Abstract
The effect of partial substitution of nickel by cobalt in perovskite LaNi1−xCoxO3 (0 ≤ x ≤ 0.6) oxides, synthesized by the sol–gel method, using nickel and/or cobalt nitrates, lanthanum nitrate, citric acid as chelating agent has been studied. The obtained samples were characterized by several techniques. XRD and IR analysis show that the formation of the perovskite phase occurs at 750 °C. Scanning electron microscopy (SEM) revealed that powders were composed of particles with different shape and size. The increase in the fraction of the doped-Co leads to highly agglomeration of particles while the porosity decreases. The electrochemical measurements shows that the presence of cobalt leads to a decrease of the catalytic activity attributed to the Co–Ni alloy formation. The highest electrode performance is achieved with LaNi0.6Co0.4O3 electrode. The band gap energy was calculated from the UV–Visible and it is found above 3 eV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Perovskite-type mixed oxides are ceramic materials that combine metals elements with non-metallic ones usually can be described by the general stoichiometric formula ABO3, where A represents a lanthanide or alkaline earth ion and B a trivalent ion of a transition metal. The perovskite lattice can accommodate multiple cationic substitutions with only small changes since the values of the structure factors (t) is between 0.75 and 1 [1]. In this structure, the properties are mainly determined by the occupancy of B sites, which usually are partially substituted [1, 2]. During these last years, theoretical and technological studies for mixed oxides with perovskite structure have developed based on their physical (ferroelectric, piezoelectric, magnetic, electro-optic, dielectric, conducting and superconducting properties and catalytic properties) [3,4,5]. The perovskite type catalysts containing nickel, such as LaNiO3 have received much attention because of their potential application as electrode materials in solid oxide fuel cells, gas sensors. They have a structure relatively stable when heated and a good activity for the steam reforming which produces CO2 and H2O under oxidative conditions [6, 7]. The effect of the routes of preparation and calcination (temperature, time and atmosphere) and substitutions of A and/or B sites on the phase composition and crystalline structure of LaNiO3 materials are considered important because of their effects on the electronic properties, which affect the conductivity and catalytic properties of the final product [8,9,10,11].
There are several chemical methods which can be used to synthesize metal oxide nanoparticles. Among these preparation methods, the sol–gel synthesis method has several advantages over other methods including good homogeneity, low cost, high purity and low heat treatment [12,13,14].
LaNiO3 oxide has been much studied by doping of a nickelate site for practical applications. It was found that catalytic activities of LaNi1−xMxO3 (M = Mn, Al, Co) complex oxides were much higher than that of LaFeO3 sample due to the increase of the valence of B-site cations and lattice oxygen content [15]. In recent years, some research on the LaNi1−xCoxO3 system was conducted. Valderrama et al. [16] have prepared LaNi1−xCoxO3 (0 ≤ x ≤ 1) oxides by calcining the precursors at 750 °C for 4 h using the propionic acid as the solvent via a sol–gel resin method, used them as catalysts in the drying reforming of methane. Moreover, Androulakis et al. [17] have studied the electrical resistivity and the magneto-resistance of the metallic members of the LaNi1−xCoxO3 series with 0.3 ≤ x ≤ 0.6, synthesized via Pechini (citrate–gel) method. Some years later, Silva et al. [18] have synthesized a series of LaNi1−xCoxO3 compounds (x = 0, 0.2 and 0.4) by the combustion method and used them as catalysts precursors in the partial oxidation of methane (POM). Recently, Zhong et al. [19] have prepared the series oxides of LaNi1−xCoxO3 (x = 0, 0.1, 0.3, 0.7, 1.0) by a co-precipitation method, investigated the catalytic oxidation of NO. Furthermore, Zhao et al. [20] have reported the catalytic properties for steam reforming of ethanol using LaNi1−xCoxO3 as catalysts obtained by calcining the precursors at 700 °C for 5 h via one step-citrate complexing method.
To our best knowledge, there is no works in the literature on the thermal stability, oxygen evolution reaction in alkaline media and optical properties of LaNi1−xCoxO3 oxides.
In this present work, we started ours investigations on LaNi1−xCoxO3 (0 ≤ x ≤ 0.6) oxides by the thermal evolution of the precursor powder prepared via sol–gel method, to the point of the formation of the perovskite phase, followed by means of thermal analysis, IR spectroscopy and powder X-ray diffraction (XRD). Thereafter, electrochemical measurements were performed with cyclic voltammetry at a scan rate of 20 mV/s in the potential range 0–0.8 V, aiming to determine the best material composition for the oxygen evolution reaction. Finally, optical band gap values were estimated using UV–Visible spectroscopy to measure the potential of our materials to be used as photo-catalysts.
2 Experimental Method
2.1 Preparation
LaNi1−xCoxO3 (0 ≤ x ≤ 0.6) perovskites were synthesized by sol–gel method using lanthanum, nickel and cobalt nitrates as starting materials and citric acid as a complexing agent. Precursor solutions were prepared from stoichiometric amounts of precursor salts La(NO3)3·6H2O (biochem), Ni(NO3)2·6H2O (biochem), and Co(NO3)2·6H2O (biochem) and along with citric acid were dissolved in ethanol. The solution with metal ions and citric acid in the molar ration 1:1:2 (lanthanum–nickel, cobalt–citric acid) are heated at 60–70 °C for 3 h with a continuous stirring until a gel was achieved. To remove residual ethanol, the gel was dried at 100 °C for 24 h. Thereafter, the powder was calcined in an oven at different temperatures (550–850 °C) for 6 h with a heating rate of 5 °C min−1.
2.2 Powder Characterization
Several techniques were used for characterization of the perovskites. The thermal decomposition process of the precursor powder was carried out by thermogravimetric and differential thermal analysis TG/DTA using a SETARAM LABSYS evo at a heating rate of 10 °C.min−1 in air. The eventual presence of organic material after calcination was determined by infrared spectroscopy in an (FT-IR) SHIMADZU 8400 S spectrometer using KBr pellets. The phase identification of the synthesized powders, which were calcined at various temperatures, was performed from a X-ray diffraction (XRD, BRUKER-D8) by scanning the angular range 10°–80° using CuKα radiation. The identification of crystalline phases was performed using data from the cards of JCPDS (International Center for Diffraction Data). The lattice parameters of the structure were determined by refinement of the peak positions of the XRD pattern using the program CELREF. Morphological aspects of the powders were examined by using a ESEM–FEI scanning Electron microscopy (model Quanta 600 FEG).The ultraviolet and visible diffuse reflect spectra were measured at 200–850nm wavelengths with a Perkin–Elmer–Lambda 950 spectrophotometer. The electrochemical experiments for O2 evolution reaction were performed using a potentiostat/galvanostat instrument model Parstat 4000 and were carried out using three electrodes. Electrodes of oxides LaNi1−xCoxO3 were obtained by painting on nickel support. Pt and Hg/HgO were used as auxiliary and reference electrodes, respectively.
3 Results and Discussion
3.1 TG/DTA Analysis
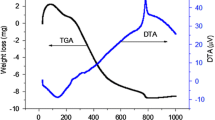
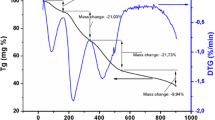
To further study the details in the whole chemical reaction process, the DTA–TG curves heating from 25 to 900 °C for LaNi0.6Co0.4O3 is shown in Fig. 1. The whole process of weight loss can be divided into four stages: (1) 51–145 °C, (2) 208–311 °C, (3) 311–436 °C, (4) 744–820 °C. At the first stage of weight loss, which is correlated with a strong DTA endothermic peak at about 126 °C representing the evaporation of water and organic compounds in the gel [21]. The second stage of weight loss can be explained by the transformation of the citrate to aconitate which is accompanied by a broad endothermic peak around 239 °C [22, 23]. The third step between 311 and 436 °C, two small endothermic peaks detected on the DTA curve are centered at 344 and 399 °C. They can be probably attributed to some chemical reactions of the organic–metal complexes with emitting some gas species like CO2 [21]. The last endothermic peak at about 782 °C, can be possibly assigned to the gradual formation of LaNi0.6Co0.4O3 crystal.
3.2 X-ray Diffraction
The XRD diffractograms of LaNi0.6Co0.4O3 oxide calcined at different temperatures (550–850 °C) are presented in Fig. 2. After calcination at 550 °C, the precursor is a mixture containing La5O7NO3 (PDF:00-038-0891), La2O3 (JCPDS: 01-074-1144), Co3O4 (JCPDS: 00-42-1467) and NiO (JCPDS: 01-089-3080). At 650 °C, the characteristic diffraction peaks of La5O7NO3 and NiO become weaker while those of Co3O4 disappear. The intensity of La2O3 peaks becomes relatively more intense whereas the characteristic peaks of the rhombohedral phase appear. With the increase of calcination temperature, the peaks attributed to La5O7NO3, La2O3 and NiO disappear totally at 750 °C, where the crystallinity of the LaNi0.6Co0.4O3 phase is improved. After further heating at 850 °C, the diffractogram presents a single perovskite phase without secondary phases. The difference in the crystallization temperature of LaNi0.6Co0.4O3 as observed in DTA and XRD could be to the difference in heating program of the samples.
XRD patterns of the LaNi1−xCoxO3 (0 ≤ x ≤ 0.6) calcined at 750 °C for 6 h in air (Fig. 3) indicated the formation of a crystalline phase of perovskite type with rhombohedric structure (PDF: 00-034-1181). There is no distinguishable difference between the XRD patterns of LaNiO3 and LaNi1−xCoxO3.
The Lattice parameters a, b and c and the unit cell volume V as a function of cobalt content (x) are summarized in (Table 1). We observe that the both parameter decrease slightly with increasing x from 0 to 0.6. This is due to the incorporation of cobalt ions with bigger ionic radius (0.078 nm) in comparison with the smaller nickel ion (0.069 nm) [24]. Similar tendency has been found already for LaNi1−xCoxO3 samples [16, 18, 25]. A linear variation with respect to the substitution degree confirming the solid solution formation.
3.3 IR Spectroscopy
The sample LaNi0.6Co0.4O3 is characterized by IR spectra in the range 400–2000 cm−1 (Fig. 4). From the spectra of the sample heated at 550 °C, several bands were observed with central point at 676, 800, 925, 1400 and 1596 cm−1. The band observed at 676 cm−1 can probably be attributed to the stretching deformation of O–Ni–O [26], which becomes more strong at higher temperature. The bands which appear at about 800 and 925 cm−1 characterize probably the carbonate [27] and nitrate anions [28], respectively. After heating (T > 500 °C), the intensity of these bands decreases and they become less visible at higher temperature (T = 850 °C). The bands observed at 1400 cm−1 were assigned to stretching vibrations of carbonate [29]. This last has weakened with increasing calcination temperature. Moreover, the band at 1596 cm−1 corresponds to the asymmetric and symmetric carbonyl group stretching vibrations of ionized carboxylate [30]. It decreases rapidly in the temperature range 650–850 °C. The decrease in the characteristic bands of the carboxylic group and nitrate anions in the IR spectra of as burnt powder shows that ions of carboxylic group and NO3 − take part in the reaction during combustion. Consequently, it can be considered as a thermally induced anionic redox reaction of the anionic gel wherein the carboxylic group acts as oxidant and nitrate ions act as reducers [31]. A weak band appears at ~468 cm−1 at 750 °C and becomes much visible at 850 °C, which corresponds to the vibration of the Ni–O bond [32].
Figure 5 shows the IR spectra of LaNi1−xCoxO3 gels calcined at 750 °C for 6 h. All these spectra are similar in shape. Tow absorption bands located at 468 and 667 cm−1 can be attributed to the stretching vibration of the Ni–O bond and the stretching deformation of the O–Ni–O, respectively. They confirm the formation of perovskite structure [26, 32].
3.4 Structural and Morphological Characterization
The average crystallite size (D) was calculated from the broadening of the XRD line width by using the Debye–Scherrer relationship (Fig. 6). For that, it was necessary to consider the peaks (202) of LaNi1−xCoxO3 oxides. The oxides present an average crystallite size in the range of 20.6–26.2 nm indicating that the perovskite powders prepared by the sol–gel method are composed of nanometric particles. It is observed that the crystallite size increases with increasing cobalt content. This is probably due to the incorporation of Co2+ into the LaNiO3 lattice, which leads to the crystallite growth. Moreover, Similar results were also found previously for LaNi1−xCoxO3 samples synthesized by co-precipitation method [19], LaNi1−xCoxO3 [25] prepared by microwave -assisted citrate method and LaCr1−xZnxO3 (x = 0−0.3) [33].
The morphologies of LaNi1−xCoxO3(x = 0−0.6) samples studied by SEM are shown in (Fig. 7). The micrographs images indicate that particles of different shapes and sizes are formed with very well pronounced agglomeration for doped samples (x > 0.2). The perovskites LaNiO3 and LaNi0.8Co0.2O3 are composed of almost parallelepiped grains, while samples with higher cobalt content (x > 0.2) various shapes of grains are observed. The increase in the fraction of the doped-Co leads to highly agglomeration of particles while the porosity decreases. Similar trend of changing in size and shape were also found previously [25, 34, 35]. Indeed, it was reported that the increasing amount of cobalt in substituted calcium hydroxyapatite (HAp) increases the tendency of crystallites to stick together, leading to the formation of large particles (aggregates).
3.5 Cyclic Voltammetry
Cyclic voltammograms were registered for the electrodes LaNi1−xCoxO3 calcined at 750 °C in 1 M KOH at 25 °C, the potential range 0 ≤ E ≤ 0.8 V with the scan rate of 20 mV s−1. Figure 8 shows the anodic current–potential curves of air electrode with different substitutions of cobalt content. We observe in general that these electrodes have a qualitatively similar behavior. The coated electrode films showed a good cohesion through polarization. All voltammograms exhibit two redox peaks. The first anodic (470.10 ≤ Epa ≤ 514.27 mV) is observed in the forward scan followed by the oxygen evolution reaction. The second one (327.26 ≤ Epc ≤ 360.53 mV) is observed in the reverse scan. These last correspond to a pseudo capacitance of the Ni(III)/Ni(II) surface redox couple [36]. The electrocatalytic activity of LaNiO3 was much higher than that of doped samples. This is probably due to that nickel is more active than cobalt [37]. The same trend was also found already by Sierra et al. [38]. It has been shown that the presence of cobalt leads to a decrease of the catalytic activity attributed to the Co–Ni alloy formation. Furthermore for samples doped with cobalt, LaNi0.6Co0.4O3 shows the high catalytic activity. This is possibly due to the porosity structure, which acts positively on the catalytic activity by increasing the number of reactant accessible catalyst sites [39].
3.6 Band Gap Study by UV–Visible Spectroscopy
The effect of doping on the energy band-gap was investigated by UV–Vis absorption spectroscopy. Figure 9 shows the Kubelka–Munk conversion spectra of as-prepared LaNi1−xCoxO3 nanoparticles in the reflectance range of 200–850nm. The range of 300–570 nm is related to the metal and metal transition [40]. These spectra show that samples have similar behavior. The optical band-gap values were found to be 3.802, 3.825, 3.826 and 3.820 eV for x = 0, 0.2, 0.4, 0.6, respectively. They are higher than 3 eV, which confirms that the perovskite compounds are semiconducting in nature. The values of Eg are ranging from 0 to 5 eV, therefore the optical absorption spectra of these materials are owing probably to the highest intraband electronic transitions related to O2p and La 5d states [41]. These results indicate that the as–prepared LaNiO3 and Co-doped LaNiO3 nanopowders can be utilized for the photocatalysis in the degradation process of organic pollutants [42].
4 Conclusion
Co-doped LaNiO3 nanopowders in the composition range x = 0–0.6 were successfully prepared via sol–gel method, employing nitrate salts of lanthanum, nickel and cobalt as cations precursors, citric acid as a chelating agent. TG-DTA curves show the thermochemical behavior of the compounds with respect to temperature. According XRD characterization, the formation of the perovskite pure phase was obtained at 750 °C with no detectable secondary phase, as well confirmed by IR spectroscopy. The morphology of samples that were examined by SEM shows that different shapes and sizes of particles are formed with very well pronounced agglomeration for doped samples (x > 0.2). In electrochemical study, LaNi0.6Co0.4O3 electrode exhibits a significantly higher catalytic activity, indicating that this material is the best electrocatalyst for oxygen evolution reactions. The UV reflectance spectra indicate that the optical band gap values of LaNi1−xCoxO3 nanoparticles are above 3 eV. This finding in optical properties indicates the potential for these doped materials to be employed in photocatalytic applications.
References
H. Tanaka, M. Misono, Curr. Opin. Solid State Mater. Sci. 5, 381 (2001)
N. Yamazoe, Y. Teraoka, Catal. Today 8, 175 (1990)
A.Y. Dobin, K.R. Nikolaev, I.N. Krivorotov, R.M. Wentzcovitch, E.D. Dahlberg, A.M. Goldman, Phys. Rev. B 68, 113408 (2003)
T. Aytug, B.W. Kang, C. Cantoni, E.D. Specht, M. Paranthaman, A. Goyal, D.K. Christen, D.T. Verebelyi, J.Z. Wu, R.E. Ericson, C.L. Thomas, C.Y. Yang, S.E. Babcock, J. Mater. Res. Bull. 16, 2661 (2001)
G. Catalan, R.M. Bowman, J.M. Gregg, Phys. Rev. B 62, 7892 (2000)
E. Pietri, A. Barrios, O. Gonzalez, M.R. Goldwasser, M.J. Pérez-Zurita, M.L. Cubeiro, J. Goldwasser, L. Leclercq, G. Leclercq, L. Gingembre, Stud. Surf. Sci. Catal. 136, 381 (2001)
F.T. Akin, J.Y.S. Lin, J. Memb. Sci. 231, 133 (2004)
E. Campagnoli, A. Tavares, L. Fabbrini, I. Rossetti, Y.A. Dubitsky, A. Zaopo, L. Forni, Appl. Catal. B 55, 133 (2005)
S. Liu, X. Tan, K. Li, R. Hughes, Ceram. Int. 28, 327 (2002)
A.A. Leontiou, A.K. Ladavos, P.J. Pomonis, Appl. Catal. A 241, 133 (2003)
K. Kleveland, M.-A. Einarsrud, T. Grande, J. Eur. Ceram. Soc. 20, 185 (2000)
M. Nazari, N. Ghasemi, H. Maddah, M.M. Motlagh, J. Nanostruct. Chem. 4, 1 (2014)
E.L. Crepaldi, P.C. Pavan, J.B. Valim, J. Braz, Chem. Soc. 11, 64 (2000)
L.P. Rivas-Vazquez, J.C. Rendon-Angeles, J.L. Rodriguez-Galicia, K. Zhu, K. Yanagisawa, Solid State Ion 172, 389 (2004)
P. Shikha, T.S. Kang, B.S. Randhawa, J. Alloys Compd. 625, 336 (2015)
G. Valderrama, A. Kiennemann, M.R. Goldwasser, Catal. Today 133, 142 (2008)
J. Androulakis, J. Giapintzakis, Phys. B 405, 107 (2010)
C.R.B. Silva, L. da Conceição, N.F.P. Ribeiro, M.M.V.M. Souza, Catal. Commun. 12, 665 (2011)
S. Zhong, Y. Sun, H. Xin, C. Yang, L. Chen, X. Li, Chem. Eng. J. 275, 351 (2015)
L. Zhao, T. Han, H. Wang, L. Zhang, Y. Liu, Appl. Catal. B 187, 19 (2016)
M. Liu, D. Xue, C. Luo, J. Alloys Compd. 426, 118 (2006)
Y.-M. Hon, K.-Z. Fung, M.-H. Hon, J. Eur. Ceram. Soc. 21, 515 (2001)
R. Alcantara, P. Lavela, J.L. Tirado, R. Stoyanova, E. Kuzmanova, E. Zhecheva, Chem. Mater. 9, 2145 (1997)
S. Makhloufi, M. Omari, J. Inorg. Organomet. Polym. Mater. 26, 32 (2016)
A. Galal, N.F. Atta, S.M. Ali, Electrochim. Acta 56, 5722 (2011)
A. Wokaun, B. Schrader, Infrared and Raman Spectroscopy-Methods and Applications. (VCH, Weinheim, 1995)
J.M.D. Tascón, L.G. Tejuca, J. Chem. Soc. Faraday Trans. 1 77, 591 (1981)
A. Mali, A. Ataie, Scr. Mater. 53, 1065 (2005)
M. Hadioui, Doctoral Thesis, University of Toulouse, 2007
P. Tarte, Spectrochim. Acta Part A 23, 2127 (1967)
K. Adaika, M. Omari, J. Sol–Gel Sci. Technol. 75, 298 (2015)
A. Leleckaite, A. Kareiva, Opt. Mater. 26, 123 (2004)
I. Chadli, M. Omari, M. A. Dalo, B. A. Albiss, J. Sol–Gel Sci. Technol. 80, 598 (2016)
B. Babu, C.V. Reddy, J. Shim, R. Ravikumar, J. Park, J. Mater. Sci. 27, 5197 (2016)
Z. Stojanović, L. Veselinović, S. Marković, N. Ignjatović, D. Uskoković, Mater. Manuf. Process 24, 1096 (2009)
M. Lebid, M. Omari, Arab. J. Sci Eng. (2014)
M.C.J. Bradford, M.A. Vannice, Catal. Rev. 41, 1 (1999)
G.S. Gallego, C. Batiot-Dupeyrat, J. Barrault, E. Florez, F. Mondragón, Appl. Catal. A 334, 251 (2008)
A.R. Oveisi, K. Zhang, A. Khorramabadi-Zad, O.K. Farha, J.T. Hupp, Sci. Rep. 5, (2015)
T. Wang, T. Xu, S. Gao, S.-H. Song, Ceram. Int. 43, 4489 (2017)
L. Guan, B. Liu, L. Jin, J. Guo, Q. Zhao, Y. Wang, G. Fu, Solid State Commun. 150, 2011 (2010)
A. Hernández-Ramirez, I. Medina-Ramirez, Photocatalytic Semiconductors: Synthesis, Characterization, and Environmental Applications (Springer, New york, 2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Omari, E., Makhloufi, S. & Omari, M. Preparation by Sol–Gel Method and Characterization of Co-doped LaNiO3 Perovskite. J Inorg Organomet Polym 27, 1466–1472 (2017). https://doi.org/10.1007/s10904-017-0604-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0604-y