Abstract
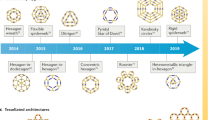
This overview describes our research progression from the study of heterocycle synthesis to dendritic architecture to simple metallomacrocycles, and finally to complex, fractal-based, suprametallomolecular materials. Construction of the first terpyridine-modified dendritic scaffold was followed by the construction and self-assembly of polyterpyridine monomers. Preparation of tristerpyridine building blocks used in concert with bisterpyridine analogs facilitated access to a Sierpiński gasket, as verified by direct visual observation with UHV-STEM, as well as other techniques. Numerous examples of heteroleptic self-assembly are described that include the quantitative preparation of a Sierpiński triangle. Addition of angled spacer moieties within polyterpyridine building blocks has generated a new family of 3D spherical materials that has facilitated the construction of hybrid fractal-dendritic materials that exhibit the unexpected concentration mediated, architectural transformations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
This adventure started in 1849 with the first reported isolation of a mono-N-heterocycle, namely pyridine, in a pure form from coal tar by Anderson [1]. Then in 1888, Blau [2] reported a 2,2′-bipyridine Fe2+ complex and later in 1932, Morgan and Burstall described [3] the synthesis and laborious isolation of the 2:2′:2"-tripyridyl (known today as [2,2′:6′,2″] terpyridine) from 20 or more products obtained by the dehydrogenation of pyridine with anhydrous FeCl3 under high temperature (340 °C) and pressure (50 atm). In 1937, they then reported [4] the formation and properties of numerous metallic salts generated using this terpyridine base. Our initial introduction to terpyridine came later in 1965, when we reported that the pyrolysis of (hetero)aryl α-methylenic ketone N,N,N-trimethylhydrazonium fluoroborates (1) gave 2,6-di(hetero)arylpyridines (2) in ca. 50% yield (Scheme 1) [5, 6]. It was, however, the Kröhnke-type procedures [7] that expanded the ease of preparation of terpyridines; thus, opening the door to their ready accessibility and the subsequent construction of supra(macro)molecular assemblies.
In 1978, Vögtle et al. reported [8] for the first time the formation of several small, 1 → 2 branched, molecular cascades (3) but stopped after two iterations because of laborious purification difficulties. Then in 1985, 1 → 2 [9] (4; “dendrimers”: Greek for trees) and 1 → 3 [10] (5; “arborols”: Latin for trees) branched tree-like macromolecules (Fig. 1) possessing three- or greater generations were reported [11]. This opened the door to the ever-expanding field of highly branched polymers [12], née dendrimers. Notably, this was also probably the introduction of chemistry (before its time) to the now rapidly growing, diverse field of Biomimicry [13].
2 Dendritic Exploration
As the field of dendrimers rapidly expanded over the past third of a century, there were several inherent synthetic problems associated with their construction: the initial divergent syntheses were plagued with many repetitive steps and the cumbersome purification procedures at each step along the way, as well as in the case of PAMAMs, inherent stability [14]. The advent of the novel convergent approaches initially reported by Hawker and Fréchet [15] was timely but introduced yet another variable that involved steric hindrance issues associated with chemical connectivity. Eventually, the introduction of “Click” chemistry [16] was a clever way to circumvent some of the bonding problems but still steric constraints and chemical irreversibility can be inherently difficult to address. Subsequently, along came commercially available dendrimers which, in part, buried the problems of structural purity at higher generations, since in many cases, it was simpler to buy the desired dendrimer-of-available-choice, however purchasing it does not make it structurally ideal—only convenient. The branching motif is also interesting, since the 1 → 2 mode of connectivity is used by most scientists in the dendrimer arena rather than the 1 → 3 motif, but why? Thus, if one starts with a 4-directional core with any 1 → 2 or 2(1 → 1) branching designs, it would take, in essence, 6 steps to generate a dendrimer with 32 branches whereas, with a 1 → 3 (convergent-like) monomer, it would take only 4 steps to get to a dendrimer with 36 branches; the latter is a much more efficient pathway, as demonstrated by the elegant works of Professor Astruc in his route to the 8th generation metallodendrimers (16 nm) [17].
The blending of a slight excess of 1 → 3 monomer (6, Scheme 2) and a four-directional core (7) each possessing a 4′-[2,2′;6′,2″] terpyridinyl moiety permitted the construction of the first dendrimer (8) possessing <tpy–Ru2+–tpy > connectivity [18]. The advantage derived here is from the tpy–Ru bond stability; thus, one can easily ascertain the structures homogeneity as noted in the “lock and key” assembly (9) [19]. The interesting part of using this <tpy–Ru2+–tpy> mode of connectivity is that it is irreversible and its NMR spectral analysis can easily confirm its purity and subsequently the directionality of the linker. Other metals can be utilized e.g., Os2+ and Fe2+ to easily connect the core with the chosen dendron; these metal centers can also instill utilitarian aspects, such as solar collection, catalysis, and electron-transfer properties, depending on the specific metal or combinations thereof.
3 Metallomacrocycles and Fractal Architecture
In 1999, the first series of shape-persistent [<tpy–MII–tpy>] monomers were constructed in an effort to ascertain the ease of controlling the ultimate structure [20]. The simplest route to the desired functionalized terpyridine was via condensation of a 2-acetylpyridine and 1,3-diformylbenzene in the presence of base, followed by treatment with NH4OAc to generate, in reasonable yield, the desired bisterpyridine (10, Scheme 3), possessing a rigid 120° directed bond angle. With an excess of RuCl3·nH2O, 10 was easily transformed to the bisRuIII adduct (11); treatment of 10 with a slight excess of the bisRuIII adduct gave the desired hexamer 12 in reasonable overall yields. The use of FeCl2 was also easily incorporated; thus, specific metal combinations could be instilled into the hexameric and other related cyclic structures [21]. Other combinations of this simple chemistry opened the door to families of precisely shaped, multi-ion metallocycles. The introduction of labile functionality at the 5-aryl bridging position permitted the facile attachments of dendrons (13) and other moieties into the tailored hexameric design. Notably, the simple RuII cyclic hexamer possessed an overall 12+ charge. One posits, what would happen if one adds a simple G1 dendrimer possessing a dodecacarboxylate coating(?); a dendrimer-metallocycle composite (14) in the form of needles was spontaneously and quantitatively created [22].
The expansion of this architecture to generate the Sierpiński gasket (15, Fig. 2) was easily achieved; thus, opening the door to a novel, non-branched, fractal family. This nearly planar fractal gasket was prepared in two-steps from previously synthesized tectons and had a precise molecular weight of 38,724.38 amu, as pictured on a low temperature STEM [23].
Treatment of 4,5-dibenzyloxy-1,2-bisterpyridylphenylbenzene (16, Scheme 4) and a trimethoxy-1,3-bisterpyridylphenylbenzene (17) gave a mixture of products with the major constituent being the cyclic trimer (18). However, when an equal mixture of 4,5,6-trimethoxy-1,2,3-tristerpyridyl-phenylbenzene (19) was treated with 16 in the presence of either Zn2+ or Cd2+, the molecular rhombus 20 was generated in quantitative yield [24]. A related Ru2+ metallo-organic ligand possessing three free terpyridine moieties was treated with Zn2+ to give a helical structure, which when treated with a 1,2,3-trispyridine aryl ligand rearranges to give the stable bis-planar rhomboidal pentacomplex [25]. This further supports the stability of triangular assembly. Utilizing a 60°-directed bisterpyridine monomer, accessed from a substituted 1,2-dihalobenzene, upon treatment with Cd2+ or Zn2+ opened a facile entrée to molecular triangles (e.g., 18) and the triangular framework has distinct advantages in the stability arena [24]. The incorporation of directed triangles led to predictable products—all in quantitative or nearly quantitative yields. The use of zinc or cadmium introduces the ability to equilibrate and the triangular matrix added the organizational constancy to generate predicable, precise structural products. This polyterpyridine directivity has opened the door to a general pathway to high yield routes to pure metallofractals, as well as to interesting architecturally diverse supramacromolecular constructs [26, 27]. For example, when a 1:1 mixture of 4,5-dimethoxy-1,2-bis(4′-terpyridylphenyl)benzene (“V” monomer) and 1,2,3,4-tetrakis(4′-terpyridylphenyl)-5,6-dimethoxybenzene (“K” monomer) was treated with 3 equivalents of Cd(NO3)2 at 25 °C, after 30 min the desired Sierpiński fractal triangle 21 was isolated in >95% yield [28]. A larger saturated Sierpiński triangle has been created by the application of combinations of these same monomers [29]. The stepwise synthesis and self-assembly of pentagonal and hexagonal metallo-supramacromolecules with precise star-shaped motifs have also been designed and characterized [30].
Thus, when tris(4-bromophenyl)benzene was treated with 3′-boronatophenyl [2,2′:6′,2″]-terpyridine, the desired trisligand 22 (Scheme 5) was formed in 70% yield. Subsequent reaction of 22 with Ru(DMSO)4Cl2 gave rise in one-step to a three-dimensional, highly symmetric and stable nanosphere 23, as a red solid [31]. A related tristerpyridine monomer 24 in which the central 1,3,5-benzene core is replaced with a trigonal-N and then reacted with Ru(DMSO)4Cl2 in a 2:3 ratio generated the related pseudo-octahedral complex 25. Whereas, when 24 was dimerized to form the mono-Ru2+ complex 26, which now possesses four terminal terpyridine moieties. Subsequent treatment with Fe2+ at 25 °C gave one isomer (27) while at an elevated 80 °C generated a different isomer (28). Thus, reaction temperature can play a critical role in the structural outcome and provides insight into overall thermodynamic stability [32]. This mode of macromolecular assembly has also been applied for the first time to the creation of a 2D- [33] and 3D- [34] structures possessing three different metal centers within a cyclic framework.
4 3D Fractal and Hybrid Materials
Directivity of the terpyridine termini and the use of the equilibrating metals are critical to obtain the desired structural outcome. This is demonstrated by the quantitative, single step, self-assembly of the shape-persistent, Archimedean-based monomer (29, Scheme 6), which upon treatment with precisely two equivalents of Zn(NO3)2 in a stirred MeOH and CHCl3 (1:1) solution at 25 °C for one hour gave >98% of the desired cuboctahedron 30 [35]. The Cd2+ analog (31) was similarly prepared and shown to undergo an interesting novel dynamic interconversion upon dilution from the cuboctahedron 31 to precisely two equivalents of an octahedron 32; upon concentration or attempted isolation, 32 reverted to 31; this reversibility was demonstrated by 1H NMR and ESI-MS. Notably, there is no spectral evidence for any intermediates during this interconversion.
The one-step, self-assembly of the related shape-persistent tetrakisterpyridine 33 (Scheme 7) possessing a much more flexible crown-ether core also gives the initial Archimedean-based cuboctahedron 34 [36]; which upon dilution and/or exchange of counterions underwent a quantitative transformation to two identical octahedrons (35), which upon further dilution generates four superposed, bistriangular complexes 36. Increasing the concentration reverses the process to regenerate 34. Notably, there is again no NMR or MS evidence for any intermediates in these transformations.
The key question is: how does one build large dendritic structures in quantitative yield in one-step from designer monomers? Although there are numerous potential schemes to answer this simple question, but from the above studies, equilibrating metal centers and multiple sites exhibiting triangular directivity are key points although there remains at least one question for the attachment of the triangular connectivity. The answer lays in vertex and facial connectivity. The Sierpiński triangle addresses the facial connectivity (21); whereas, vertex connectivity is demonstrated by the construction of the simple [4]-triangulane (37, Scheme 8) [37, 38]. The “V” monomer (see 21) was previously noted in that it is the corner of the Sierpiński triangle; it is dimerized by its initial conversion to the mono-Ru2+ complex and then treatment with excess “V” generated the monoRu2+ dimer (38), whose conversion to its bis-Ru3+ reagent (39) followed known procedures. Treatment of 3 equivalents of 39 with the “X” building block 40, in the presence of 3 equivalents Zn2+ quantitatively generated triangulane 37. As one might anticipate, as the size and metal content increases, it is critical to instill hydrophobic moieties to increase the overall solubility.
5 Conclusion
The fluorinated analogue of 39 (44) with the three-directional core 45 generated 46 in high yields, then treatment with Zn2+ gave the surface-fluorinated cuboctahedron and thus the creation of a simple dendrimer 43 (Scheme 9) via the quantitative generation of the dendrimers core. This demonstrates the ability to form a dendron, which is capable of the one-step assembling of the desired core rather than start with the core and appended upon it. Since the cuboctahedral core was previously proven by single crystal X-ray analysis [35], the high yield generation of the branched nanostructure possessing a dendritic framework in essence opens the door to perfect metallodendrimers via the construction of the internal core itself [39].
The assembly of the basic internal cuboctahedral core also creates potential new internal unimolecular micelles [10, 40], which has the novel ability to contract to the octahedron shape under dilution conditions. This new mode of dendritic monomers to quantitatively assemble precise Archimedean-based cores opens new routes to structurally precise metallodendrimers.
References
T. Anderson, Earth Environ. Trans. R. Soc. Edinb. 16, 123 (1846)
F. Blau, Ber. Dtsch. Chem. Ges. 21, 1077 (1888)
G.T. Morgan, F.H. Burstall, J. Chem. Soc. 20 (1932)
G.T. Morgan, F.H. Burstall, J. Chem. Soc. 1649 (1937)
D.L. Fishel, G.R. Newkome, J. Am. Chem. Soc. 88, 3654 (1966)
G.R. Newkome, D.L. Fishel, J. Org. Chem. 37, 1329 (1972)
F. Kröhnke, Synthesis 1976, 1 (1976)
E. Buhleier, W. Wehner, F. Vögtle, Synthesis 9, 155 (1978)
D.A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, P. Smith, Polym. J. 17, 117 (1985)
G.R. Newkome, Z.-q. Yao, G.R. Baker, V.K. Gupta, J. Org. Chem. 50, 2003 (1985)
W. Worthy, Chem. Eng. News 66, 19 (1988)
F.M. Menger, Top. Curr. Chem. 136, 1 (1986)
J.R. Lloyd, P.S. Jayasekara, K.A. Jacobson, Anal. Methods 8, 263 (2016)
C.J. Hawker, J.M.J. Fréchet, J. Am. Chem. Soc. 112, 7638 (1990)
H.C. Kolb, M.G. Finn, K.B. Sharpless, Angew. Chem. Int. Ed. 40, 2005 (2001)
J. Ruiz, G. Lafuente, S. Marcen, C. Ornelas, S. Lazare, E. Cloutet, J.-C. Blais, D. Astruc, J. Am. Chem. Soc. 125, 7250 (2003)
G.R. Newkome, F. Cardullo, E.C. Constable, C.N. Moorefield, A.M.W.C. Thompson, J. Chem. Soc. Chem. Commun. 11, 925 (1993)
G.R. Newkome, R. Güther, C.N. Moorefield, F. Cardullo, L. Echegoyen, E. Pérez-Cordero, H. Luftmann, Angew. Chem., Int. Ed. Engl. 34, 2023 (1995)
G.R. Newkome, T.J. Cho, C.N. Moorefield, G.R. Baker, M.J. Saunders, R. Cush, P.S. Russo, Angew. Chem. Int. Ed. 38, 3717 (1999)
G.R. Newkome, T.J. Cho, C.N. Moorefield, R. Cush, P.S. Russo, L.A. Godínez, M.J. Saunders, P. Mohapatra, Chem. Eur. J. 8, 2946 (2002)
P. Wang, C.N. Moorefield, K.-U. Jeong, S.-H. Hwang, S. Li, S.Z.D. Cheng, G.R. Newkome, Adv. Mater. 20, 1381 (2008)
G.R. Newkome, P. Wang, C.N. Moorefield, T.J. Cho, P. Mohapatra, S. Li, S.-H. Hwang, O. Lukoyanova, L. Echegoyen, J.A. Palagallo, V. Iancu, S.-W. Hla, Science 312, 1782 (2006)
X. Lu, X. Li, J.-L. Wang, C.N. Moorefield, C. Wesdemiotis, G.R. Newkome, Chem. Commun. 48, 9873 (2012)
D. Liu, Z. Jiang, M. Wang, X. Yang, H. Liu, M. Chen, C.N. Moorefield, G.R. Newkome, X. Li, P. Wang, Chem. Commun. 52, 9773 (2016)
J.M. Ludlow, III, T.-Z. Xie, Z. Guo, K. Guo, M.J. Saunders, C.N. Moorefield, C. Wesdemiotis, G.R. Newkome, Chem. Commun. 51, 3820 (2015)
J.M. Ludlow III, M.J. Saunders, M. Huang, Z. Guo, C.N. Moorefield, S.Z.D. Cheng, C. Wesdemiotis, G.R. Newkome, Supramol. Chem. 29, 69 (2017)
R. Sarkar, K. Guo, C.N. Moorefield, M.J. Saunders, C. Wesdemiotis, G.R. Newkome, Angew. Chem. Int. Ed. 53, 12182 (2014)
Z. Jiang, Y. Li, M. Wang, D. Liu, J. Yuan, M. Chen, J. Wang, G.R. Newkome, W. Sun, X. Li, P. Wang, Angew. Chem. Int. Ed. 56, 11450 (2017)
Z. Jiang, Y. Li, M. Wang, B. Song, K. Wang, M. Sun, D. Liu, X. Li, J. Yuan, M. Chen, Y. Guo, X. Yang, T. Zhang, C.N. Moorefield, G.R. Newkome, B. Xu, X. Li, P. Wang, Nat. Commun. 8, 1 (2017)
T.-Z. Xie, S.-Y. Liao, K. Guo, X. Lu, X. Dong, M. Huang, C.N. Moorefield, S.Z.D. Cheng, X. Liu, C. Wesdemiotis, G.R. Newkome, J. Am. Chem. Soc. 136, 8165 (2014)
S. Chakraborty, W. Hong, K.J. Endres, T.-Z. Xie, L. Wojtas, C.N. Moorefield, C. Wesdemiotis, G.R. Newkome, J. Am. Chem. Soc. 139, 3012 (2017)
Y. Yao, S. Chakraborty, S. Zhu, K.J. Endres, T.-Z. Xie, W. Hong, E. Manandhar, C.N. Moorefield, C. Wesdemiotis, G.R. Newkome, Chem. Commun. 53, 8038 (2017)
M. Chen, J. Wang, D. Liu, Z. Jiang, Q. Liu, X. Yang, W. Yu, J. Yan, G.R. Newkome, P. Wang, Chem. Commun. (2017). doi:10.1039/C7CC05577C
T.-Z. Xie, K. Guo, Z. Guo, W.-Y. Gao, L. Wojtas, G.-H. Ning, M. Huang, X. Lu, J.-Y. Li, S.-Y. Liao, Y.-S. Chen, C.N. Moorefield, M.J. Saunders, S.Z.D. Cheng, C. Wesdemiotis, G.R. Newkome, Angew. Chem. Int. Ed. 54, 9224 (2015)
T.-Z. Xie, K.J. Endres, Z. Guo, J.M. Ludlow III, C.N. Moorefield, M.J. Saunders, C. Wesdemiotis, G.R. Newkome, J. Am. Chem. Soc. 138, 12344 (2016)
S. Chakraborty, R. Sarkar, K. Endres, T.-Z. Xie, M. Ghosh, C.N. Moorefield, M.J. Saunders, C. Wesdemiotis, G.R. Newkome, Eur. J. Org. Chem. 5091 (2016)
T. Wu, Y.S. Chen, M. Chen, Q. Liu, X. Xue, Y. Shen, J. Wang, H. Huang, Y.T. Chan, P. Wang, Inorg. Chem. 56, 4065 (2017)
G.R. Newkome, C.N. Moorefield, et al., US Pat. Applied, 2015
G.R. Newkome, C.N. Moorefield, G.R. Baker, M.J. Saunders, S.H. Grossman, Angew. Chem. Int. Ed. Engl. 30, 1178 (1991)
Acknowledgements
The authors gratefully acknowledge the funding from the National Science Foundation (CHE-1151991), the James and Vanita Oelschlager funding via the University of Akron, and the numerous undergraduate and graduate students, postdoctoral fellows, and visiting faculty, who were part of this adventure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newkome, G.R., Moorefield, C.N. & Chakraborty, S. A Long Pathway to the Quantitative Assembly of Metallodendrimers. J Inorg Organomet Polym 28, 360–368 (2018). https://doi.org/10.1007/s10904-017-0676-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0676-8