Abstract
In the present paper, the use of a nanocomposite system based on bis(2-hydroxy-acetophenone)ethylenediimine vanadyl and carbon nanotubes prepared by a simple and rapid method for the catalysis oxidation of levodopa (LD) was described. Cyclic voltammetry was used to investigate the redox properties of this composite. A pair of well-defined quasi reversible redox peaks of vanadyl complex was obtained at the modified carbon paste electrode by direct electron transfer between the vanadyl complex and the electrode. Dramatically enhanced electrocatalytic activity was exemplified at the modified electrode, as an electrochemical sensor to study the electro oxidation of LD. At the optimum pH of 7.0, the oxidation of LD occurs at a potential about 300 mV less positive than that of an unmodified electrode. Based on differential pulse voltammetry, the oxidation of LD exhibited a dynamic range between 0.8 and 1000 µM and a detection limit (3σ) of 0.43 µM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Many people around the world suffer from Parkinson’s disease. The disease is caused by Dopamine hormone deficiency in the brain. Dopamine cannot cross the blood–brain barrier, so dopamine taken as a drug does not directly affect the central nervous system [1] to return the DA to normal level. Levodopa is a medicine which is converted to dopamine by the brain [2]. L-dopa (levodopa, 3, 4-dihydroxy-l-phenylalanine, LD) is widely used as a source of dopamine in the treatment of most patients suffering from Parkinson’s disease and epilepsy. This drug can be principally metabolized by an enzymatic reaction to dopamine compensating for the deficiency of dopamine in the brain [3]. In order to achieve a better medicinal effect and lower toxicity, it is very important to control the content of levodopa in pharmaceutical tablets. Therefore, it is important to establish a simple, inexpensive, fast, sensitive and accurate detection method for the determination of this drug. Various methods have been described in literature for the determination of levodopa in various biological samples and pharmaceutical preparations. Nevertheless, these methods often have diverse disadvantages such as high cost, low selectivity, the use of organic solvents, complex sample preparation procedures or long analysis time. In contrast, electrochemical methods can offer several advantages, such as inexpensive and simple analytical method with remarkable detection sensitivity, reproducibility, and ease of miniaturization [4–7]; they are significant in the analytical determination of levodopa and carbidopa [8–10].
Carbon paste electrodes, due to their ease of construction, renewability, and compatibility with various types of modifiers, have been widely used as a suitable matrix for preparation of modified electrodes. Furthermore, they show rather a low background current compared to the solid graphite or noble metal electrodes [11]. The chemical modifications of bare electrodes with a suitable electroactive species as the mediator offer significant advantages in the design and development of electrochemical sensors and biosensors [12–16]. A good mediator should be insoluble in aqueous media, be retained on it, react rapidly with the analytes, be stable in the reduced and oxidized forms, be non-reactive to oxygen, and finally it must be non-toxic. Moreover, the regeneration of the oxidized form should occur at low voltage.
Over the last few years, nanomaterials and nanoscience have been growing very rapidly and added a new dimension to electro analysis and electrode development nano tech performance range of benefits that they are due to increased. Carbon nanotubes (CNT) were discovered in 1991 by Iijima [17]. The CNT have got a lot of attention and have become of interest to material scientists because of their unique optical, electronic and mechanical properties [18]. CNTs have high aspect ratio in many fields because of good electrical, and physical properties, which makes them one of the best choices for nanoscale electron devices, such as field emission displays, atomic force microscope tips, hydrogen storage cells, electronic switches, and high super conductivity material [19–22].
In this work the electrochemical behavior of a bis(2-hydroxy-acetophenone)ethylenediimine vanadyl (abbreviated as BHV) was studied at CNT modified carbon paste electrode. The presence of CNTs at the modified electrode improved the electrochemical behavior and increased the electron transfer rate constant of vanadyl complex. This work shows the vanadyl complex and CNTs modified CPE with good redox behavior and relatively high electron transfer rate constant is suitable for electrocatalysis of LD. The calibration plot and selectivity of the modified electrode versus LD were evaluated by differential pulse voltammetry (DPV).
2 Experimental
2.1 Apparatus and Chemicals
The electrochemical measurements were performed with a potentiostat/galvanostat (SAMA 500, electroanalyzer system, Iran). A conventional three electrode cell was used at 25 ± 1 °C. An Ag/AgCl/KCl (3.0 M) electrode, a platinum wire, and the BHV-CNTCPE were used as the reference, auxiliary and working electrodes, respectively. All potentials mentioned in this paper were referred to this reference electrode. A Metrohm 691 pH/ion meter was used for pH measurements. SEM images obtained using a KYKY EM3200 using an accelerating voltage of 20 kV.
All solutions were freshly prepared with double distilled water. LD, l-lysine, glucose, lactose, fructose, sucrose, D-penicillami, carbidopa, tryptophan, folic acid, uric acid and guanine and all other reagents were of analytical grade from Merck (Darmstadt, Germany). The bis(2-hydroxyacetophenone)ethylenediimine vanadyl was synthesized and used after purification [23]. Graphite powder and paraffin oil (DC 350, density = 0.88 g cm−3) as the binding agents (both from Merck, Darmstadt, Germany) were used for preparing the pastes. The buffer solutions were prepared from orthophosphoric acid and its salts in the pH range of 2.0–11.0.
2.2 Preparation of Electrode
The BHV-CNTCPE were prepared by dissolving 0.015 g of BHV in 3 mL ethanol and then added in 0.5 g graphite powder and 0.04 g CNT with a mortar and pestle hand mixing for a few minutes. Then, ∼0.7 mL of paraffin was added to the above mixture and mixed for 20 min until a uniformly-wetted paste was obtained. The paste was then packed into the end of a glass tube (ca. 3.4 mm i.d. and 10 cm long). A copper wire inserted into the carbon paste provided the electrical contact. For each measurement, when necessary, a new surface was obtained by pushing an excess of the paste out of the tube and polishing with a weighing paper. For comparison, BHV modified CPE electrode (BHV-CPE) without CNT, CNT paste electrode (CNTCPE) without BHV, and unmodified CPE in the absence of CNT nanoparticles were also prepared in the same way.
3 Results and Discussion
3.1 SEM Characterization
Typical SEM images of different electrodes were shown in Fig. 1. Figure 1a shows the layer of irregularly flakes of graphite powder present on the surface of CPE. After CNTs added to the carbon paste, it can be seen that CNTs were distributed on the paste (Fig. 1b), with special three-dimensional structure, indicating that the CNTs were successfully modified on the BHV-CNTNCPE.
SEM images of a CPE (magnification: 11.9 kx, operational voltage: 20 kV, working distance: 11.63 mm, scale bare: 2 µm, detector: secondary electron detector), b BHV CNTCPE (magnification: 23.0 kx, operational voltage: 20 kV, working distance: 11.86 mm, scale bare: 1 µm, detector: secondary electron detector)
3.2 Electrochemical Properties of Modified BHV-CNTNCPE
To the best of our knowledge there is no prior report on the electrochemical properties and, in particular, the electrocatalytic activity of BHV in aqueous media. Therefore, we prepared modified electrodes based on BHV-CNTCPE and studied their electrochemical properties in a buffered aqueous solution (pH 7.0) using CV. It should be noted that one of the advantages of BHV as an electrode modifier is its insolubility in aqueous media. Experimental results showed reproducible, well-defined, anodic and cathodic peaks with Epa and Epc of 0.30 and 0.23 V versus Ag/AgCl/KCl (3.0 M), respectively. This substance can be used as mediator for the electrocatalysis of some important biological compounds with slow electron transfer. The observed peak separation potential, ∆Ep = (Epa−Epc) of 70 mV, was greater than the value of 59/n mV expected for a reversible system, suggesting that the redox couple of BHV in BHV-CPE has a quasi-reversible behavior in aqueous medium. The effect of the potential scan rate (ν) on electrochemical properties of the BHV-CPE was also studied by CV (Fig. 2). Plots of both anodic and cathodic peak currents (Ip) were linearly dependent on ν in the range of 20–1000 mV s−1 (Fig. 2a), indicating that the redox process of BHV at the modified electrode is diffusionless in nature.
CV experiment has shown us some applications of materials such as the apparent change transfer rate constant, ks, and the transfer coefficient, α, of a surface-confined redox couple. According to the procedure of Laviron [24], by using the variation of anodic and cathodic peak potentials with logarithm of scan rate, all of this parameters can be evaluated from CV experiments. Figure 2b shows such plots, indicating that the Ep values are proportional to the logarithm of scan rate for ν values higher than 0.1 V s−1 (Fig. 2b). To extract the kinetic parameters αc and αa (cathodic and anodic transfer coefficients, respectively) we can use the slopes of the plots in Fig. 2b. The slope of the linear segments are equal to −2.303RT/αnF and 2.303RT/(1 − α)nF for the cathodic and anodic peaks, respectively. The evaluated value for the αa is 0.48 Also, Eq. (1) can be used to determine the electron transfer rate constant between modifier (BHV) and CNTCPE:
where (1 − α)nα = 0.52, ν is the sweep rate and all other symbols have their conventional meanings. By using Eq. (1) the value of ks was evaluated to be 3.5 ± 0.1 s−1.
3.3 Electrocatalytic Oxidation of LD at a BHV-CNTCPE
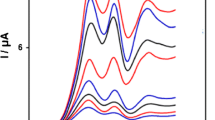
In order to test the potential electrocatalytic activity of the BHV-CNTCPE, its cyclic voltammetric response was obtained in buffer solution (pH 7.4) containing 0.25 mM LD. Figure 3 show cyclic voltammograms of BHV-CNTCPE for the electrochemical oxidation of 250 µM LD at unmodified CPE (curve b), CNTCPE (curve d), BHV-CPE (curve e) and BHV-CNTCPE (curve f). As it is seen, while the anodic peak potential for LD oxidation at the CNTCPE, and unmodified CPE are 600 and 650 mV, respectively, the corresponding potential at BHV-CNTCPE and BHV-CPE is ∼300 mV. These results indicate that BHV can act as a good mediator and peak potential for LD oxidation at the BHV-CNTCPE and BHV-CPE electrodes shift by ∼300 and 350 mV toward negative values compared to CNTCPE and unmodified CPE, respectively. However, BHV-CNTCPE shows much higher anodic peak current for the oxidation of LD compared to BHV-CPE (41.8 µA for BHV-CPE and 77.1 µA for BHV-CNTCPE), indicating that the combination of CNT and the vanadyl complex (BHV) has significantly improved the performance of the electrode toward LD oxidation. In fact, BHV-CNTCPE in the absence of LD exhibited a well-behaved redox reaction (Fig. 3, curve c) in 0.1 M phosphate buffer (pH 7.0). However, there was a significant increase in the anodic peak current in the presence of 250 µM LD (curve f), which can be related to the strong electrocatalytic effect of the BHV-CNTCPE toward this compound [25]. From these results, an electrocatalytic behavior is observed for LD oxidation at the surface of BHV-CNTCPE via an EC´ catalytic mechanism. In this mechanism, LD is oxidized in the catalytic chemical reaction (C′) by the oxidized form of BHV (BHVox) which produced via an electrochemical reaction (E). Therefore, when the BHV is oxidized at the potential of 300 mV, the levodopa can be oxidized too in this potential. Thus the LD is oxidized at the potential of 300 mV at the BHV-CNTCPE while it is oxidized at 650 mV at the bare electrode. Meanwhile CD and Trp are oxidized at the electrode surface via an electrochemical (E) reaction. The advantages of BHV-CNTCPE had been elucidated with higher conductivity, fast electron transfer rate, good anti-fouling properties and inherent catalytic ability of BHV.
The effect of scan rate on the electrocatalytic oxidation of LD at the BHV-CNTNCPE was investigated by CV (Fig. 4). As can be observed in Fig. 4, the oxidation peak potential shifted to more positive potentials with increasing scan rate, confirming the kinetic limitation in the electrochemical reaction. Also, a plot of peak height (Ip) versus the square root of scan rate (ν1/2) was found to be linear in the range of 10–95 mV s−1, suggesting that, at sufficient over potential, the process is diffusion rather than surface controlled (Fig. 4a). A plot of the scan rate-normalized current (Ip/ν1/2) versus scan rate (Fig. 4b) exhibits the characteristic shape typical of an EC′ process [25].
CVs of BHV-CNTCPE in 0.1 M phosphate buffer solution (pH 7.0) containing 250 µM LD at various scan rates; from inner to outer scan rates of 10, 25, 35, 45, 65, 85 and 95 mV s−1, respectively. Insets: variation of a anodic peak current versus ν1/2; b Tafel plot derived from the rising part of voltammogram recorded at a scan rate of 10 mV s−1; c normalized current (Ip/ν1/2) versus ν
Tafel plot was drawn from data of the sharp rising part of the voltammogram as shown in inset C of Fig. 4 if deprotonation of LD is a sufficiently fast step, the tofel plot can be used to estimate the number of electrons involved in the rate determining step. A Tafel slope of 0.1141 V decade−1 was obtained which agrees well with the involvement of one electron in the rate determining step of the electrode process [25], assuming a transfer coefficient, (α) is about 0.48.
3.4 Chronoamperometric Measurements
The chronoamperometry as well as the other electrochemical methods was also employed for the investigation of electrode processes at chemically modified electrodes.
Chronoamperometric measurements of LD at BHV-CNTCPE were carried out at the working electrode potential of 400 mV for various concentrations of LD in PBS (pH 7.0). For an electroactive material (LD in this case) with a diffusion coefficient of D, the current observed for the electrochemical reaction at the mass transport limited condition is described by the Cottrell equation [25]. Experimental plots of I versus t−1/2 were drawn, and the best fits for different concentrations of LD were determined as shown in Fig. 5a.
Chronoamperograms obtained at BHV-CNTCPE in 0.1 M phosphate buffer solution (pH 7.0) for different concentrations of LD. The numbers 1–5 correspond to 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1.0 mM of LD. Insets: a plots of I versus t−1/2 obtained from chronoamperograms, b plot of the slope of the straight lines against the LD concentration
The slopes of the resulting straight lines were then plotted versus LD concentration (Fig. 5b). From the resulting slope and Cottrell equation the mean value of the D was found to be (6.25 ± 0.2) × 10−5 cm2 s−1 according to the method of Galus [26]. Chronoamperometry can also be used to evaluate the catalytic rate constant, k, for the reaction between LD and the BHV-CNTCPE:
where IC is the catalytic current of LD at the BHV-CNTCPE, IL is the limited current in the absence of LD and γ = kCbt is the argument of the error function (Cb is the bulk concentration of LD). In cases where γ exceeds the value of 2, the error function is almost equal to 1 and therefore, the above equation can be reduced to:
where t is the time elapsed. The above equation can be used to calculate the rate constant, k, of the catalytic process from the slope of IC/IL versus t1/2 at a given LD concentration. From the values of the slopes, the average value of k was found to be (3.52 ± 0.45) × 104 M−1 s−1.
3.5 Calibration Plot and Limit of Detection
To evaluate the sensitivity and dynamic range of the BHV-CNTCPE, routine samples with various concentrations of LD were assayed under optimal conditions. In DPV method, the peak current is related to the concentration of electroactive species (LD in this case) [25]. As shown in Fig. 6, the Differential pulse voltammetry (DPV) response of the sensor increased with the increment of LD concentration, and exhibited a very good linear relationship with the LD concentration from 0.8 to 1000 µM. The plot of peak current versus LD concentration consisted of two linear segments with slopes of 0.371 and 0.031 µA µM−1 in the concentration ranges of 0.8–80 and 80–1000 µM, respectively. The decrease in sensitivity (slope) of the second linear segment is likely due to kinetic limitation. The detection limit (3σ) for LD in the lower range region was found to be 0.43 µM.
Differential pulse voltammograms of the BHV-CNTCPE in 0.1 M PBS (pH 7.0) containing different concentrations of LD, from inner to outer, correspond to 0.8, 2, 10, 20, 60, 80, 200, 400, 500 700, 900 and 1000 µM of LD. Plot of the peak currents as a function of concentration of LD in two linear ranges of 0.8–80 and 80–1000 µM
3.6 Interference Study
The influence of various foreign species on the determination of 1.0 × 10−4 M LD was investigated. The tolerance limit was taken as the maximum concentration of the foreign substances, which caused an approximately ±5 % relative error in the determination. The tolerated concentration of foreign substances was 1.0 × 10−1 M for Na+, Cl−, F−, S2−, CO3 2−, NO3 −, HCO3 − and K+; 5.0 × 10−2 M for Mg2+, Ba2+, Cd2+, Cu2+, Pb2+, Ni2+, Al3+and Ca2+; 5.0 × 10−3 M for l-lysine, glucose, lactose, fructose, sucrose, D-penicillami, folic acid, uric acid and guanine.
4 Conclusions
Electrochemical behavior of vanadyl complex (BHV) was investigated at carbon paste electrode modified with it and CNTs. The results show that the presence of CNT at the modified electrode improves the charge transfer rate constant of the BHV. Oxidation of LD is catalyzed at pH 7.0, whereas the peak potential of LD is shifted by 300 mV to a less positive potential at the surface of the BHV-CNTCPE. The catalytic peak currents obtained from DPV were linearly dependent on the LD concentration in the range of 0.8–1000 μM. The detection limit (3σ) for LD was 0.43 μM.
References
R.D. Palmiter, Trends Neurosci. 30, 375 (2007)
C. Dowding, C. Shenton, S. Salek, Drugs Aging 23, 693 (2006)
D.R. Robertson, N.D. Wood, H. Everest, K. Monks, D.G. Waller, A.G. Renwick, C.F. George, Br. J. Clin. Pharmacol. 28, 61 (1989)
M. Mazloum-Ardakani, A. Khoshroo, J. Electroanal. Chem. 717–718, 17 (2014)
M. Mazloum-Ardakani, A. Khoshroo, L. Hosseinzadeh, Sensor. Actuator. B. Chem. 214, 132 (2015)
T. Isaji, M. Abe, T. Amaya, T. Hirao, J. Inorg. Organomet. Polym Mater. 25, 145 (2015)
Z. Taleat, A. Khoshroo, M. Mazloum-Ardakani, Microchim. Acta 181, 865 (2014)
M. Mazloum-Ardakani, A. Khoshroo, Anal. Chim. Acta 798, 25 (2013)
M. Mazloum-Ardakani, Z. Taleat, A. Khoshroo, H. Beitollahi, H. Dehghani, Biosens. Bioelectron. 35, 75 (2012)
M. Mazloum-Ardakani, M. Zokaie, A. Khoshroo, Ionics 21, 1741 (2015)
R. Adams, Anal. Chem. 30, 1576 (1958)
M. Mazloum-Ardakani, F. Sabaghian, A. Khoshroo, M. Abolhasani, H. Naeimi, Ionics 21, 239 (2015)
M. Mazloum-Ardakani, A. Khoshroo, Electrochim. Acta 103, 77 (2013)
M. Mazloum-Ardakani, A. Khoshroo, L. Hosseinzadeh, Sensor. Actuator. B. Chem. 204, 282 (2014)
S. Duan, S. Xu, X. Xu, C. Zhou, J. Inorg. Organomet. Polym Mater. 21, 886 (2011)
M. Mazloum-Ardakani, A. Khoshroo, Electrochim. Acta 130, 634 (2014)
S. Iijima, nature 354, 56 (1991)
Z. Yao, C.-C. Zhu, M. Cheng, J. Liu, Comput. Mater. Sci. 22, 180 (2001)
H. Dai, Acc. Chem. Res. 35, 1035 (2002)
M. Mazloum-Ardakani, M.A. Sheikh-Mohseni, Carbon nanotubes in electrochemical sensors (InTech, Croatia, 2011)
M. Moghim, S. Zebarjad, J. Inorg. Organomet. Polym. Mater. (2015). doi:10.1007/s10904-015-0235-0
M. Mazloum-Ardakani, A. Khoshroo, Electrochem. Commun. 42, 9 (2014)
D.M. Boghaei, S. Mohebi, J. Mol. Catal. A: Chem. 179, 41 (2002)
E. Laviron, J.Electroanal.Chem 101, 19 (1979)
A.J. Bard, L.R. Faulkner, Electrochemical methods, 2nd edn. (Wiley, New York, 2001)
Z. Galus, G.F. Reynolds, S. Marcinkiewicz, Fundamentals of electrochemical analysis (Ellis Horwood, New York, 1976)
Acknowledgments
The authors would like to thank Yazd University Research Council, IUT Research Council and Excellence in Sensors for financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazloum-Ardakani, M., Amin-sadrabadi, E., Khoshroo, A. et al. Electrocatalytic Properties of Vanadyl Complex in Graphite Nanocomposite and its Enhanced Electrochemical Catalysis Properties for Levodopa Oxidation. J Inorg Organomet Polym 25, 1576–1581 (2015). https://doi.org/10.1007/s10904-015-0277-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0277-3