope cervicapra | M-10270, M-10732, M-14139, M-14140, M-20778, M-28827, M-35058, M-35218, M-35305, M-35527, M-35712, M-35957, M-54486, M-81689, M-100362, M-180039 |
Grant’s gazelle | Nanger granti | M-35299, M-82053, M-82055, M-82056, M-82057, M-82152, M-85153 |
Dama gazelle | N. dama | M-80091, M-113808 |
Soemmerring’s gazelle | N. soemmerringii | M-80111 |
Thomson’s gazelle | Eudorcas thomsonii | M-82058, M-82059, M-82061 |
Springbok | Antidorcas marsupialis | M-35864, M-70450, M-81739, M-81740, M-81743, M-81745, M-83549, M-233055 |
Gerenuk | Litocranius walleri | M-81170, M-87214, M-87215, M-87216, M-88401, M-88409, M-183302, M-187829 |
Saiga | Saiga tatarica | M-85301, M-85302, M-85303, M-85304, M-119649, M-171758, M-180279 |
Oribi | Ourebia ourebi | M-34763, M-34764, M-53394, M-53307, M-53312, M-53317, M-53330, M-80258, M-80545, M-82069, M-82071, M-216388 |
Steenbok | Raphicerus campestris | M-34728, M-80538 |
Mongolian gazelle | Procapra gutturosa | M-46444, M-46453 |
Rights and permissions
About this article
Cite this article
Gunji, M., Endo, H. Growth Pattern and Functional Morphology of the Cervical Vertebrae in the Gerenuk (Litocranius walleri): The Evolution of Neck Elongation in Antilopini (Bovidae, Artiodactyla). J Mammal Evol 26, 225–235 (2019). https://doi.org/10.1007/s10914-017-9396-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-017-9396-7