Abstract
The steady-state method is used to study the effect of temperature on the fluorescence characteristics of 7-(diethylamino)-3-(1-methyl-1H-benzo[d]imidazol-2-yl)-2H-chromen-2-one (7DA3MHBI-2HChromen-2-one) laser dye in glycerol solvent for the temperature range 293-343 K. Absorption and emission characteristics are affected by varying temperatures due to induced thermal effects. Transition probabilities mechanism of non-radiative and radiative are studied and frequency dependent parameters are estimated. Dipole moments in the ground and excited state are estimated using the thermochromic shift method over general solvatochromic methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spectral properties and dipole moments estimation are useful in understanding the molecular structure of organic laser dyes in excited states. Knowledge about dipole moments in excited state is useful in understanding the nature of excited states, photochemical transformation, and designing non-linear material, fabricating opto-electronic devices and thermal sensors. Spectral properties and dipole moments are changed by the immediate surrounding environment. Solvents and their properties (ε, n, \({{\text{E}}}_{{\text{T}}}^{{\text{N}}},\) Hydrogen bond donor (HBD),Hydrogen bond acceptor (HBA), viscosity, concentration, pH, temperature, quenchers, etc., affect the spectral characteristics of laser dyes [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Though various parameters affect the laser spectral properties, in recent years the effect of temperature has drawn the great attention of researchers. A large group of researchers extensively studied organic laser dye's spectral properties in different solvent environments by varying temperatures [17,18,19,20,21,22,23,24,25,26,27] and reported that there is no much significant change in absorption and emission wavelength maxima; however fluorescence intensities decrease with an increase in temperature. These intensity changes are due to changes in probabilities of non-radiative and radiative transitions due to thermal effects initiated by varying temperature. To understand underlying deactivation mechanism of these temperature dependence transitions from an excited state. In this paper, we have studied the effect of temperature on spectral properties and estimated dipole moment of7DA3MHBI-2HChromen-2-one laser dye in glycerol solvent. Also, spectral and decay rate parameters such as relative fluorescence intensities, lifetime, rate decay constant, activation energy, theoretically lifetime value at 20 °C are presented in this paper. Dipole moments are generally estimated using solvatochromic shift methods using different solvents with increasing polarities. However, in this paper we used advanced thermochromic shift method in estimating the dipole moment in single solvent. Glycerol solvent being associative and highly viscous solvent, we chose glycerol for our studies. This study will enhance the knowledge about the thermal sensitivity of laser spectral characters in different environments.
Theory
Temperature Effect on Fluorescence Intensity
Fluorescence intensity varies with varying temperatures but fluorescence lifetime is found to be independent of it. Fluorescence intensity decreases with an increase in temperature and can be explained by intersystem crossing between singlet (S1) and triplet (T1) states. However, any decrease in the fluorescence lifetime indicates an increase in the probabilities of non-radiative deactivation of the singlet excited state. Various mechanisms are used to explain the decrease in the fluorescence lifetime [22, 23, 27] with temperature such as internal conversion, inter-system crossing, disassociation, loss of molecular planar structure due to thermal energy, etc.
For 10 – 70 °C small temperature range it is assumed that only a non-radiative deactivation mechanism is dominant. The fluorescence lifetime dependence on temperature is given by [19]
where, \(\uptau ({\text{t}})\) – fluorescence lifetime at different temperatures, \({{\text{n}}}_{1}\) and \({{\text{n}}}_{2}\) – radiative and non-radiative transition probabilities respectively, \({{\text{n}}}_{2}^{0 }\)– deactivation process factor, E-activation energy in de-activation process, T-absolute temperature, R-universal gas constant. The fluorescence rate of decay constant is given by
where, \({{\text{k}}}_{{\text{f}}}=\frac{1}{\uptau ({\text{t}})}\), kf, and \({{\text{k}}}^{\mathrm{^{\prime}}}\) are fluorescence rate decay constant at different temperatures, \({{\text{k}}}^{0}\) – fluorescence rate decay constant at 0 °C, temperature independent rate decay constant, frequency assisted thermal process respectively, \(\mathrm{\Delta E}\) – free energy activation component. Fluorescence lifetime at different temperatures is given by
where, \(\uptau ({\text{t}})\), \({\uptau }_{0}\left(20\right), {{\text{I}}}_{0}({\text{t}})\) and \({{\text{I}}}_{0}^{20}\) are the fluorescence life time at different temperatures fluorescence lifetime at 20 °C, fluorescence intensities at different temperatures and at 20 °C respectively. A plot of ln \({{\text{k}}}_{{\text{f}}}\) v/s T gives a straight line with \(ln \,{k}^{\prime}\) as intercept and slope gives \((-\mathrm{\Delta E}/{\text{RT}})\). The magnitude of \({{\text{k}}}^{\prime}\) measures how the fluorescence intensity and lifetime vary with temperature.
Thermochromic Shifts and Dipole Moment
The dipole moment in the excited is estimated by using solvatochromic spectral shifts of absorption and fluorescence in different solvents of increasing polarity. The solvatochromic shifts method is suitable for estimating dipole moments of both small and big-sized molecules. In this method different interactions are involved and data spread is a bit quite large. Instead of using different solvents, an improved thermochromic solvatochromic method [28,29,30,31,32,33,34,35] suggests using a single suitable solvent and varying the ε and n by varying the temperature. The experimental collected data spread is very small and specific interactions are eliminated. Thus, this method is found to be more accurate for the measurement of change in dipole moment in the ground and excited states.
Basic Equations for Estimation of Dipole Moment
The following thermochromic solvatochromic equations are used to estimate the dipole moment in the ground and singlet excited state [28,29,30,31,32,33,34,35].
where, \({\overline{\upnu } }_{{\text{A}}}-{\overline{\upnu } }_{{\text{f}}}\), \({\overline{\upnu } }_{{\text{A}}}+{\overline{\upnu } }_{{\text{f}}}\) are difference and sum of absorption and fluorescence maximum wave number respectively,
where, ε – dielectric constant, n—refractive index, \({{\text{m}}}_{1}\), \({{\text{m}}}_{2}\) are the slopes determined using Eqs. (4) and (5), \({\upmu }_{{\text{e}}}\) and \({\upmu }_{{\text{g}}}\)– dipole moments in excited and ground states respectively, h – Planck’s constant, c – velocity of light in vacuum, a – Onsager cavity radius, \(\alpha\) – solute polarizability.
If the dipole moment of ground and excited is not parallel the angle between them is given by
\({{\text{f}}}_{{\text{BK}}}\left(\upvarepsilon ,{\text{n}}\right)\) And \({\upphi }_{{\text{BK}}}\left(\upvarepsilon ,{\text{n}}\right)\) values at different temperatures are calculated using
where, \(\mathrm{\alpha }\), \(\upbeta\) and \(\upgamma\) are the coefficients of T, T2, T3 respectively of dielectric of varying temperature. a, b are coefficients of T, T2 respectively of refractive index of varying temperature.
Experimental Details
Materials
7DA3MHBI-2HChromen-2-one laser dye was purchased from sigma Aldrich Chemicals Pvt. Ltd., HPLC grade glycerol solvent from S.D. Fine Chemicals Ltd., India. Solute and solvent were used without any additional purification.
Computational Method
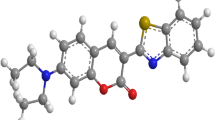
For solute properties 7-(diethylamino)-3-(1-methyl-1H-benzo[d]imidazol-2-yl)-2H-chromen-2-one (7DA3MHBI-2HChromen-2-one) laser dye is geometrical optimized (Fig. 1) using DFT-B3LYP/6–31 basis set and R-closed wave function using Gaussian Software 0.9 version. Solute polarizability α = 40.32 × 10–30 and the ratio of α/a3 is found to be 0.536.
Steady State Measurements
For stable and reliable spectra, the concentration of the solute in the solvent is kept low (1 × 10–6 mol/L). The absorption spectra were recorded using UV–VIS spectrophotometer Hitachi U-3310.The absorption maximum wavelength is chosen for excitation wavelength. Fluorescence intensity was recorded using Hitachi F-7000 fluorescence spectrophotometer. For temperature studies, cuvette temperature is controlled by using thermocouple within ± 0.3 °C between 20–70 °C. Fluorescence lifetime at 20 °C is measured using Edinburgh instrument model El-199.
Results and Discussion
Fluorescence intensities of 7DA3MHBI-2HChromen-2-one in glycerol solvent as a function of temperature have been measured. The typical absorption and fluorescence spectra of 7DA3MHBI-2HChromen-2-one in glycerol solvent as a function of temperature (20–70 °C) are shown in Figs. 2 and 3. It is found that absorbance wavelength maxima (418.2 ± 2.5 nm) more or less remain the same and doesn’t change much with increase in temperature as shown in Table 1. Fluorescence maxima shift slight towards lower wavelength of 0 –1 nm as shown Table 1, this specify that excited singlet emission state is slightly modified on heating and compound basicity in the excited state is increased [36]. Many thermal induced phenomena’s affects fluorescence intensity, decrease in intensity with increase in temperature is either due to increase in non-radiative deactivation process involvement i.e. intercrossing from singlet state (S1) to triplet state (T1) or molecular dissociation. For any small changes in fluorescence intensity with less than 25% with temperature is explained in terms of conversion of electronic to vibrational energy. If it’s greater than 25% its essential to look into non-radiative deactivation mechanism from singlet to triplet state. Relative fluorescence intensity (It/I20) is estimated for different temperature and It/I20v/s temperature is plotted as shown in Fig. 4 with linear fit (Adj. R2 = 0.996) Figs. 5 and 6. It shows It/I20 linearly decrease with increase in temperature. To understand the underlying mechanism of non-radiative deactivation i.e. intersystem crossing mechanism, values of S1 and T2 are to be determined using crossing point for absorption and fluorescence spectra and \(T_2=\mathrm{\Delta E}+S_1\) respectively [22]. S1 is found by intersecting point between absorption and emission spectra of 0–0 band (Fig. 7) whose value is found to be 21,885.2 cm−1.The value of T2 is estimated using the estimated values of decay rate parameters. The decay rate parameter Kf at different temperature is found by taking the reciprocal of \(\uptau ({\text{t}})\) (using Eq. 2) by using \({\uptau }_{0}\) at 20 °C which is found experimentally as shown in Table 2. The fluorescence lifetime and Kf of 7DA3MHBI-2HChromen-2-one decrease with increase in temperature. A plot of fluorescence lifetime and Ln Kf v/s temperature is plotted as shown in Figs. 5 and 6 with good linear fit who’s Adj.R2values are found to be 0.996 and 0.990 respectively. Using linear least square fit method the value of \(k^{\prime}\) and \(\Delta \mathrm{E}\) were calculatedand represented in Table 3 with correlation value of 0.990. Using the value of \(\mathrm{\Delta E}\) and S1, T2 is estimated whose value is less than S1 (Table 3) Henceforth, T2 state lies above S1indicating that transition to ground state (S0) from the excited singlet state occurs via triplet T2 state [22, 23, 27]. With increasing in temperature \(\mathrm{\Delta E}\) is found to be small indicating that T2 energy level lies closely to S1 (Fig. 8).
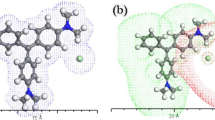
Advance thermochromic solvatochromic method is used to estimate dipole moments of 7DA3MHBI-2HChromen-2-one laser dye. Table 1 and Figs. 2 and 3 show that with increase in temperature a blue shift in the absorption and emission wavelength. It is found that solvent polarities (ε, n) and solvent polarity parameters \({{\text{f}}}_{{\text{BK}}}\left(\upvarepsilon ,{\text{n}}\right)\) and \({\upphi }_{{\text{BK}}}\left(\upvarepsilon ,{\text{n}}\right)\mathrm{ }\) decrease with increase in temperature. Neglecting higher orders the decrease in ε and n values are calculated using Eqs. 14 and 15 and Table 4. Solvent polarity parameters also depend on mean polarizability and Onsager cavity radius of the solute. Edward’s increment method [37] is used to estimate the cavity radius and solute polarizability by Gaussian 9.0 software (α/a3 = 0.543). Solvent polarity parameters \({{\text{f}}}_{{\text{BK}}}\left(\upvarepsilon ,{\text{n}}\right)\) and \({\upphi }_{{\text{BK}}}\left(\upvarepsilon ,{\text{n}}\right)\) are estimated (Eqs. 9 and 10) by using α/a3 = 0.536.A plot of difference and sum of thermochromic band shifts (\({\overline{\upnu } }_{{\text{A}}}-{\overline{\upnu } }_{{\text{f}}}\) and \({\overline{\upnu } }_{{\text{A}}}+{\overline{\upnu } }_{{\text{f}}}\)) wave number versus solvent polarity parameters \({{\text{f}}}_{{\text{BK}}}\left(\upvarepsilon ,{\text{n}}\right)\) and \({{\text{f}}}_{{\text{BK}}}\left(\upvarepsilon ,{\text{n}}\right)+2{{\text{g}}}_{{\text{BK}}}\left({\text{n}}\right)\) are plotted as shown in Figs. 9 and 10 respectively. A linear regression fit of these points was carried out. Shows a good correlation of adj. R2 = 0.99 and 0.98 respectively. The slopes \({{\text{m}}}_{1}\) and \({{\text{m}}}_{2}\) from graphs Figs. 9 and 10.are tabulated in Table 5. Using \({{\text{m}}}_{1}\) and \({{\text{m}}}_{2}\) values \({\upmu }_{{\text{e}}}\) isestimated using Eqs. 11 and 12. Ground state and excited state dipole estimated values are found to be 0.6 D and 4.54 D respectively. For same a = 4.221 A0 Renuka et al. [38] it is reported that in a series of alcohols solvents using solvatochromic shift method the dipole moments are \({\upmu }_{{\text{g}}}\) and \({\upmu }_{{\text{e}}}\) values are found to be 1.41 D and 4.51 D respectively. For same a = 4.221 A0 using thermochromic shift method the value of \({\upmu }_{{\text{e}}}\) is in close agreement with the reported value and \({\upmu }_{{\text{g}}}\) value shows a deviation of 0.81 D. The cosine angle between the \({\upmu }_{{\text{e}}}\) and \({\upmu }_{{\text{g}}}\) is found to 5.45 radians (using Eq. 13). To understand the type of interaction and locate optimal site of interaction between solute and solvent, molecular electrostatic potential of 7DA3MHBI-2HChromen-2-one molecule in glycerol solvent using Gaussian software 9.0 is calculated and plotted as shown in Fig. 11. Green color indicates positive region or nucleophilic active region and red indicates negative region or electrophilic active region. Negative region is localized over 1-methyl 4,5-dihydro-1H-pyrazole region of compound and suggests that the specific hydrogen bonding interaction occurs between –OH group of glycerol and 1-methylpyrolidine [39].
Conclusions
The spectral characters of 7DA3MHBI-2HChromen-2-one laser dye have been studied in glycerol solvent by varying the temperature (20—70 °C). Fluorescence maxima negligibly shift towards shorter wavelength (0–1 nm) and relative intensity decreases linearly with increase in temperature. Increase in temperature results in decrease in fluorescence lifetime of 7DA3MHBI-2HChromen-2-one molecule suggesting involvement of non-radiative transition phenomenon. Fluorescence frequency decay parameters are used to evaluate \({{\text{k}}}^{\prime}\) and \(\Delta {\mathrm{E}}\). Using \(\mathrm{\Delta E}\) and S1, T2 energy level is evaluated. It is noted that T2 energy levels lies very close to singlet state (S1) indicating that deactivation from excited singlet to ground state involves in intersystem crossing (S1 → T2). The advanced method thermochromic shifts over solvatochromic shifts is used to estimate the dipole moment in the ground and excited state. The excited state dipole moment estimated using thermochromic shifts is nearly same and deviation in ground state dipole moment of about 0.81 D in excited state is observed when compared with solvatochromic shifts method for same value of Onsager cavity radius. Also, ground state and excited state dipole moment are nearly parallel. This study helps in determining dipole moments in singlet excited state using single solvent for varying temperature over normal solvatochromic shift method and also to understand the thermal sensitivity of laser dyes with temperature.
Availability of Data and Materials
Not applicable.
References
Patil NR, Melavanki RM, Thipperudrappa J, Afi UO (2013) Effect of temperature on the fluorescence emission of ENCTTTC in different nonpolar solvents. Can J Phys 91:971–975. https://doi.org/10.1139/cjp-2013-0025
Nadaf YF, Renuka CG (2015) Analysis of rotational diffusion of coumarin laser dyes. Can J Phys 93:3–6. https://doi.org/10.1139/cjp-2014-0020
Nagaraja D, Melavanki RM, Patil NR, Kusanur RA (2014) Solvent effect on the relative quantum yield and fluorescence quenching of 2DAM. Spectrochim Acta A Mol Biomol Spectrosc 130:122–128. https://doi.org/10.1016/j.saa.2014.03.063
Zhang H, Yu A (2014) Photophysics and photocatalysis of carbon nitride synthesized at different temperatures. J Phys Chem C 118:11628–11635. https://doi.org/10.1021/jp503477x
Dobek K, Karolczak J, Kubicki J (2014) Temperature effects on excitation and deactivation processes of coumarin 102. A comparison with coumarin 153. Dyes Pigm 100:222–231. https://doi.org/10.1016/j.dyepig.2013.09.008
Basavaraja J, Inamdar SR, Suresh Kumar HM (2015) Solvents effect on the absorption and fluorescence spectra of 7-diethylamino-3-thenoylcoumarin: Evaluation and correlation between solvatochromism and solvent polarity parameters. Spectrochim Acta A Mol Biomol Spectrosc 137:527–534. https://doi.org/10.1016/j.saa.2014.08.118
Zhang D, Martín V, García-Moreno I et al (2011) Development of excellent long-wavelength BODIPY laser dyes with a strategy that combines extending π-conjugation and tuning ICT effect. Phys Chem Chem Phys 13:13026–13033. https://doi.org/10.1039/c1cp21038f
Ruiz CC, Hierrezuelo JM, Molina-Bolivar JA (2015) Analysis of the Photophysical Behavior and Rotational-Relaxation Dynamics of Coumarin 6 in Nonionic Micellar Environments: The Effect of Temperature. Molecules 20:19343–19360. https://doi.org/10.3390/molecules201019343
Bhavya P, Melavanki R, Sharma K et al (2019) Exploring the spectral features and quantum chemical computations of a novel biologically active heterocyclic class of compound 2MEFPBA dye: Experimental and theoretical approach. Chemical Data Collections 19:100182. https://doi.org/10.1016/j.cdc.2019.100182
Deepa HR, Kumar HMS, Basanagouda M, Thipperudrappa J (2014) Influence of silver nanoparticles on absorption and fluorescence properties of laser dyes. Can J Phys 92:163–167. https://doi.org/10.1139/cjp-2013-0133
Basavaraja J, Suresh Kumar HM, Inamdar SR, Wari MN (2016) Estimation of ground and excited state dipole moment of laser dyes C504T and C521T using solvatochromic shifts of absorption and fluorescence spectra. Spectrochim Acta A Mol Biomol Spectrosc 154:177–184. https://doi.org/10.1016/j.saa.2015.10.020
Gautam RK, Chatterjee A, Seth D (2019) Photophysics, rotational dynamics and fluorescence lifetime imaging study of coumarin dyes in deep eutectic solvent. J Mol Liq 280:399–409. https://doi.org/10.1016/j.molliq.2019.01.129
Renuka CG, Nadaf YF, Sriprakash G, Rajendra Prasad S (2018) Solvent Dependence on Structure and Electronic Properties of 7-(Diethylamino) - 2H–1- Benzopyran-2- one (C-466) Laser Dye. J Fluoresc 28:839–854. https://doi.org/10.1007/s10895-018-2249-9
Han J, Cao B, Li Y et al (2020) The role played by solvent polarity in regulating the competitive mechanism between ESIPT and TICT of coumarin (E-8-((4-dimethylamino-phenylimino)-methyl)-7-hydroxy-4-methyl-2H-chromen-2-one). Spectrochim Acta A Mol Biomol Spectrosc 231:118086. https://doi.org/10.1016/j.saa.2020.118086
Bhavya P, Melavanki R, Manjunatha MN (2018) Solvent effects on the photophysical properties of coumarin dye. AIP Conf Proc. 1953–080022–1–5. https://doi.org/10.1063/1.5032828
Pramod AG, Renuka CG, Nadaf YF, Rajaramakrishna R (2019) Impact of solvents on energy gap, photophysical, photometric properties for a new class of 4-HCM coumarin derivative: Nonlinear optical studies and optoelectronic applications. J Mol Liq 292:111383. https://doi.org/10.1016/j.molliq.2019.111383
Mannekutla JR, Mulimani BG, Inamdar SR (2008) Solvent effect on absorption and fluorescence spectra of coumarin laser dyes: evaluation of ground and excited state dipole moments. Spectrochim Acta A Mol Biomol Spectrosc 69:419–426. https://doi.org/10.1016/j.saa.2007.04.016
Zakerhamidi MS, Ghanadzadeh A, Tajalli H et al (2010) Substituent and solvent effects on the photo-physical properties of some coumarin dyes. Spectrochim Acta A Mol Biomol Spectrosc 77:337–341. https://doi.org/10.1016/j.saa.2009.12.060
Ghazy R, Azim SA, Shaheen M, El-Mekawey F (2004) Experimental studies on the determination of the dipole moments of some different laser dyes. Spectrochim Acta A Mol Biomol Spectrosc 60:187–191. https://doi.org/10.1016/s1386-1425(03)00205-1
Zakerhamidi MS, Ghanadzadeh A, Moghadam M (2011) Effect of anisotropic and isotropic solvent on the dipole moment of coumarin dyes. Spectrochim Acta A Mol Biomol Spectrosc 78:961–966. https://doi.org/10.1016/j.saa.2010.12.002
Giri R, Rathi SS, Machwe MK (1991) Influence of solvents and temperature on the excited state characteristics of coumarin laser dyes. Acta Physica Hungarica 70:3–13. https://doi.org/10.1007/BF03054204
Deepa HR, Thipperudrappa J, Kumar HMS (2021) Spectral properties of laser dyes at varying temperatures. Can J Phys 99:88–93. https://doi.org/10.1139/cjp-2020-0087
Deepa HR, Thipperudrappa J, Suresh Kumar HM (2020) Effect of temperature on fluorescence quenching and emission characteristics of laser dyes. J Phys Conf Ser 1473:012046. https://doi.org/10.1088/1742-6596/1473/1/012046
Estrada-Pérez CE, Hassan YA, Tan S (2011) Experimental characterization of temperature sensitive dyes for laser induced fluorescence thermometry. Rev Sci Instrum 82:074901. https://doi.org/10.1063/1.3590929
Thipperudrappa J, Biradar DS, Manohara SR et al (2008) Solvent effects on the absorption and fluorescence spectra of some laser dyes: estimation of ground and excited-state dipole moments. Spectrochim Acta A Mol Biomol Spectrosc 69:991–997. https://doi.org/10.1016/j.saa.2007.06.002
Nad S, Kumbhakar M, Pal H (2003) Photophysical Properties of Coumarin-152 and Coumarin-481 Dyes: Unusual Behavior in Nonpolar and in Higher Polarity Solvents. J Phys Chem A 107:4808–4816. https://doi.org/10.1021/jp021543t
Jana B, Inamdar SR, HM SK (2017) Effect of quencher and temperature on fluorescence intensity of laser dyes: DETC and C504T. Spectrochim Acta A Mol Biomol Spectrosc 170:124–130. https://doi.org/10.1016/j.saa.2016.07.010
Kawski A, Kukliński B, Bojarski P, Diehl H (2000) Ground and Excited State Dipole Moments of LAURDAN Determined from Solvatochromic and Thermochromic Shifts of Absorption and Fluorescence Spectra. Zeitschrift für Naturforschung A 55:817–822. https://doi.org/10.1515/zna-2000-9-1011
Kawski A, Kukliński B, Bojarski P (2005) Dipole moment of aniline in the excited S1 state from thermochromic effect on electronic spectra. Chem Phys Lett 415:251–255. https://doi.org/10.1016/j.cplett.2005.09.008
Kawski A, Kukliński B, Bojarski P (2002) Thermochromic Absorption, Fluorescence Band Shifts and Dipole Moments of BADAN and ACRYLODAN. Zeitschrift für Naturforschung A 57:716–722. https://doi.org/10.1515/zna-2002-0812
Kawski A, Kukliński B, Bojarski P (2006) Dipole moment of benzonitrile in its excited S1 state from thermochromic shifts of fluorescence spectra. Chem Phys Lett 419:309–312. https://doi.org/10.1016/j.cplett.2005.12.007
Kawski A, Kukliński B, Bojarski P (2006) Excited S1 state dipole moments of nitrobenzene and p-nitroaniline from thermochromic effect on electronic absorption spectra. Chem Phys 330:307–312. https://doi.org/10.1016/j.chemphys.2006.09.002
Kawski A, Kukliński B, Bojarski P (2006) Electronically excited dipole moment of 4-aminobenzonitrile from thermochromic absorption and fluorescence measurements. Chem Phys Lett 425:257–261. https://doi.org/10.1016/j.cplett.2006.05.043
Kawski A, Kukliński B, Bojarski P (2006) Excited state dipole moments of N, N-dimethylaniline from thermochromic effect on electronic absorption and fluorescence spectra. Chem Phys 320:188–192. https://doi.org/10.1016/j.chemphys.2005.07.007
Kawski A, Kukliński B, Bojarski P (2009) Photophysical properties and thermochromic shifts of electronic spectra of Nile Red in selected solvents. Excited states dipole moments Chem Phys 359:58–64. https://doi.org/10.1016/j.chemphys.2009.03.006
Renuka CG (2005) Spectroscopic and picosecond time resolved studies of structurally similar coumarin organic laser dyes, Doctoral thesis, Karnatak University Dharwad, India. https://hdl.handle.net/10603/96528
Edward JT (1970) Molecular volumes and the Stokes-Einstein equation. J Chem Educ 47:261. https://doi.org/10.1021/ed047p261
Raikar US, Tangod VB, Renuka CG, Mastiholi BM (2010) Dynamical behavior of coumarin compounds in alcohol solvents. Afr J Pure Appl Chem 4(2010):51–57. https://doi.org/10.5897/AJPAC.9000058
Baze Y, Tóth J, Fizer M, Sidey V (2021) Balogh I (2021) Estimation of Ground and Excited-State Dipole Moments of Three Symmetric Carbocyanine Dyes via the Analysis of Luminescence Properties. J Mol Liq 337:116476. https://doi.org/10.1016/J.MOLLIQ.2021.116476
Funding
None.
Author information
Authors and Affiliations
Contributions
A.K. Prepared - Conceptulization, Methodology, Data Analysis, Figures, Tables A.K. Prepared - Original draft, C.G.R- Reviewing, Supervision
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A., Renuka, C.G. Temperature Effect on Fluorescence Intensity and Dipole Moment Using Thermochromic Shift Method of 7DA3MHBI-2HChromen-2-one Laser Dye in Highly Viscous Glycerol Solvent. J Fluoresc (2024). https://doi.org/10.1007/s10895-023-03569-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03569-6