Abstract
Superparamagnetic zinc ferrite (ZnFe2O4) nanoparticles were prepared by a surfactant assisted hydrothermal method and subjected to the heat treatment. The structure, vibrational, morphology, and magnetic properties of synthesized product were characterized by XRD, FT-IR, HR-SEM, and VSM measurements. XRD result confirms the formation of regular spinel structured ZnFe2O4 with space group of Fd3m and an average crystalline size was calculated as 21 nm and 28 nm for the samples annealed in air atmosphere at 300 °C and 600 °C. The HR-SEM image shows that the particles are in spherical shape with small aggregation. A room temperature superparamagnetic behavior was observed for both samples. The saturation magnetization (M s) of 12.0 emu/g and 9.10 emu/g were observed for the samples annealed in air atmosphere at 300 °C and 600 °C, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent times, intensive research is being carried out in the controlled growth of nanostructures in various fields, such as electronics, optics, magnetic materials and energy conversion [1]. Spinel ferrites with the formula MFe2O4 (where M = Fe, Zn, Mn, Ni, Co etc.) are important magnetic materials, which have attracted plenty of attention due to its size and shape dependent properties [2]. Among these, the zinc-based ferrite (ZnFe2O4) nanoparticles have drawn much more attention because of their potential applications in the field of photocatalysis [3], magnetic resonance imaging (MRI) [4], ferrofluids [5], information storage [6], and gas sensors [7]. Until now various synthesis methods have been reported for the preparation of ZnFe2O4 nanoparticles such as sol-gel [8], Co-precipitation [9], combustion [10], thermal decomposition [11] and hydrothermal method [12]. Naseri et al. have reported synthesis of superparamagnetic ZnFe2O4 nanoparticles by thermal treatment method using a capping agent and investigated the magnetic properties of the zinc ferrite with respect to size of the particles [13]. Wang et al. have reported different annealed temperature synthesis of superparamagnetic ZnFe2O4 nanoparticles by thermal decomposition method and investigated magnetic properties [14]. The hydrothermal technique is most useful due to its simplicity, low cost, and nontoxic route. Furthermore, it offers the possibility to deal with a great number of experimental strategies by modification of parameters such as solvent, precipitant agent concentration or temperature [15]. In the present work, the superparamagnetic ZnFe2O4 nanoparticles were prepared by a hydrothermal method, using ethylamine as a surfactant, and the product was annealed at two different temperatures. The structural, vibrational, morphological, and magnetic studies were investigated by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), high resolution scanning electron microscopy (HR-SEM), and the vibrating sample magnetometer (VSM) techniques.
2 Materials and Methods
2.1 Materials
Zinc nitrate hexahydrate [Zn (NO3)2⋅6H2O], ferric nitrate nonahydrate [Fe (NO3)3⋅9H2O], and ethylamine (C2H7N) were purchased from Sigma-Aldrich chemical reagent Co. (MO, USA) and were used without further purifications.
2.2 Preparation of Superparamagnetic ZnFe2O4 Nanoparticles
ZnFe2O4 nanoparticles were prepared by the surfactant assisted hydrothermal method. Zinc nitrate and ferric nitrate were used as reagents. An appropriate amount of metal nitrates with molar ratio of 1:2 were first dissolved in 40 ml de-ionized (DI) water. Then 2 ml of ethylamine was added to the solution by drop wise under magnetic string at room temperature. Finally we obtained precipitated solution. The mixture was continuously stirred for half an hour and transferred to 50 ml Teflon-lined autoclave. The autoclave was sealed and maintained at 160 °C for 15 h then allowed it to reach the room temperature. Finally, the brown precipitates were collected by centrifugation using DI water and absolute ethanol washed three times and dried at 60 °C for 2 h at hot air oven. Then product was crushed by mortar and annealed in air atmosphere at 300 °C and 600 °C for 10 h. The final products were named sample-a and sample-b.
2.3 Characterizations
Crystal structure of the synthesized products was identified by powder X-ray diffraction pattern of the samples recorded using PAN analytical X′ pert pro X-ray diffractometer with CuKα radiation (1.5406 Å). FTIR spectra were recorded from 400 cm−1 to 4000 cm−1 using Perkin Elmer spectrometer by the KBr pellet technique. The morphology of the annealed samples was inspected using a FEI Company, Quanta 200 FEG scanning electron microscope with an accelerating voltage of 10 kV. The magnetic hysteresis loops were recorded by a vibrating sample magnetometer VSM lakeshore-7410 at room temperature.
3 Results and Discussion
3.1 Structural Analysis
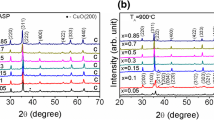
The crystallographic structure of the synthesized products was identified of powder XRD measurement. The powder XRD patterns sample-a and sample-b are presented in Fig. 1. All the diffraction peaks were indexed to the face centered regular spinel cubic structure with space group of Fd3m, consistent with lattice parameter of α=8.412 Å (JCPDS card No. 89-1009). No other impurity peaks were detected, which confirm the purity of the materials. The strong sharp peaks revealed that ZnFe2O4 nanoparticles were well crystallized. The average crystallite size of ZnFe2O4 was calculated using Scherrer’s equation,
where D is the size of the axis parallel to (hkl) plane, K is a constant taken as 0.89, θ is the diffraction angle, β is the broadening of diffraction line measured at half maximum intensity (radians), and λ=1.5406 Å, the wavelength of CuKα. The line broadening of the X-ray diffraction pattern gives clear evidence for the nanocrystalline nature of the synthesized samples. The calculated average crystallite sizes were 21 nm and 28 nm for sample-a and sample-b, respectively.
3.2 Functional Group Analysis
Figure 2 shows regular spinel ZnFe2O4 structure in the annealed zinc ferrite sample is further supported by FT-IR spectrum. The broad band absorption peak at 3433 cm−1 (bending mode of H2O) indicated the presence of water molecules on the surface of ZnFe2O4 nanoparticles. The very weak band observed at 1640 cm−1 was related to stretching vibration of C=C, presence of EA on surface of the ZnFe2O4 nanoparticles. The strong peaks appearing at 542 cm−1 and 467 cm−1 represent tetrahedral and octahedral modes of ZnFe2O4; this was assigned to Fe–O vibration and Zn–O vibration, respectively [16].
3.3 Morphological Analysis
The microstructure of synthesized products was acquired using HR-SEM measurement. Figure 3 shows that the nanoparticles have almost uniform spherical shape with small aggregation. From SEM image, it can also be seen that sample-b has dispersed aggregation of spherical nanoparticles than the sample-a. This may be due to the increased annealing temperature causing the particle sizes growth.
3.4 Magnetic Analysis
Magnetic hysteresis loops (M-H loop) were measured by vibrating sample magnetometer. Figure 4 shows the hysteresis loops of the ZnFe2O4 nanoparticles at room temperature. The coercive force and remanent magnetization of ZnFe2O4 nanoparticle for the two sample were almost negligible indicating superparamagnetic behavior, which appears only when the particle size decrease below a critical value [17]. The saturation magnetization (M s) values of sample-a and sample-b are 12.0 emu/g and 9.10 emu/g, respectively, which are smaller than the bulk value of 88.4 emu/g [18]. It is seen that saturation magnetization sample-a is higher than that of sample-b and this can be attributed to the heat treatment in air causing reduction in oxygen vacancies leading to reduced magnetic moment [19] and also to the growth of crystal size at higher temperature and the redistribution of cations in the nanocrystalline ZnFe2O4 [20].
4 Conclusions
Superparamagnetic ZnFe2O4 nanoparticles were successfully prepared by surfactant assisted hydrothermal method. The powder XRD analysis revealed the high purity and single phase of the zinc ferrite sample, average crystallite sizes are 21 nm and 28 nm for the samples annealed in air atmosphere at 300 °C and 600 °C. The FTIR data support the formation of spinel phase octahedral and tetrahedral site. It was found from HR-SEM image that the synthesized nanoparticles were spherical in shape. Further, VSM measurements confirmed the superparamagnetic behavior of the synthesized samples, with a saturation magnetization (M s) of 12.0 and 9.10 emu/g.
References
Yin, Y., Rioux, R.M., Erdonmez, C.K., Hughes, S., Somorjai, G.A., Alivisatos, A.P.: Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 304, 711–714 (2004)
Park, J., An, K., Hwang, Y., Park, J.G., Noh, H.J., Kim, J.Y., Park, J.H., Hwang, N.M., Hyeon, T.: Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 3, 891–895 (2004)
Fan, G., Gu, Z., Yang, L., Li, F.: Nanocrystalline zinc ferrite photo catalysts formed using the colloid mill and hydrothermal technique. Chem. Eng. J. 155, 534–541 (2009)
Wan, J., Jiang, X., Li, H., Chen, K.: Facile synthesis of zinc ferrite nanoparticles as non-lanthanide T1 MRI contrast agents. J. Mater. Chem. 22, 13500–13505 (2012)
Raj, K., Moskowitz, B., Casciari, R.: Advances in ferrofluid technology. J. Magn. Magn. Mater. 149, 174–180 (1995)
Yu, S.H., Yoshimura, M.: Direct fabrication of ferrite MFe2O4 (M = Zn, Mg)/Fe composite thin films by soft solution processing. Chem. Mater. 12, 3805–3810 (2000)
Niu, X., Du, W., Du, W.: Preparation and gas sensing properties of ZnM2O4 (M = Fe, Co, Cr). Sens. Actuators B, Chem. 99, 405–409 (2004)
Chatterjee, A., Das, D., Pradhan, S.K., Chakravorty, D.: Synthesis of nanocrystalline nickel-zinc ferrite by the sol-gel method. J. Magn. Magn. Mater. 127, 214–218 (1993)
Shenoy, S.D., Joy, P.A., Anantharaman, M.R.: Effect of mechanical milling on the structural, magnetic and dielectric properties of coprecipitated ultrafine zinc ferrite. J. Magn. Magn. Mater. 269, 217–226 (2004)
Xue, H., Li, Z.H., Wang, X.X., Fu, X.Z.: Facile synthesis of nanocrystalline zinc ferrite via a self-propagating combustion method. Mater. Lett. 61, 347–350 (2007)
Yao, C., Zeng, Q., Goya, G.F., Torres, T., Liu, J., Wu, H., Ge, M., Zeng, Y., Wan, Y., Jiang, J.Z.: ZnFe2O4 nanocrystals: synthesis and magnetic properties. J. Phys. Chem. C 111, 12274–12278 (2007)
Yu, S.H., Fujino, T., Yoshimura, M.: Hydrothermal synthesis of ZnFe2O4 ultrafine particles with high magnetization. J. Magn. Magn. Mater. 256, 420–424 (2003)
Naseri, M.G., Saion, E.B., Hashim, M., Sharri, A.H., Ahangar, H.A.: Synthesis and characterization of zinc ferrite nanoparticles by a thermal treatment method. Solid State Commun. 151, 1031–1035 (2011)
Wang, M., Zhihui, Ai., Zhang, L.: Generalized preparation of porous nanocrystalline ZnFe2O4 superstructures from zinc ferrioxalate precursor and its super paramagnetic property. J. Phys. Chem. C 112, 13163–13170 (2008)
Rameshbabu, R., Ramesh, R., Kanagesan, S., Karthigeyan, A., Ponnusamy, S.: Synthesis of superparamagnetic ZnFe2O4 nanoparticle by surfactant assisted hydrothermal method. J. Mater Sci.: Mater Electron, in press
Wang, M., Zhihui, Ai., Zhang, L.: Generalized preparation of porous nanocrystalline ZnFe2O4 superstructures from zinc ferrioxalate precursor and its super paramagnetic property. J. Phys. Chem. C 112, 13163–13170 (2008)
Stewart, S.J., Figueroa, S.J.A., Sturla, M.B., Scorzelli, R.B., Garcia, F., Requejo, F.G.: Magnetic ZnFe2O4 nanoferrites studied by X-ray magnetic circular dichroism and Mössbauer spectroscopy. Physica B, Condens. Matter 389, 155–158 (2007)
Goya, G.F., Rechenberg, H.R.: Ionic disorder and Néel temperature in ZnFe2O4 nanoparticles. J. Magn. Magn. Mater. 196–197, 191–192 (1999)
Ayyappan, S., Raja, S.P., Venkateswaran, C., Philip, J., Raj, B.: Room temperature ferromagnetism in vacuum annealed ZnFe2O4 nanoparticles. Appl. Phys. Lett. 96, 143106–143108 (2010)
Li, F.S., Wang, H.B., Wang, L., Wang, J.B.: Magnetic properties of ZnFe2O4 nanoparticles produced by a low-temperature solid-state reaction method. J. Magn. Magn. Mater. 309, 295–299 (2007)
Acknowledgements
The authors would like to thank SRM University for the financial support and also thank the Department of Physics and Nanotechnology, Nanotechnology Research Centre (NRC), and SRM College of Pharmacy for XRD, FE-SEM, FTIR measurements. They are thankful to DST-FIST (SR/FST/PSI-155/2010) for providing the facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rameshbabu, R., Ramesh, R., Kanagesan, S. et al. Synthesis and Study of Structural, Morphological and Magnetic Properties of ZnFe2O4 Nanoparticles. J Supercond Nov Magn 27, 1499–1502 (2014). https://doi.org/10.1007/s10948-013-2466-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-013-2466-z