Abstract
Genetic testing for inherited cancer risk has recently improved through the advent of multi-gene panels and the addition of deletion and duplication analysis of the BRCA genes. The primary aim of this study was to determine which factors influence the intent of individuals with a personal history of breast and/or ovarian cancer and negative or uncertain BRCA1 and BRCA2 testing to return to a hereditary cancer program for additional genetic risk assessment, counseling, and testing. Surveys were sent to 1197 individuals and 257 were returned. Of those participants who were planning to return to clinic, most cited having family members who could benefit from the test result as the primary motivation to return. Many participants who were not planning to return to clinic cited the cost of testing as a barrier to return. Cost of testing and concerns about insurance coverage were the most commonly cited barriers for the group of participants who were undecided about returning to clinic. Results from this study may be used to guide re-contact efforts by clinicians to increase patient uptake to return to clinic for up-to-date genetic risk assessment, counseling, and testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2016, there were approximately 246,000 new diagnoses of breast cancer and greater than 40,000 breast cancer-related deaths in Americans (Howlader et al. 2016). Greater than 22,000 women receive a new diagnosis of ovarian cancer and about 14,000 women die from ovarian cancer annually in the USA (Howlader et al. 2016). Approximately 15% of all ovarian cancer cases, 5 to 10% of all breast cancer cases, and more than half of hereditary breast cancer cases are caused by mutations in the BRCA1 and BRCA2 genes (Castera et al. 2014; Krainer et al. 1997). Improvements in genetic testing methodologies have allowed for increased testing sensitivity. For instance, BRCA testing has improved in recent years with enhanced detection of large deletions and duplications, which is now included as standard of care for BRCA testing. Approximately 10% of mutations in BRCA1 and BRCA2 are due to large rearrangements (Judkins et al. 2012; Petrucelli et al. 2016).
In addition, development of next-generation sequencing (NGS) techniques has led to the creation of breast and ovarian cancer risk multi-gene panels (Hiraki et al. 2014) that allow for concurrent testing of multiple genes at once. Mutations in the BRCA genes are the most common genetic cause of hereditary breast cancers, but mutations in other breast cancer susceptibility genes may collectively be responsible for up to half of all hereditary breast cancers (Castera et al. 2014; Moran et al. 2016; Susswein et al. 2016). Multi-gene panels have been shown to provide increased detection of causative mutations compared to single-gene tests (Fecteau et al. 2014; Hiraki et al. 2014; Kurian et al. 2014; Mauer et al. 2014).
Patients who are found to have a mutation in BRCA1, BRCA2, or another cancer susceptibility gene may be candidates for heightened surveillance or risk-reduction strategies (Kurian et al. 2014; Minion et al. 2014). A molecular diagnosis can also provide a patient’s family with valuable risk information and the possibility of undergoing cascade testing. For patients who previously tested negative for BRCA1 and BRCA2 mutations, cancer multi-gene panel detection rates vary in the literature from 2.9 to 11.4% (Kurian et al. 2014; Minion et al. 2014; Tung et al. 2015; Vaccari et al. 2015). Many healthcare centers are considering inviting patients who had genetic testing prior to the advent of multi-gene panels to return for additional genetic risk assessment, counseling, and testing (Hampel 2009).
Past studies have been conducted to characterize motivations and barriers to clinical follow-up. Motivating factors include high interpersonal social support, perceived benefit of follow-up, and a positive relationship with the healthcare provider. Barriers to follow-up include time constraints, low income, and lack of transportation (Augusto et al. 2013; Buchberg et al. 2015; Pratt et al. 2015; Wevers et al. 2014). Bakos et al. (2008) found that patients with a negative BRCA result still worried about carrying a harmful mutation that had not been discovered and felt that their risk of getting cancer was higher than the general population. Flores et al. (2016) conducted a study to examine the factors that impact interest in genetic testing for modest and moderate breast cancer risk genes in female first-degree relatives of breast cancer patients who tested negative for BRCA1 and BRCA2 mutations. Interest in testing was reportedly high at 70% and was higher if the results could guide risk-reducing behaviors. Factors that were independently associated with testing interest were perceived lifetime risk of developing cancer and high cancer worry.
Purpose of the Study
Although the detection rate and utility of multi-gene cancer panels have been discussed in the literature, motivations and barriers for those considering multi-gene panel testing after receiving uninformative BRCA results have not been well explored. Multi-gene panel testing could have clinical utility for patients with either a previous negative result or a variant of uncertain significance (VUS) identified in the BRCA genes. Therefore, uninformative BRCA results include both negative and VUS results for the purposes of this study. The primary aim of this study was to determine which factors influence the intent of individuals with a personal history of breast and/or ovarian cancer and negative or uncertain BRCA1 and BRCA2 testing to return to a hereditary cancer program for additional genetic risk assessment, counseling, and testing.
Methods
Design
A comparative, paper-based survey of patients previously seen by genetic counselors in a large academic Hereditary Cancer Program (HCP) from 1996 to 2012 (see Appendix A) was designed by the study team. The survey was primarily quantitative in design with one qualitative question. This study was approved by Cincinnati Children’s Hospital Medical Center’s (CCHMC) Institutional Review Board (IRB approval no. 2015–2168).
Participants
Our target population included patients with a personal history of breast and/or ovarian cancer who had tested negative for BRCA1 and BRCA2 gene mutations or had a variant of uncertain significance (VUS) identified in either gene, who were seen in CCHMC’s HCP clinic between January 1, 1996 and December 31, 2012. Both males and females were included in our study. Individuals were eligible to participate if they met the above criteria, were over the age of 18, and could read and write in English.
Instrumentation
A 38-item survey was developed by the study team that included close-ended questions assessing demographic characteristics, psychosocial factors (e.g., family support, having children, fear of finding a mutation, or availability of resources), and medical information (e.g., personal and familial cancer history, prophylactic surgeries, or regular cancer screening). Format included multiple-choice, fill-in, and Likert scale. The survey also included an open-ended question about the factor that had the most influence on the respondent’s intent to return to the HCP clinic. The survey was piloted on colleagues to assess the face validity and comprehension of questions and revised based on feedback, prior to mailing to participants. The survey required approximately 10 minutes to complete.
Procedures
Between August 2015 and September 2016, eligible individuals were first mailed a clinical letter inviting them to return to the HCP for further evaluation including updated risk assessment and possible additional genetic testing. A brief description of multi-gene panels and the clinic’s contact information was included. Approximately 2 weeks later, another mailing with the study recruitment letter and survey was mailed. Non-responders were mailed another copy of the recruitment letter and survey 3 weeks after their first survey was mailed. Data from returned surveys were stored in REDCap. Demographic and limited clinical information were collected on eligible individuals who did not respond to the survey. Individuals were given the option to opt-out of this study.
Data Analysis
Descriptive statistics were used to summarize the demographic, psychosocial, and medical characteristics of the study population. Means and standard deviations were calculated for continuous variables, while frequencies or proportions were reported for categorical variables. Likert scale items were treated as continuous variable (strongly disagree = 1, disagree = 2, agree = 3, strongly agree = 4). Multinomial logistic regression was used to test if any survey item was associated with intent to return. T test or Fisher’s exact test was used to test demographic or clinical differences between responders and non-responders. Two researchers independently reviewed all responses to the open-ended question (question three). Themes were developed using deductive codes based on the survey question and inductive codes based on participants’ responses (Ayres et al. 2003; Landis and Koch 1977). A participant’s response may have included multiple themes. The two coders discussed discrepancies and reached consensus. Consensus coding was reviewed by the research team and frequencies for each theme were calculated. Fisher’s exact test was used to test the association between each theme and intent to return. Given the exploratory nature of this study, a nominal p value threshold (p < 0.05) was applied for significance for all comparisons. All the quantitative analyses were performed in R software, version 3.22 (https://www.r-project.org).
Results
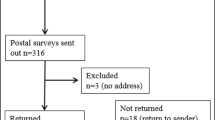
Of 1197 persons who were mailed a clinical letter and subsequent survey, addresses were outdated and no forwarding address was available for 186 persons; 30 were deceased. Of the remaining 918 individuals, 2 persons contacted the center to have their data removed from the study, and 257 surveys were returned (response rate of 26.2%). Seven participants (0.03% of study population) recalled having a BRCA VUS result. Detailed demographic information for those who responded to the survey is reported in Table 1.
Close-Ended Questions Assessing Factors that Impact Decision to Return to Clinic
Participants who were planning to return to clinic or had already made an appointment were included in the “yes” group (n = 110; 43.1%). Those who were not planning to return to clinic in the near future or ever were included in the “no” group (n = 87; 34.1%). The third group of participants were included in the “undecided” group and were unsure whether they wanted to return to clinic (n = 58; 22.8%). The amount of time (in years) between the most recent visit and the time of the study was not different for participants in the yes (7.6 ± 2.6), no (7.9 ± 3.1), and undecided groups (7.2 ± 2.5) (p = 0.3). The yes, no, and undecided groups did not differ significantly on any demographic factors except for having children. Those without children were more likely to be part of the undecided or no groups (p = 0.007) (see Table 2).
There were 15 Likert scale items used to rate agreement with each statement. Response options included strongly disagree, disagree, agree, and strongly agree or not applicable. For seven items, the no group differed significantly from the yes group. The more the participants agreed with these seven statements, the less likely they were to be part of the no group (i.e., less likely to not plan to return to clinic) (see Table 3). These statements include “People with a family history of cancer should get tested for genes that may be related to increased cancer rates in their family (OR = 0.37, p = 1.2 × 10−4),” “My family history of cancer makes me afraid I will get cancer again (OR = 0.40, p = 1.1 × 10−5),” “My personal history of cancer makes me afraid I will get cancer again (OR = 0.52, p = 6.6 × 10−4),” “My genetic test results could benefit my family members (OR = 0.19, p = 5.0 × 10−8),” “My genetic test results could benefit me (OR = 0.15, p = 9.5 × 10−10),” and “I am scared of what genetic tests may find (OR = 0.52, p = 5.9 × 10−4).” For the statement “My family is supportive of me getting tested for genes that may increase my cancer risk,” both the undecided group (OR = 0.43, p = 0.01) and the no group (OR = 0.26, p = 8.2 × 10−6) differed significantly from the yes group.
Participant-Reported Factors Impacting Decision to Return to Clinic
Factors impacting decision to return to clinic were elicited by free response. Question no. 3 stated “What do you feel is the most important reason for your answer to question 2 (Do you plan to return to the HCP clinic)?” One hundred and four individuals in the yes group provided responses that were categorized into 17 themes, 79 individuals in the no group provided responses that were categorized into 25 themes, and 56 individuals in the undecided group provided responses that were categorized into 19 themes. The most frequently reported responses for the yes, no, and undecided groups can be found in Tables 4, 5, and 6 respectively.
Family benefit, information seeking, personal benefit and cancer prevention, altruism, and high risk perception are the most frequent themes in the yes group. For these themes, the difference in response rates among the three groups is statistically significant (all p < 0.05) (see Table 4). Cost of testing, previous negative genetic test result, insurance coverage, low risk perception, feeling too busy, no change in personal history of cancer, perceived value of testing or appointment as important, institutional barriers, doctor input, personal or family updated genetic testing, and having no family to benefit are the most popular responses in the no group. For all of these themes except for institutional barriers, doctor input, and personal or family updated genetic testing, the response rates differed significantly among the three groups (p < 0.05) (see Table 5). Cost of testing, insurance coverage, feeling too busy, needing more information to make a decision, institutional barriers, family benefit, and previous negative genetic test results are the most popular responses in the undecided group. For each of these themes, except institutional barriers, there is a significant difference in the response rates among the three groups (all p < 0.05) (see Table 6). Cost of testing, insurance coverage, and feeling too busy were commonly reported by participants in both the no and undecided groups while rarely reported in the yes group. Although 3.8% of those who were not planning to return to clinic cited previously performed prophylactic surgeries as a reason to not return to clinic, there was no significant difference between the yes, undecided, and no groups with regard to surgical history (history of surgery and laterality of mastectomy and salpingo-oophorectomy were assessed with close-ended questions) (data not shown).
Survey Responders and Non-responders
Responders were compared to non-responders on demographic characteristics. The two groups did not differ significantly by age (p = 0.08). Responders and non-responders did differ significantly with regard to race; responders had a higher proportion of individuals who identified as white than non-responders (96.3 vs. 92.5%, p = 0.03). The average number of years passed since the most recent HCP appointment was less for responders than for non-responders (7.6 vs. 8.1 years, p = 0.02). The highest level of education completed differed significantly between the two groups, with the responders having higher levels of education (p = 0.005) (see Table 7).
Discussion
The existing literature supports re-evaluation of patients who previously tested negative for BRCA1 and BRCA2 mutations in light of advancements in genetic testing technology and availability of clinical testing for newer genes. Results from this study elucidate reasons why people are motivated to return to or are deterred from return to clinic. In addition, the results may aid genetic counselors and other care providers in determining what information to provide patients during re-contact efforts and appointments that might increase the likelihood of patients returning for evaluation.
Patients with a personal history of cancer who have children were significantly more likely to return to clinic for follow-up and additional genetic testing. Having a strong family history of cancer and having family support for genetic testing also had an impact on the decision to return. Of these factors, having a family who is supportive of genetic testing for cancer risk had a particularly strong influence; both the undecided and no groups differed significantly from the yes group in this regard. Free-response data further confirm the importance of family in making a decision to return to clinic. Having children or other family members who could benefit from the information was the most frequently mentioned reason for intent to return to clinic. Interestingly, some participants from the undecided and no groups still cited having family members who could benefit from the genetic test result, again reflecting that concern for family members has a huge impact on decision-making for this population. For those in the undecided or no groups, having family members who could benefit from testing may not be as compelling a motivator as it is for those planning to return to clinic. Other factors, such as cost of testing, may have a greater influence on decision-making in these groups. As those who do not have children or other close relatives are less likely to plan on returning to clinic, providers may want to stress the benefits from the risk information that multi-gene panel testing may be able to provide for these individuals beyond the benefit to family members.
Personal benefits to be gained from the results of genetic testing, such as eligibility for high-risk screening procedures, were noted as a motivator. Desiring as much information as possible about one’s cancer risk (coded as “information seeking”) was also frequently reported as a motivator. Those who intend to return to clinic may have higher anxiety about cancer recurrence than those who are undecided or do not plan to return to clinic, as was noted in the study by Bakos et al. (2008). Alternatively, having information may be a way to cope with stress for these individuals, regardless of their anxiety levels.
For those who do not intend to return to clinic, the cost of testing was the most frequently cited barrier. One participant from the no group stated “I spent over $3000 to find out I was BRCA negative 8 years ago. I don’t understand what I could learn coming back again or how much it would cost me.” Cost of testing and worry about insurance coverage were frequent barriers for the undecided group. However, the cost of genetic testing has dropped dramatically over the last several years, more insurance policies cover genetic testing, and many labs now will pre-authorize testing with insurance companies to clarify out-of-pocket costs before running tests. Some laboratories have lower costs for patients who plan to pay out-of-pocket, and others have reduced rates or no-cost policies for those who previously had genetic testing with their lab.
Patients are not likely to be aware of these changes and flexible payment options if they do not work in genetics or the healthcare field. These options are particularly helpful to those who are uninsured or under-insured and individuals of lower socio-economic status. The clinical letter inviting participants to return to clinic only stated that the genetic testing would be pre-authorized through their insurance company. Participants may have been discouraged by the out-of-pocket cost of previous genetic testing. Despite everyone in this study population having health insurance, health insurance coverage was a popular concern in both the no and undecided groups. The frequency of concerns about cost and insurance coverage may be related to the rise in popularity of high deductible plans and paying out of pocket for genetic testing up to deductible amount.
Several participants cited a previous negative genetic test result as a barrier to return. Some participants thought no further action was required regarding genetic testing after completing sequencing for BRCA1 and BRCA2. Relevant quotes include “I assumed that I didn’t need to come back since my results were negative” (a no group participant) and “Since my original results were negative, I thought that was all I needed to do” (an undecided group participant). Having previous negative results may have inaccurately lowered these participants’ risk perception. Having a low risk perception for future cancers and experiencing no change in one’s personal cancer history are also barriers to clinic return. These individuals may not have thought much about their cancer risk since completing treatment. Additional concerns in this population included feeling too busy to come to clinic for another medical appointment and institutional barriers (such as scheduling difficulties or not receiving a response to a phone call or voicemail). Some participants in the no group noted that a follow-up appointment with genetics was important or valuable, but still they did not intend to return. It is possible that additional information or support might increase the likelihood that these patients return to clinic. Indeed, some participants in the group that were undecided about returning to clinic explicitly stated that they wanted more information to make their decision.
Study Limitations
Most participants in this study were well-educated, high socio-economic status (SES), middle-aged white women, and all of our participants had some form of health insurance. There was a high ratio of non-responders in our study. Although there was no statistically significant difference between the responders and the non-responders in mean age, there were differences in regard to race, time passed since their most recent HCP appointment, and highest level of education completed. Given the potential for an ascertainment bias in our sample, the findings may have limited generalizability. A larger study sample with a greater variety of races, ages, sex, and cancer history would be more robust and increase the external validity of the findings. Additionally, we did not do an assessment for appropriateness of genetic testing prior to sending an invitation to return to clinic or as part of this research study. Details about the participants’ family history of cancer were not collected or reviewed. Thus, it is possible that some individuals in our study, regardless of their intent to return to clinic, may not be considered “high risk” under the current NCCN guidelines for genetic testing. Data from individuals with a VUS were not analyzed separately from those with a negative test result as there were only seven individuals who reported having a VUS. Due to this small sample size, there was concern as to whether any differences between this group and those with a negative test result would be meaningful. These individuals may have different motivations and barriers than those with a negative result.
The survey used in this study was created by the study team and was not formally validated. After collecting data using the survey, we noted that although we asked for the most important reason for participants’ decision whether or not to return to clinic, many reported more than one reason for their decision. Participants who only listed one reason may have had other reasons that were important that they did not report due to the wording of the question.
Practice Implications
The results of this study have elucidated characteristics and concerns of patients who had negative or uncertain BRCA testing and were now considering updated risk assessment and genetic testing. These data can be used to help determine what kinds of information, when provided to patients, may help maximize return rates during a re-contact effort. For example, clinicians could provide additional information upfront to help patients understand the importance of returning to clinic for further evaluation and risk assessment. Additional information in the invitation letter, a supplemental FAQ sheet, or offering pre-visit tele-counseling may provide the additional information, support, or flexibility needed by those individuals who are having trouble making their decision whether or not to return. Providing patients with common red flags for hereditary cancer would allow them to briefly assess their own personal and family histories for risk factors that encourage consideration of clinical follow-up and potential genetic testing. Discussing the benefits of a follow-up genetics visit and/or multi-gene panel testing for both the patient and their family members, as well as being transparent about insurance coverage, potential out-of-pocket costs, and the billing policies of one’s preferred lab before the patient returns to clinic all may help increase the likelihood of return by addressing common concerns.
Ongoing contact and education efforts with referring providers and primary care providers could aid in the dissemination of new scientific information over longer periods of time. Non-genetics care providers, such as oncologists, are more likely to have regular access to their patients and have up-to-date contact information. Several participants in our study made it clear that the opinion of their doctor was valuable in making their decision to return. One participant who was undecided about returning to clinic stated, “I would come back if professionals believe it is in my best interest or in my children’s best interest.” A participant who was not planning to return to clinic reported, “I have been counseled by my surgeon and will follow her advice regarding future testing.” One participant who was planning to return wrote, “I talked to my oncologist about [the] test. She thought it was a good idea, not just for me but for my children as well.” Non-genetics providers are a valuable and accessible resource for patients at high risk for hereditary cancer syndromes, so ensuring providers know of updates in the field of cancer genetics is likely an efficient way of disseminating this information to these patients. Although a genetic counseling referral is recommended in these cases, education efforts by genetics professionals to ensure that non-genetics providers are well-versed in these updates and testing options may help those who have to prioritize their care to reduce costs related to additional specialist visits.
Anticipatory guidance for patients regarding the rapid growth of scientific knowledge in the field of cancer genetics may be helpful along with introducing the concept that a negative test does not mean that a mutation in a different cancer susceptibility gene is not present. These should be addressed at the point of initial contact. Encouraging patients to re-contact genetics professionals in the future for updated information that could inform their risk for cancer may help alleviate the challenge facing providers of reaching those patients who were seen many years prior. The responsibility of clinical follow-up would then be shared with the patient. These education points would be particularly important for individuals and families with negative genetic testing but concerning personal and/or family histories.
Research Recommendations
Further research with a more diverse population would aid in clarifying the generalizability of this study’s results. A multi-site study may be a way to increase diversity in future studies. Including a question on the survey such as “Have any of your healthcare providers discussed genetic testing options with you?” may help clarify the role of non-genetics care providers’ input in patient decision-making. Future studies could include detailed collection of the number of people affected with cancer and/or the severity of the diagnoses to help define what factors in a family history drive patients to return. Further study is needed to determine what makes family support so important and what kind of support is most beneficial (e.g., financial or emotional support). Future studies could compare return rates for groups provided information with different levels of detail during their recruitment effort to indicate which topics are most important to cover for patients. It may also be useful to compare return rates for “high-risk” and “low-risk” groups based on cancer risk models or for those with a VUS result compared to those with a negative test result. A qualitative interview study may be able to elicit more detailed information from patients.
Conclusions
Because gene sequencing technology and the understanding of the significance of different gene mutations are improving at a rapid pace, a person’s previous uninformative genetic testing result may now be insufficient. Therefore, it may be informative if patients who had a VUS or tested negative for mutations in BRCA1 and BRCA2 but have a positive personal history of cancer are invited back to genetics clinic for up-to-date risk assessment and genetic testing such as multi-gene cancer panel (Haanpaa et al. 2013). The results of this study elucidate characteristics and concerns of patients who had BRCA testing with negative or uncertain results and are now considering updated risk assessment and genetic testing. Of those participants who were planning to return to clinic, most cited having family members who could benefit from the test result as the primary motivation to return. Cost of testing and concerns about insurance coverage were the most commonly cited barriers by participants who were not planning to return to clinic or were undecided. If patients return to a hereditary cancer program (HCP) clinic for reinterpretation of results and/or additional genetic testing, healthcare providers can help facilitate delivery of the most appropriate risk information, medical management, and available testing and payment options. If follow-up testing were informative, clinical care recommendations may be adjusted accordingly, including appropriate screening and risk-reduction strategies. These results have important implications for healthcare providers involved in the care of patients who may be at risk of inherited cancer.
References
Augusto, E. F., Rosa, M. L., Cavalcanti, S. M., & Oliveira, L. H. (2013). Barriers to cervical cancer screening in women attending the family medical program in Niteroi, Rio de Janeiro. Archives of Gynecology and Obstetrics, 287(1), 53–58. https://doi.org/10.1007/s00404-012-2511-3.
Ayres, L., Kavanaugh, K., & Knafl, K. A. (2003). Within-case and across case approaches to qualitative data analysis. Qualitative Health Research, 13(6), 13.
Castera, L., Krieger, S., Rousselin, A., Legros, A., Baumann, J. J., Bruet, O., et al. (2014). Next-generation sequencing for the diagnosis of hereditary breast and ovarian cancer using genomic capture targeting multiple candidate genes. European Journal of Human Genetics, 22(11), 1305–1313. https://doi.org/10.1038/ejhg.2014.16.
Bakos, A. D., Hutson, S. P., Loud, J. T., Peters, J. A., Giusti, R. M., & Greene, M. H. (2008). BRCA mutation-negative women from hereditary breast and ovarian cancer families: A qualitative study of the BRCA-negative experience. Health Expectations, 11(3), 220–231. https://doi.org/10.1111/j.1369-7625.2008.00494.x.
Buchberg, M. K., Fletcher, F. E., Vidrine, D. J., Levison, J., Peters, M. Y., Hardwicke, R., Yu, X., & Bell, T. K. (2015). A mixed-methods approach to understanding barriers to postpartum retention in care among low-income, HIV-infected women. AIDS Patient Care and STDs, 29, 126–132. https://doi.org/10.1089/apc.2014.0227.
Fecteau, H., Vogel, K. J., Hanson, K., & Morrill-Cornelius, S. (2014). The evolution of cancer risk assessment in the era of next generation sequencing. Journal of Genetic Counseling, 23(4), 633–639. https://doi.org/10.1007/s10897-014-9714-7.
Flores, K. G., Steffen, L. E., McLouth, C. J., Vicuna, B. E., Gammon, A., Kohlmann, W., et al. (2016). Factors associated with interest in gene-panel testing and risk communication preferences in women from BRCA1/2 negative families. Journal of Genetic Counseling, 26, 480–490. https://doi.org/10.1007/s10897-016-0001-7.
Haanpaa, M., Pylkas, K., Moilanen, J.S., & Wingvist, R. (2013). Evaluation of the need for routine clinical testing of PALB2 c.1592delT mutation in BRCA negative Northern Finnish breast cancer families. BMC Medical Genetics, 14(82). http://www.biomedcentral.com/1471-2350/14/82. Accessed 28 Feb 2018.
Hampel, H. (2009). Recontacting patients who have tested negative for BRCA1 and BRCA2 mutations: how, who and why? Journal of Genetic Counseling, 18(6), 527–529. https://doi.org/10.1007/s10897-009-9254-8.
Hiraki, S., Rinella, E. S., Schnabel, F., Oratz, R., & Ostrer, H. (2014). Cancer risk assessment using genetic panel testing: considerations for clinical application. Journal of Genetic Counseling, 23(4), 604–617. https://doi.org/10.1007/s10897-014-9695-6.
Howlader, N., Noone, A.M., Krapcho, M., Miller, D., Bishop, K., Altekruse, S.F., et al. (2016) SEER cancer statistics review, 1975–2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016.
Judkins, T., Rosenthal, E., Arnell, C., Burbidge, L. A., Geary, W., Barrus, T., Schoenberger, J., Trost, J., Wenstrup, R. J., & Roa, B. B. (2012). Clinical significance of large rearrangements in BRCA1 and BRCA2. Cancer, 118(21), 5210–5216. https://doi.org/10.1002/cncr.27556.
Krainer, M., Silva-Arrieta, S., FitzGerald, M. G., Shimada, A., Ishioka, C., Kanamaru, R., MacDonald, D. J., Unsal, H., Finkelstein, D. M., Bowcock, A., Isselbacher, K. J., & Haber, D. A. (1997). Differential contributions of BRCA1 and BRCA2 to early-onset breast cancer. The New England Journal of Medicine, 336(20), 1416–1421. https://doi.org/10.1056/nejm199705153362003.
Kurian, A. W., Hare, E. E., Mills, M. A., Kingham, K. E., McPherson, L., Whittemore, A. S., McGuire, V., Ladabaum, U., Kobayashi, Y., Lincoln, S. E., Cargill, M., & Ford, J. M. (2014). Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. Journal of Clinical Oncology, 32(19), 2001–2009. https://doi.org/10.1200/JCO.2013.53.6607.
Landis, J. R., & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174.
Mauer, C. B., Pirzadeh-Miller, S. M., Robinson, L. D., & Euhus, D. M. (2014). The integration of next-generation sequencing panels in the clinical cancer genetics practice: an institutional experience. Genetics in Medicine, 16(5), 407–412. https://doi.org/10.1038/gim.2013.160.
Minion, L., Dolinsky, J., Chao, E., & Monk, B. (2014). Hereditary predisposition to ovarian cancer, looking beyond BRCA1/BRCA2. Gynecologic Oncology, 135(2), 383–384. https://doi.org/10.1016/j.ygyno.2014.07.012.
Moran, O., Nikitina, D., Royer, R., Poll, A., Metcalfe, K., Narod, S. A, et al. (2016). Revisiting breast cancer patients who previously tested negative for BRCA mutations using a 12-gene panel. Breast Cancer Research and Treatment, 1–8. doi: 10.1007/s10549-016-4038-y.
Petrucelli, N., Daly, M. B., Pal, T. (2016). BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. 1998 Sep 4 [Updated 2016 Dec 15]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1247/.
Pratt, K. J., Collier, D. N., Walton, N. L., Lazorick, S., & Lamson, A. L. (2015). Predictors of follow-up for overweight youth and parents. Families, Systems & Health, 33, 55–60. https://doi.org/10.1037/fsh0000103.
Susswein, L. R., Marshall, M. L., Nusbaum, R., Vogel Postula, K. J., Weissman, S. M., Yackowski, L., Vaccari, E. M., Bissonnette, J., Booker, J. K., Cremona, M. L., Gibellini, F., Murphy, P. D., Pineda-Alvarez, D. E., Pollevick, G. D., Xu, Z., Richard, G., Bale, S., Klein, R. T., Hruska, K. S., & Chung, W. K. (2016). Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genetics in Medicine, 18(8), 823–832. https://doi.org/10.1038/gim.2015.166.
Tung, N., Battelli, C., Allen, B., Kaldate, R., Bhatnagar, S., Bowles, K., Timms, K., Garber, J. E., Herold, C., Ellisen, L., Krejdovsky, J., DeLeonardis, K., Sedgwick, K., Soltis, K., Roa, B., Wenstrup, R. J., & Hartman, A. R. (2015). Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer, 121(1), 25–33. https://doi.org/10.1002/cncr.29010.
Vaccari, E., Yackowski, L., Hussong, M., Murphy, P., Cremona, M., Booker, J. et al. (2015). Characterizing the clinical presentation of individuals with pathogrnic variants in a breast/ovarian cancer gene panel: GeneDx.
Wevers, A., Wigboldus, D. H., de Kort, W. L., van Baaren, R., & Veldhuizen, I. J. (2014). Characteristics of donors who do or do not return to give blood and barriers to their return. Blood Transfusion, 12(Suppl 1), s37–s43. https://doi.org/10.2450/2013.0210-12.
Acknowledgements
All research activities were conducted while the first author was enrolled in the Genetic Counseling Program, College of Medicine, University of Cincinnati and Division of Human Genetics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH. Representatives from GeneDx were not involved in data collection or analysis. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The research team would like to thank Danielle Rolfes and Meghan Tipsword for their contributions to the research, including preparing and sending mailings, scheduling appointments, tracking updated patient contact information, and coding free-response data.
Funding
Funding to conduct this research was provided by GeneDx.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sara Knapke is an employee of GeneDx, Inc., a wholly owned subsidiary of OPKO Health, Inc.
Sarah E. Chadwell received a research grant from GeneDx, Inc., a wholly owned subsidiary of OPKO Health, Inc. to conduct this research.
Hua He, Jaime Lewis, Rebecca Sisson, and Jennifer Hopper declare that they have no conflicts of interest.
Human Studies and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
Electronic Supplementary Material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Chadwell, S.E., He, H., Knapke, S. et al. Factors Influencing Clinical Follow-Up for Individuals with a Personal History of Breast and/or Ovarian Cancer and Previous Uninformative BRCA1 and BRCA2 Testing. J Genet Counsel 27, 1210–1219 (2018). https://doi.org/10.1007/s10897-018-0241-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-018-0241-9