Abstract
This research investigates the excimerisation of acriflavine dye in ethylene glycol and glycerol solvents. Acriflavine, a member of the acridine dye family, exhibits unique fluorescence properties with applications in various fields, including cellular nucleus observation, nucleic acid analysis, and dye laser active media etc. The study explores the impact of solvent and concentration on acriflavine's emission properties, with a focus on excimer formation, which can influence its suitability as a dye laser active medium. UV-Visible absorption spectroscopy reveals concentration-dependent absorption profiles, with distinctive monomer bands. Steady-state fluorescence studies demonstrate the emergence of red-shifted excimer fluorescence bands as concentrations increase in both solvents. Temperature-dependent fluorescence studies reveal the dynamics of excimer formation, suggesting dynamic diffusion as the excimerisation mechanism. Time-resolved fluorescence spectroscopy confirms the singlet character of both monomer and excimer states, providing insights into the excimerisation process. Critical concentration values are determined, representing the equilibrium between monomeric and excimeric forms. The study also explores pH-induced spectral shifts, highlighting the influence of acidity on fluorescence properties. Overall, this research deepens our understanding of acriflavine's excimerisation in ethylene glycol and glycerol, offering insights that can enhance its diverse applications, especially in laser technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The photophysical characteristics of dye molecules in different solvent environments and their interactions with solvents are critical considerations for potential applications [1]. Fluorescence spectroscopy has historically served as a powerful tool for investigating and interpreting the photophysical features resulting from dye-solvent interactions into practical applications. Among fluorescent dyes, acriflavine, a member of the acridine family, has garnered significant global interest due to its unique fluorescence properties. It finds applications in diverse fields such as fluorescence-based cellular nucleus observation [2], analysis of nucleic acid structures [3], functioning as an active medium in laser dyes [4], and catalyzing various intermolecular electron transfer processes [5]. Acriflavine's high-emission yield qualifies it for use as a photosensitizer [6], a fluorescent probe, and a donor/acceptor agent in Förster resonance energy transfer (FRET) processes for efficient sensing [7,8,9].
Since 1966, when organic compound-based emissions for lasers were introduced [10], the search for effective luminescent organic compounds has become prominent. Among these, organic dyes stand out due to their robust luminescence and conjugated π-bond structures [11]. Their broad emission bands facilitate wavelength tuning within the emission range [12], which can be further influenced by varying dye concentration and solvent choice when used in laser applications [13]. Adjusting dye concentration in specific solvents can lead to dye molecule aggregation, forming dimers or excimers in the solvent, potentially affecting emission wavelengths [14]. Commonly used organic dyes for dye lasers include coumarin, xanthene, and pyrromethene [15]. Acriflavine, a high quantum yield acridine dye, has also been explored as a dye laser active medium [2]. Effective laser emissions from organic dyes rely on their dissolution in appropriate solvents, such as water, alcohol, ethylene glycol, and glycerol [16] etc. Thus, investigating acriflavine dye's emission properties in different solvents by varying concentration and solvent composition is crucial for optimizing its efficiency as a laser dye medium.
Our prior work has explored into the fluorescence-based photophysical properties of acriflavine in water and alcohol, providing historical context and chronological development of acriflavine excimerisation [17]. In that work we elucidated acriflavine aggregation in water and alcohol in terms of critical concentration at monomer-excimer intensity equilibrium. It also elucidates the excimerisation process's origin (static or dynamic diffusion) through temperature-dependent steady-state and time-resolved fluorescence studies. These insights are vital for acriflavine excimerisation's optical, spectroscopic, and laser applications. Furthermore, our previous work revealed longer wavelength excimer emission bands and shorter wavelength monomer bands with varying acriflavine dye concentrations in different solvents, offering innovative dye laser tuning possibilities. Expanding beyond water and alcohol, commonly used solvents like glycerol and ethylene glycol play a crucial role in dye dissolution for laser gain media. Thus, investigating acriflavine fluorescence properties in these solvents is vital for understanding its lasing efficiency.
Continuing from our prior research [17], in this work, we present a comprehensive investigation into the excimerisation of acriflavine dye within the media of ethylene glycol and glycerol. We conducted spectroscopic analyses using UV-visible and steady-state and time-resolved fluorescence spectroscopy on pure acriflavine in these solvents. Our work deepens the understanding of photoexcimerisation through temperature-dependent steady-state and time-resolved fluorescence studies. This investigation offers fundamental insights into acriflavine excimerisation in glycerol and ethylene glycol, potentially enhancing its diverse applications.
Materials and Methods
Materials
The materials employed in this study encompass high-purity acriflavine along with the solvents: glycerol, ethylene glycol procured from Sigma-Aldrich.
Sample Preparation
The sample solutions were meticulously prepared using acriflavine dye at varying concentrations, with glycerol and ethylene glycol serving as the respective solvents. The preparation procedure entailed the following steps:
-
(a)
A total of eleven distinct concentrations (3.85 \(\times\) 10-6 mol/l, 9.62 \(\times\) 10-6 mol/l, 1.92 \(\times\) 10-5 mol/l, 3.85 \(\times\) 10-5 mol/l, 9.62 \(\times\) 10-5 mol/l, 0.0003 mol/l, 0.0011 mol/l, 0.0013 mol/l, 0.0015 mol/l, 0.0017 mol/l, 0.0019 mol/l) of acriflavine solutions were prepared in glycerol.
-
(b)
Simultaneously, eleven diverse concentrations (3.85×10-6 mol/l, 9.62×10-6 mol/l, 1.92×10-5 mol/l, 3.85×10-5 mol/l, 6.73×10-5 mol/l, 0.0003 mol/l, 0.0007 mol/l, 0.0011 mol/l, 0.0015 mol/l, 0.0019 mol/l, 0.0026 mol/l) of acriflavine solutions were prepared in ethylene glycol.
Experimental Procedures
UV-Visible Absorption Spectroscopy
The UV-Visible absorption characteristics of acriflavine in glycerol and ethylene glycol were systematically investigated using a UV-Visible spectrophotometer (Shimadzu, model: UV-1900i) operating within a scanning range spanning from 200 nm to 1100 nm. To distinguish the absorption profiles, each specified solvent was subjected to incremental concentrations of acriflavine, commencing from the lowest concentration (as outlined in Section Sample Preparation). Spectral acquisitions were pursued until a point of saturation was evident in the respective spectrum for each solvent. All spectra were meticulously acquired under controlled room temperature conditions.
Steady State Fluorescence Spectroscopy
Steady state fluorescence spectra of acriflavine within diverse solvents were acquired using two distinct methodologies: firstly, by varying concentrations while maintaining a constant temperature (room temperature); and secondly, by altering the temperature while maintaining a fixed concentration.
Fluorescence spectra were captured utilizing a spectrometer equipped with a 405 nm excitation source. The collimated diode laser system responsible for the 405 nm excitation, sourced from Laser Glow Technologies, delivered an average power of 118.5 mW. The employed spectrometer is a concave grating spectrometer boasting a resolution of 0.9 nm and a wavelength range spanning from 320 nm to 900 nm. It is outfitted with a Toshiba linear CCD array detector, and data acquisition and display are facilitated through the utilization of spectra analyte V2.26 software. To mitigate potential inner filter effects, an optically thin sample cell (quartz cuvette with a path length of 5 mm) was employed in the experimentation. The solution within this quartz cuvette underwent excitation by the laser beam, with the resulting emitted light collected at an angle of 90° relative to the incident beam. This collection process was facilitated by an adjustable slit affixed to the spectrometer. To eliminate the impact of reflected light on the collected spectra, we carefully designed the experimental setup. In particular, we arranged the system to ensure that the incident laser beam consistently maintained a 30-degree angle relative to the cuvette plane holding the sample. The experimental setup was securely fixed during entire experiments.
The steady-state fluorescence spectra of acriflavine were acquired across a range of temperatures while maintaining fixed concentrations. Specifically, concentrations of 0.0015 mol/l, 0.0019 mol/l were employed for the glycerol and ethylene glycol, respectively. These acriflavine solutions were subjected to controlled heating, raising their temperatures up to 130 °C for glycerol and 110 °C for ethylene glycol. Subsequently, to achieve a target temperature of -20 °C, the identical solutions underwent a controlled cooling process within a freezer environment characterized by a temperature range spanning from 8 °C to -25 °C.
Time Resolved Fluorescence Spectroscopy
The time-resolved fluorescence spectra were acquired using a fluorescence lifetime spectrometer, namely the LifeSpect II (Edinburgh), which is housed within the Central Instrument Facility (CIF) at the Indian Institute of Technology, Guwahati. The excitation source employed was a 405 nm pulsed diode laser (Edinburgh Photonics EPL) operating at a repetition rate of 10 MHz, and it delivered pulses with a duration of 90 ps. The emission slit possessed a width of 20 nm. As a detector, a microchannel plate-photomultiplier tube was utilized, characterized by a response width of less than 25 ps and an Instrument Response Function (IRF) of less than 130 ps. Post re-convolution, the retrieved shortest lifetime is approximately one-tenth of the instrument response function.
Fixed temperature experiments were conducted under ambient conditions.
Result and Discussion
UV-Visible Absorption Study
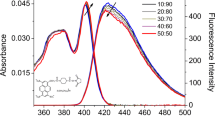
To assess the feasibility of forming diverse types of aggregates across a range of concentrations spanning from 3.85×10-6 mol/l to 9.62×10-5 mol/l in glycerol and 3.85×10-6 mol/l to 6.73×10-5 mol/l in ethylene glycol, UV-Visible absorption spectra were recorded for glycerol and ethylene glycol, as shown in Fig. 1a and b respectively. Notably, beyond the concentrations of 9.62×10-5 mol/l in glycerol and 6.73×10-5 mol/l in ethylene glycol, the absorption spectra exhibited saturation. Fig. 1a and b illustrate that acriflavine consistently exhibits two prominent bands at 464 nm and 260 nm, along with two weaker shoulders at 283 nm and 232 nm, across all concentrations in both solvents.
UV-Visible absorption spectra of pure acriflavine solution in a glycerol and b ethylene glycol. Acriflavine exhibits two intense bands at 464 nm and 260 nm, as well as two weak shoulders at 283 nm and 232 nm for all concentrations in both solvents. The Lambert-Beer plots for the intensity of the 464 nm bands are presented here alongside their respective absorption spectra
It is important to note that acriflavine dye consists of two components: 3,6-diamino-10-methylacridinium chloride hydrochloride (Euflavine) and 3,6-diaminoacridine (Proflavine). Fig. 2 shows the structures of Euflavine and Proflavine. The absorption peaks at 464 nm, 260 nm, and 283 nm correspond to the monomeric absorption band of proflavine [18, 19], while the shoulder at 232 nm is attributed to the absorption band of euflavine [20]. The ratio of these two components in the compound varies from 3:1 to 7:1 depending on the manufacturer [21].
The intensity of the 464 nm band was plotted against the concentration of acriflavine solutions and is presented in Fig. 1a and 1b, alongside their respective absorption spectra. These plots reveal a clear distinction in the relationship between absorbance and concentration for glycerol (Fig. 1a; R2 = 0.98186) and ethylene glycol (Fig. 1b; R2 = 0.99395). The data for ethylene glycol shows a linear relationship, confirming its adherence to the Lambert-Beer Law. In contrast, a slight deviation from linearity is observed in the case of glycerol, indicating that it does not strictly adhere to the Lambert-Beer law as compared to the ethylene glycol [22]. In this study, an excitation wavelength of 405 nm was selected. As a result, only the 464 nm band contributes to the emission spectra of acriflavine, making it suitable for the Lambert-Beer plot. These findings align with results of previous research [2, 17, 23]. Furthermore, an increase in concentration does not give rise to any new blue- or red-shifted absorption bands in the spectra presented in Fig. 1a and b. Since changes in concentration do not alter the absorbed spectral distribution, it suggests the absence of primary stable aggregates or dimers in the ground state [17, 24, 25].
Steady State Fluorescence Study
Variable Concentration and Constant Temperature
The steady-state fluorescence spectra of acriflavine were examined at room temperature across various concentrations in the solvent glycerol and ethylene glycol, as illustrated in Figs. 3 and 4 respectively. In Fig. 3, the spectra are denoted as G1-G6, corresponding to acriflavine concentrations of 0.0003 mol/l, 0.0011 mol/l, 0.0013 mol/l, 0.0015 mol/l, 0.0017 mol/l, and 0.0019 mol/l in glycerol, as outlined in Table 1. Similarly, Fig. 4 shows spectra denoted as EG1-EG6, associated with acriflavine concentrations of 0.0003 mol/l, 0.0007 mol/l, 0.0011 mol/l, 0.0015 mol/l, 0.0019 mol/l, and 0.0026 mol/l in ethylene glycol, as indicated in Table 2.
In both solvents, only one peak is observed around 500 nm for the 0.0003 mol/l concentration (represented by spectra G1 and EG1 in Figs. 3 and 4 respectively). For concentrations of acriflavine lower than 0.0003 mol/l in both solvents (as detailed in "Section Preparation of Sample"), the spectra exhibit consistent characteristics with a single peak around 500 nm. Consequently, these spectra are not included in Figs. 3 and 4. This particular 500 nm peak is identified as the monomer fluorescence band, a characteristic observed by other researchers previously [23, 26, 27] as well as in our own work [17].
Figures 3 and 4 reveal the emergence of an additional red-shifted emission band between 560 nm and 581 nm for acriflavine solutions with concentrations exceeding 0.0003 mol/l in all solvents. This red-shifted band was similarly observed in water, propanol, methanol, ethanol, and butanol, spanning the wavelength range of 549 nm to 587 nm, and was previously established as the excimer fluorescence band in our earlier study [17]. Therefore, we attribute the red-shifted band in glycerol and ethylene glycol, appearing between 560 nm and 581 nm, to the excimer fluorescence band.
Tables 1 and 2 present the peak wavelengths and intensities of these two fluorescence bands for glycerol and ethylene glycol respectively. Notably, as depicted in Figs. 3 and 4 and confirmed by data in Tables 1 and 2, the intensity of the monomer peak at around 500 nm decreases, exhibiting a red-shift within the range of 503 to 536 nm. Concurrently, the intensity of the excimer fluorescence band shows a consistent increase across all solvents as the concentration increases. This trend remains consistent without self-absorption effects until reaching optimal concentrations of 0.0019 mol/l for glycerol and 0.0026 mol/l for ethylene glycol. Beyond these concentrations, both monomer and red-shifted band intensities decline uniformly. We propose that this reduction is due to the onset of the "secondary inner-filter effect" [28], resulting from fluorescence reabsorption. Consequently, data acquisition for fluorescence spectra was limited to the 0.0019 mol/l and 0.0026 mol/l optimal concentrations, for glycerol and ethylene glycol, respectively.
Fixed Concentration and Varying Temperature
To ascertain the origin of the red-shifted spectral band spanning the wavelength range from 560 nm to 581 nm, a comprehensive investigation was conducted using steady-state fluorescence experiments. The experimental protocol outlined in the "Experimental Procedures" was followed, maintaining a fixed concentration of acriflavine within the solvents while systematically varying the temperature. Notably, previous works by Stevens [29] and Oladepo [30] suggested that alterations in the system's temperature could modulate the equilibrium between the monomer and excimer species of fluorophores. Elevating the temperature results in heightened molecular collisions, inducing the excimer molecule to transition into a vibrationally excited state within its first excited state. In this vibrational state, the excimer molecule accumulates ample energy to effectuate a transition to a singlet mode of the excited monomeric state, subsequently leading to excimer dissociation into an excited monomer molecule [31]. Thus, a temperature increase significantly favors the disintegration of the excimer into monomeric constituents [17].
Figure 5a and b illustrate the variations in the intensity of the excimeric and monomeric fluorescence peaks at various temperatures for acriflavine concentrations of 0.0015 mol/l in glycerol and 0.0019 mol/l in ethylene glycol, respectively. At these concentrations, the monomer and excimer fluorescence peaks appear at 521 nm and 574 nm for glycerol, and at 526 nm and 575 nm for ethylene glycol. The temperature-dependent variations in the intensity ratio between the monomeric fluorescence peaks (IM) at the highest temperature and the excimeric fluorescence peaks (IE) at different temperatures are presented in Table 3 for glycerol and Table 4 for ethylene glycol. Evidently, as portrayed in Fig. 5a and b, increasing the temperature within both solvent environments corresponds to an upsurge in monomeric fluorescence intensity, associated with a diminishing intensity of the red-shifted peak. Consequently, this alteration in intensity ratios highlights the evolving equilibrium dynamics between monomeric and excimeric states. This is pivotal as, were the variations in peak intensities due to impurities or the presence of an alternative vibronic component within the monomeric state, the intensity ratio would remain unchanged.
Steady-state fluorescence spectra of pure acriflavine solutions: a 0.0015 mol/l in glycerol, temperature range: -20 °C to 130 °C, b 0.0019 mol/l in ethylene glycol, temperature range: -20 °C to 110 °C. Monomer and excimer peaks observed at 521 nm and 574 nm for glycerol, and 526 nm and 575 nm for ethylene glycol respectively. In both the solvents, excimer peak intensity decreases while the monomer peak intensity increases with rising temperature
These findings underscore a significant transformation in the equilibrium existing between the monomeric band around the spectral range of approximately 503 nm to 536 nm and the red-shifted band spanning 560 nm to 581 nm, resulting from temperature perturbation. Thus, the compelling deduction can be drawn that the red-shifted band's emergence is intrinsically tied to the formation of the acriflavine molecule's excimeric species. Consequently, the distinctive red-shifted band observable within the acriflavine solution in both solvents can be aptly designated as the excimeric fluorescence/phosphorescence band. The definitive classification as fluorescence or phosphorescence is subsequently established through a meticulous examination of time-resolved fluorescence, as detailed in the ensuing section.
Time Resolved Fluorescence Spectroscopy Study
The relaxation process of the excited states was investigated, and the resulting monomer and excimer peaks, observed in steady-state fluorescence spectroscopy, were analyzed using time-resolved fluorescence spectra. For this study, acriflavine solutions with concentrations of 0.0015 mol/l in glycerol and 0.0019 mol/l in ethylene glycol were employed, consistent with the acriflavine concentration used in the steady-state fluorescence analysis at varying temperatures.
Figures 6 and 7 depict the fluorescence time profiles of the monomer and excimer emissions for glycerol and ethylene glycol, respectively. The quality of the model was assessed by examining residuals versus time and the Instrument Response Function (IRF) profiles of the time-resolved fluorescence spectra, as presented in these figures. Each figure comprises four components: the upper left image displays the fluorescence time profile along with the fitting curve, the bottom left image depicts the fitting residuals, the right image exhibits the calculated fluorescence lifetimes, and numerical values at the bottom provide the magnitude of the fitting parameters.
Fluorescence time profile of a 0.0015 mol/l concentration of acriflavine solution in glycerol. The excitation source used was a diode laser with a 405 nm wavelength and a 90 ps (picosecond) pulse duration, a Monomer fluorescence peak at a wavelength of 521 nm. b Excimer fluorescence peak at a wavelength of 574 nm. The numerical values annotated at the base of the images signify the magnitude of fitting parameters, where \({\chi }^{2}\) denotes the goodness of fit, \(B\) represents the amplitude of the lifetime component, \(f\) corresponds to the fractional contribution or amplitude of decay components, and Δ indicates the standard deviation associated with each parameter. The term 'IRF' denotes the Instrument Response Function, which is less than 130 ps
Fluorescence time profile of a 0.0019 mol/l concentration of acriflavine solution in ethylene glycol. The excitation source used was a diode laser with a 405 nm wavelength and a 90 ps (picosecond) pulse duration, a Monomer fluorescence peak at a wavelength of 526 nm. b Excimer fluorescence peak at a wavelength of 575 nm. The numerical values annotated at the base of the images signify the magnitude of fitting parameters, where \({\chi }^{2}\) denotes the goodness of fit, \(B\) represents the amplitude of the lifetime component, \(f\) corresponds to the fractional contribution or amplitude of decay components, and Δ indicates the standard deviation associated with each parameter. The term 'IRF' denotes the Instrument Response Function, which is less than 130 ps
In Fig. 6, it is evident that for glycerol solutions, both monomer and excimer fluorescence time profiles are well-described by a double-exponential function. Specifically, in Fig. 6a, the monomer lifetimes are \({\tau }_{1}=3.737 \;ns, {\tau }_{2}=4.800 \;ns\), while in Fig. 6b, the excimer lifetimes are \({\tau }_{1}=6.966 \;ns, {\tau }_{2}=7.922 \;ns\). Similarly, in Fig. 7, it is observed that for ethylene glycol solutions, both monomer and excimer fluorescence time profiles conform to a double-exponential function. In Fig. 7a, the monomer lifetimes are \({\tau }_{1}=1.147 \;ns, {\tau }_{2}=4.876 \;ns\), while in Fig. 7b, the excimer lifetimes are \({\tau }_{1}=5.913 \;ns, {\tau }_{2}=6.847 \;ns\).
In fluorescence time profiles fitted with a double exponential function, two distinct time components are observed, as depicted in Fig. 6 a, b, and 7 a, b. The first component, termed as the rise time or excitation time (\({\tau }_{1}\)), signifies the duration of excitation. The second component, termed as the decay time (\({\tau }_{2}\)), represents the subsequent relaxation process. In our experiment, both the rise and decay times were found to be of the order of nanoseconds duration in both solvents. Notably, compared to the Instrument Response Function (IRF), the rise time (\({\tau }_{1}\)) in both solvents appears significantly prolonged. This observation suggests that the excimer formation occurs slowly, likely through a dynamic diffusion process between a photoexcited and a ground-state monomer, as previously suggested [17, 32]. In a prior study, we also predicted excimer formation of acriflavine dye via dynamic diffusion in ethanol and butanol [17].
The average lifetimes for the monomer peak are found to be 4.80 ns and 4.93 ns, while for the excimer peak, they are 6.35 ns and 5.49 ns in glycerol and ethylene glycol, respectively (Fig. 8). Consequently, there are no significant differences in the decay times between the monomer and excimer peaks. Given that these decay times are in the nanosecond range, the excited states correspond to singlet states for both the monomer and the excimer. The energy level structure and corresponding transitions for monomer and excimer fluorescence are likely to be consistent with our previous work [17].
Normalized fluorescence decay curves excited by a diode laser with a 405 nm wavelength and a 90 ps (picosecond) pulse duration for acriflavine solutions at concentrations of 0.0015 mol/l in glycerol and 0.0017 mol/l in ethylene glycol. In glycerol: a Monomer fluorescence decay curve at 521 nm with an average lifetime of 4.80 ns, and b Excimer fluorescence decay curve at 574 nm with a lifetime of 6.35 ns. In ethylene glycol: a Monomer fluorescence decay curve at 526 nm with an average lifetime of 4.93 ns, and b Excimer fluorescence decay curve at 575 nm with a lifetime of 5.49 ns. The Instrument Response Function (IRF) for the experiment was less than 130 ps
Equilibrium of Acriflavine Excimerisation in Pure Solution
After confirming the excimerisation and fluorescence emissions of pure acriflavine solutions in both glycerol and ethylene glycol at ambient conditions, it is essential to establish an equilibrium state between the monomer and the excimer. To facilitate this, we introduce a novel term, referred to as the "critical concentration," which serves as a quantitative measure of the equilibrium between the fluorophore's monomeric and aggregated forms within the solvents [17, 33]. The determination of the critical concentration for acriflavine's excimerisation involves evaluating the intensity ratio of excimer to monomer fluorescence peaks (denoted as \(\frac{{I}_{E}}{{I}_{M}}\), where, where \({I}_{E}\) represents excimer fluorescence peak intensity, and \({I}_{M}\) stands for monomer fluorescence peak intensity, as outlined in Tables 1 and 2). This intensity ratio is plotted against the concentration of acriflavine in both glycerol and ethylene glycol, as depicted in Fig. 9a and b, respectively. The concentration at which this intensity ratio becomes unity is defined as the critical concentration, which has been calculated to be 0.0015 mol/l for glycerol and 0.0017 mol/l for ethylene glycol, from the plots shown in Fig. 9a and b. At these critical concentrations, the monomer and excimer forms of acriflavine exist in equilibrium, with their fluorescence intensities being equal [33]. Importantly, it is noteworthy that the critical concentration is lower for glycerol compared to ethylene glycol. This discrepancy in critical concentrations is indicative of varying aggregation strengths [33], thereby implying that excimerisation of acriflavine is more favored in glycerol than in ethylene glycol. This observation finds further support in the Lambert-Beer plot for glycerol (as shown in Fig. 1a), where a minor deviation from perfect linearity is evident due to the aggregation or interaction of acriflavine molecules occurring at lower concentrations compared to those in ethylene glycol. It is also evident from Figs. 3 and 4 that, as the concentration of acriflavine increases in both solvents, the intensity of the monomer peak decreases while the peak wavelength shifts towards the red end of the spectrum. Conversely, the intensity of the excimer peak increases with increasing acriflavine concentration in the solutions, accompanied by a red-shift in its peak wavelength. The ensuing section will provide a detailed elucidation and further exploration of these variations.
Plot of excimer to monomer fluorescence peak intensity ratio (\(\frac{{I}_{E}}{{I}_{M}})\) against different concentration of acriflavine in glycerol and ethylene glycol. a In glycerol, the relationship follows a linear equation: \(y=-0.0467+698.75 x\), with \({R}^{2}=0.96352\) where \(x\) represents the concentration of acriflavine in glycerol, and \(y\) represents the excimer to monomer fluorescence peak intensity ratio. The critical concentration is determined to be 0.0015 mol/l for glycerol. b In ethylene glycol, the plot also follows a linear equation: \(y=0.3879+350.93 x\), with \({R}^{2}=0.99038\) where \(x\) represents the concentration of acriflavine in ethylene glycol, and \(y\) represents the excimer to monomer fluorescence peak intensity ratio. The critical concentration is found to be 0.0017 mol/l for ethylene glycol. (Critical concentration: the concentration at which the excimer to monomer fluorescence peak intensity ratio (\(\frac{{I}_{E}}{{I}_{M}})\) becomes unity, \({I}_{E}=\) excimer fluorescence peak intensity and \({I}_{M}=\) monomer fluorescence peak intensity)
pH-Induced Spectral Changes in Acriflavine Fluorescence Emission
With the increase of acriflavine concentration in the respective solvent environments, notable red-shifts are observed in both the monomer fluorescence peak (approximately 33 nm) and the excimer fluorescence peak (around 23 nm). This phenomenon aligns with recent findings illustrating spectral red-shifts in the emission spectra of other fluorophores, such as pyrene monomer, rhodamine B dimer, and acriflavine excimer, attributed to variations in pH [17, 34, 35].
To elucidate the influence of pH on the spectral properties of acriflavine in glycerol and ethylene glycol, we conducted fluorescence emission experiments using acriflavine solutions with a concentration of 0.0003 mol/l (denoted as G1 and EG1 for glycerol and ethylene glycol, respectively) in two distinct pH conditions. At this concentration in both solvents, only the monomer fluorescence is present. Subsequently, we extended our investigation to concentrations of 0.0017 mol/l (G5) and 0.0026 mol/l (EG6) of acriflavine in glycerol and ethylene glycol, respectively, again under two pH conditions. At these higher concentrations, both monomer and excimer fluorescence contributions are evident. The pH of these solutions was altered by the addition of a minute quantity of Hydrochloric Acid (HCl).
Our observations in Fig. 10 reveal significant red-shifts, up to 14 nm in monomer fluorescence and 11 nm in excimer fluorescence, due to changes in pH. As the concentration of the experimental samples increases, the solutions tend toward an acidic nature, leading to an increased accumulation of hydrogen ions. This elevated hydrogen ion concentration serves to stabilize the excited monomer and excimer molecules within the vibrational level of their corresponding excited states [17, 35]. Consequently, transitions from lower vibrational levels of the upper excited state become increasingly probable, leading to the observed red-shifts in both monomer and excimer fluorescence peaks with increasing concentration.
Steady-state fluorescence spectra of acriflavine solutions in glycerol (a1 and a2) and ethylene glycol (b1 and b2). In glycerol, a1 and a2 displays the monomer and monomer + excimer fluorescence respectively, at concentrations of 0.0003 mol/l (G1) and 0.0017 mol/l (G5) (see Table 1). In ethylene glycol, b1 and b2 displays the monomer and monomer + excimer fluorescence respectively, at concentrations of 0.0003 mol/l (EG1) and 0.0026 mol/l (EG6) (see Table 2). A spectral red-shift is evident in both monomer and excimer peaks in all images, resulting from pH adjustment through the addition of a small amount of hydrochloric acid (HCl) to the solutions
In addition to pH-induced shifts, we also consider the possibility of ground state aggregates (dimers) contributing to the red-shift of the excimer peak, particularly at higher concentrations. However, the verification of ground state aggregates relies on the detection of blue-shifted absorption peaks at elevated concentrations [17]. In our absorption spectroscopy measurements (Fig. 1), we captured spectra up to the highest concentrations tested, namely 9.62×10-5 mol/l in glycerol and 6.73×10-5 mol/l in ethylene glycol. Beyond these concentrations, if dimers were to form, their excited state energy would deviate significantly from that of the excimers, resulting in distinct fluorescence wavelengths. Consequently, at higher concentrations, the emergence of additional fluorescence peaks at higher wavelengths would be necessary to confirm the existence of ground state aggregates (dimers). Furthermore, if the fluorescence peak wavelengths of the excimer and the dimer exhibit minimal differences, the latter may also contribute to the observed red-shift in the excimer fluorescence peak [17].
Conclusion
In this comprehensive investigation, we explored the excimerisation of acriflavine dye in the solvents: ethylene glycol and glycerol. Our research aimed to shed light on the impact of solvent choice and concentration on acriflavine's emission properties, with a particular focus on excimer formation, which holds significance for its utilization as a dye laser active medium. Our findings reveal a concentration-dependent absorption profile characterized by distinct monomer bands. Steady-state fluorescence studies clearly demonstrate the emergence of red-shifted excimer fluorescence bands as acriflavine concentrations increase in both glycerol and ethylene glycol. Through temperature-dependent fluorescence studies, we uncovered the dynamics of excimer formation, suggesting dynamic diffusion as the primary mechanism driving excimerisation. Time-resolved fluorescence spectroscopy provided valuable insights, confirming that both monomer and excimer states exhibit singlet character, thereby clarifying the excimerisation process. We also determined critical concentration values, which mark the equilibrium between monomeric and excimeric forms. Additionally, we explored pH-induced spectral shifts, highlighting the substantial influence of acidity on acriflavine's fluorescence properties. In conclusion, this research significantly advances our understanding of acriflavine's excimerisation behavior in glycerol and ethylene glycol solvents. By leveraging acriflavine excimer emission, this work demonstrates the potential to enhance the fine-tuning of dye laser wavelengths within a range from approximately 500 nm to 580 nm. These insights have the potential to enhance its diverse applications, particularly in laser technologies. The knowledge gained from this study contributes to the broader field of photophysics and opens up avenues for optimizing acriflavine's utilization in various scientific and technological applications.
Data Availability
The data generated and analysed during the present study are available from the corresponding author upon reasonable request.
Reference
Sinha TB, Singharoy D, Das HS, Gupta S, Khatua PK (2019) Photophysical studies of the dye 1-Anilinonapthalene-8-sulfonic acid in different solvents and its quantum chemical investigation. J. Mol. Struct. 1179:462–468. https://doi.org/10.1016/j.molstruc.2018.11.012
Sharma VK, Sahare PD, Rastogi RC, Ghoshal SK, Mohan D (2003) Excited state characteristics of acridine dyes: Acriflavine and acridine orange. Spectrochim Acta - Part A Mol Biomol Spectrosc 59:1799–1804. https://doi.org/10.1016/S1386-1425(02)00440-7
Lo̊ber G, (1981) The fluorescence of dye-nucleic acid complexes. J Lumin 22:221–265. https://doi.org/10.1016/0022-2313(81)90022-3
Maeda M (1984) Laser Dyes. Academic press, New York
Previtali CM (1980) CHAPTER VI Transfer Processes. In: International Geophysics. pp 261–320. https://doi.org/10.1016/S0074-6142(08)60507-0
Li Y, Kuwabara H, Gong YK et al (2003) Resonance energy transfer from dibucaine to acriflavine in polystyrene latex dispersions. J Photochem Photobiol B Biol 70:171–176. https://doi.org/10.1016/S1011-1344(03)00090-3
Chakraborty S, Hussain SA (2019) Fluorescence resonance energy transfer (FRET) between acriflavine and CdTe quantum dot. Mater Today Proc 46:6087–6090. https://doi.org/10.1016/j.matpr.2020.02.757
Misra V, Mishra H, Joshi HC, Pant TC (2000) Excitation energy transfer between acriflavine and rhodamine 6G as a pH sensor. Sensors Actuators, B Chem 63:18–23. https://doi.org/10.1016/S0925-4005(00)00296-3
Sahare PD, Sharma VK, Mohan D, Rupasov AA (2008) Energy transfer studies in binary dye solution mixtures: Acriflavine + Rhodamine 6G and Acriflavine + Rhodamine B. Spectrochim Acta - Part A Mol Biomol Spectrosc 69:1257–1264. https://doi.org/10.1016/j.saa.2007.07.003
Sorokin PP, Lankard JR (1966) Stimulated emission observed from an organic dye. IBM J Res Dev 10:162–163. https://doi.org/10.1147/rd.102.0162
Yamaguchi Y, Matsubara Y, Ochi T, Wakamiya T, Yoshida ZI (2008) How the π conjugation length affects the fluorescence emission efficiency. J Am Che Soc 130:13867–13869. https://doi.org/10.1021/ja8040493
Denisov LK, Uzhinov BM (1980) Heterocyclic compounds as the active media of lasers (review). Chem Heterocycl Compd 16:553–564. https://doi.org/10.1007/BF02400891
Latz HW (1976) Dye Lasers. In: Wehry EL (ed) Modern Fluorescence Spectroscopy. Modern Analytical Chemistry. Springer, Boston, pp 83-119
Ghanadzadeh A, Zeini A, Kashef A, Moghadam M (2009) Solvent polarizability and anisotropy effects on the photophysical behavior of oxazine 1: An appropriate polarizability indicator dye. Spectrochim Acta A Mol Biomol Spectrosc 73:324–329. https://doi.org/10.1016/j.saa.2009.02.029
Drexhage KH (1973) Structure and Properties of Laser Dyes. In: Schäfer FP (eds) Dye Lasers. Topics in Applied Physics, vol 1, Springer, Berlin, Heidelberg, pp 144–193
Zhang Y, Li D, Ou Y, Pu X, Sun Y (2019) The Study on Lasing Threshold Properties of Rhodamine B in Glycerol Aqueous Solution. IEEE Photonics J 11:1–9. https://doi.org/10.1109/JPHOT.2019.2907469
Swargiary H, Radiul SM, Kalita MPC, Hazarika S (2023) Photoexcimerisation of pure acriflavine dye in water and alcohol. J Photochem Photobiol A Chem. https://doi.org/10.1016/j.jphotochem.2023.114636
Levshin LV (1955) The effect of concentration on the optical properties of solutions of 3, 6- diaminoacridine. J Exper Theoret Phys USSR 28:201–212
Haugen GR, Melhuish WH (1964) Association and self-quenching of proflavine in water. Trans Faraday Soc 60:386–394. https://doi.org/10.1039/TF9646000386
3,6-Diamino-10-methylacridinium chloride hydrochloride (2022). https://pubchem.ncbi.nlm.nih.gov/compound/15558347#section=Spectral-Information. Accessed 12 Dec 2022
Matzke KH, Thiessen G (1976) The acridine dyes: Their purification, physicochemical, and cytochemical properties. Histochemistry 49:73–79. https://doi.org/10.1007/BF00490128
Serra F, Terentjev EM (2010) Nonlinear Absorption of Light in Materials with Long-lived Excited States. In: Evans T (ed) Nonlinear Dynamics, InTech, Europe, pp 1-30
Sato M, Azumi T, Azumi H (1967) Delayed thermal excimer fluorescence of acriflavine in a stretched PVA sheet. Bull Chem Soc Jpn 40:1031–1034
Birks JB, Dyson DJ, Munro IH (1963) Excimer fluorescence II. Lifetime Studies of pyrene solutions. Proc R Soc A: Math Phys Eng Sci 275:575–588
Stevens B, Hutton E (1960) Radiative life-time of the pyrene dimer and the possible role of excited dimers in energy transfer processes. Nature 186:1045–1046
Wen W, Hsu R (1962) Effect of dimerization upon the α-phosphorescence of acriflavine in glucose glass. J Phys Chem 66:1353–1354
Lim EC, Swenson GW (1962) Delayed fluorescence of acriflavine in rigid media. J Chem Phys 36:118–122
Fonin AV, Sulatskaya AI, Kuznetsova IM, Turoverov KK (2014) Fluorescence of dyes in solutions with high absorbance. Inner filter effect correction. PLoS One 9:e103878. https://doi.org/10.1371/journal.pone.0103878
Stevens B (1961) Evidence for the photo-association of aromatic hydrocarbons in fluid media. Nature 192:725–727
Oladepo SA (2021) Temperature-dependent fluorescence emission of 4-cyano-4′-pentylbiphenyl and 4-cyano-4′-hexylbiphenyl liquid crystals and their bulk phase transitions. J Mol Liq 323:114590
Horrocks DL (1970) Techniques for the study of excimers. In: Horrocks DL, Peng CT (eds) Proceedings of the International Conference on Organic Scintillators and Liquid Scintillation Counting, University of California, San Francisco, pp 75-90. https://doi.org/10.1016/B978-0-12-356250-0.50007-5
Picüarra S, Gomes PT, Martinho JMG (2000) Fluorescence study of the coil-globule transition of a poly(e-caprolactone) chain labeled with pyrenes at both ends. Macromolecules 33:3947–3950
Radiul SM, Chowdhury J, Goswami A, Hazarika S (2022) Fluorescence spectroscopy-based characterisation method for aggregation behaviour rhodamine B in water, ethanol, and propanol. Laser Phys 32 075602 (9pp)
Wang S, Zhang Z, Huang Z, Lei X, Wang Y, Li L, Yang L, Liu H, Sun F, Ma LJ (2021) A pyrene-based pH fluorescence probe with continuous multiple responses under acidic conditions and its application for environmental water systems and cells. J Photochem Photobiol A: Chem 418:113438. https://doi.org/10.1016/j.jphotochem.2021.113438
Radiul SM, Chowdhury J, Hazarika S (2023) Fluorescent H-aggregates of pure rhodamine B (RhB) in glycerol, ethylene glycol, methanol and butanol under ambient condition. J Mol Struct 1275:134606. https://doi.org/10.1016/j.molstruc.2022.134606
Acknowledgement
The authors thank the Science and Engineering Research Board, Department of Science and Technology, Government of India for the grant, No. CRG/2021/002942. Author SH wishes to thank FIST (DST) grant SR/FST/PSI-213/2016(C).
Funding
This work was supported by the Science and Engineering Research Board, Department of Science and Technology, Government of India (Project No. CRG/2021/002942).
Author information
Authors and Affiliations
Contributions
Simanta Hazarika: Conceptualisation, Methodology, Investigation, Supervision, Formal Analysis, Visualisation, Writing-original Draft. Hiren Swargiary: Investigation, Formal Analysis, Visualisation, Writing-original Draft. Seikh Mustafa Radiul: Investigation, Formal Analysis, Visualisation, Writing-original Draft.
Corresponding author
Ethics declarations
Ethics Approval
NA.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swargiary, H., Radiul, S.M. & Hazarika, S. Excimer Fluorescence of Acriflavine Dye in Glycerol and Ethylene Glycol. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03686-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03686-w