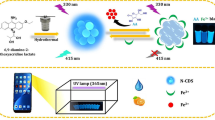
Abstract
In this study, a dual-mode ratiometric fluorometric and colorimetric platform for the determination of nitrite in pickles was proposed by exquisitely employing the fact that non-fluorescent o-Phenylenediamine (OPD) was oxidized by nitrite under acidic conditions to form fluorescent 2,3-diaminophenazine (DAP) (Em = 575), which meanwhile quench the fluorescent nitrogen-doped carbon dots (N-CDs) at 455 nm, the ratio of fluorescence intensity of DAP to N-CDs (F575/F455) changed with the increase of nitrite accompanied by visible color changes. Thus, nitrite can be quantitatively detected within a wide linear range (10–500 µM) with a low detection limit of 0.45 µM due to the high quantum yield of 39.7% of N-CDs. In addition, the colour of the N-CDs/OPD system changed from transparent to yellow when the nitrite was introduced, enabling colorimetric and on-site visual detection. The detection limit of the colorimetric method was 3.03 µM with a linear range of 10–500 µM. The proposed ratiometric fluorometric method has pleasant selectivity and good immunity to interference.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pickled vegetable is a popular food item. However, during the fermentation process of pickles, nitrite in the vegetables is rapidly increased due to bacterial contamination and the action of endogenous nitrate reductase enzymes that can convert the nitrate to nitrite [1]. Consumption of foods with high levels of nitrite can be harmful to human health owing to the fact that nitrite can reduce the oxygen-carrying capacity of haemoglobin in the body, leading to tissue hypoxia [2]. In addition, nitrites increase the risk of stomach and oesophageal cancers through the reaction with amino acids, proteins, phospholipids, and other amines in vivo to form carcinogenic nitrosamines [3]. For the past few years, several methods have been developed to detect nitrite in foods, such as the colorimetric method [4], fluorescence spectrometry [5], chromatography [6], electrochemical analysis [7], and capillary electrophoresis [8], etc. As a convenient, cost-effective, and highly sensitive analytical method, fluorescence spectrometry has a promising application in the detection of nitrite in foods. For example, Wang et al. [9], obtained a new fluorescent nanomaterial denoted as Rh 6G-SiO2 by grafting the intermediate product of the reaction between rhodamine 6G and hydrazine hydrate onto the surface of SiO2 nanoparticles. Nitrite detection was achieved utilizing the principle that the fluorescence of Rh 6G-SiO2 could be quenched by nitrite. Liu et al. [10], successfully prepared gold nanoclusters (Au NCs) using chloroauric acid as a precursor, achieving the selective and sensitive detection of nitrite based on direct fluorescence quenching. Hao et al. [11], exploited UiO-66-NH2 as a fluorescent probe for the detection of nitrite. Under acidic conditions, the fluorescence of the system was rapidly quenched due to the diazotization reaction of -NH2 with NO2−, which turned the fluorescent UiO-66-NH2 into non-fluorescent UiO-66, achieving the trace detection of nitrite. However, The above materials have their own drawbacks, such as being toxic [9], expensive [10] and unstable [11].
In comparison, carbon dots (CDs) with sizes less than 10 nm are a new type of fluorescent nanomaterial which are environmentally friendly, low-cost, well-soluble in water and have excellent fluorescence stability [12]. The excellent optical properties of CDs make them widely used for the detection of food additives, such as nitrite. The mechanisms of detection include direct quenching [13, 14] or enhancing [15] the fluorescence of CDs by nitrite proportionally. However, the interference immunity of this method is relatively inferior, resulting in reduced assay accuracy at trace levels [16]. It is common knowledge that the N atom in nitrite has an intermediate oxidation state of + 3 valence, so nitrites possess both reducing and oxidizing properties. In neutral and alkaline environments, nitrites mainly exhibit reducing properties while it is a strong oxidant in acidic environments [17]. Hence, the researchers constructed an indirect nitrite detection platform by using the oxidizing properties of nitrites, which is supposed to compensate for the shortcomings of the weak resistance to interferences. For example, Kong et al. [18], designed a fluorescent probe based on N-doped graphene quantum dots (g-CNQDs) and Fe (II)-bathophenanthroline complex (BPS-Fe2+) for the indirect detection of nitrite. The fluorescence of g-CNQDs was first quenched by BPS-Fe2+. After nitrite was added, BPS-Fe2+ was oxidized to BPS- Fe3+ complexes by nitrite, resulting in the recovery of fluorescence of g-CNQDs. Similarly, Liu et al. [19] also exploited the oxidative nature of nitrite under acidic conditions by oxidizing Fe2+ to Fe3+. Based on the quenching effect of Fe3+ on the fluorescence of CDs, they realized the accurate detection of nitrite.
OPD is a common low-cost fluorescent indicator. Interestingly, it is easily oxidized by various oxidants such as H2O2 and Cu2+ to form DAP with yellow fluorescence. Hence, a variety of sensors have been created based on this distinct fluorescent or colorimetric response [20,21,22]. Inspired by these reports, we are going to develop a novel ratiometric fluorescent and colorimetric detection platform based on N-CDs/OPD system for assaying nitrite. As shown in Scheme 1, N-CDs were prepared by a one-step hydrothermal method. Due to the doping of the N element, N-CDs possess a high quantum yield. In the detection process, non-fluorescent OPD was able to be oxidized by nitrite under acidic conditions to form fluorescent DAP, while the color of the solution changed from colorless to yellow. DAP (emission at 575 nm) can quench the fluorescence of N-CDs (emission at 455 nm). Based on the above principles, a ratiometric fluorescent and colorimetric dual-mode platform for nitrite detection was developed and applied to the sensitive and accurate detection of nitrite in pickles. Compare with the single-signal response detection method, the ratiometric fluorescent detection platform, which has the functions of self-calibrating and better immunity to interference due to its dual-signal response, provides a superior degree of accuracy. To our knowledge, a ratiometric fluorescent method based on N-CDs and OPD for the determination of nitrite has not been reported yet.
Experimental
Reagents
Citric acid, diethylenetriamine (DETA), o-phenylenediamine (OPD), sodium nitrite, sodium chloride, potassium ferrocyanide trihydrate, sodium tetraborate decahydrate, zinc acetate dihydrate were purchased from Aladdin Co., Ltd. (Shanghai, China). The pH range (2.0–12.0) of acetate buffer solution was prepared using CH3COONa and CH3COOH and adjusted by HCl or NaOH, which were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). Copper (II) sulfate pentahydrate, calcium chloride, potassium chloride, magnesium chloride hexahydrate, barium chloride dihydrate, cobalt(II) acetate tetrahydrate, lead(II) acetate trihydrate, Sodium bicarbonate and sodium carbonate were obtained from Sigma-Aldrich, China. Ultra-pure water was prepared by a Milli-Q purification system (Millipore, USA). All the above mentioned reagents were of analytical grade without further purification.
Instruments
The morphology of N-CDs was analyzed using a transmission electron microscope (TEM, JEOL 2010, Japan). X-ray photoelectron spectroscopy (XPS) of N-CDs was performed with a K-Alpha spectrometer (Thermo Fisher, USA). Fourier transform infrared (FTIR) spectrum of N-CDs was obtained on a Nicolet iS10 spectrometer (Thermo Fisher, USA). A UV-2600 spectrophotometer (Shimadzu, Japan) was applied to measure UV–Vis absorption spectra. All the fluorescence spectra were obtained on a Max iD3 multiscan Spectrum (Molecular Devices, USA).
Synthesis of N-CDs
Typically, 0.76 g of citric acid and 200 µL of DETA were added into 40 mL of deionized water and stirred for 3 min. Then, the mixed working solution was transferred into a Teflon-lined autoclave and heated to 180 °C and kept for 8 h. Next, the resulting N-CDs solution was centrifuged at 10,000 rpm for 20 min after naturally cooling down to ambient temperature. Finally, the N-CDs were collected and filtered through a 0.22 μm PES membrane and dialyzed using a 500 Da dialysis bag for 12 h.
Ratiometric Fluorometric and Colorimetric Detection of NO2 −
The detection of nitrite was carried out in an acetate buffer solution (0.1 M, pH = 3.5). Typically, 100 µL of N-CDs, 100 µL of 5 mM OPD and 700 µL of acetate buffer solution were added to a 1.5 mL centrifuge tube. Then 100 µL of nitrite with various concentrations were added. The mixtures were vortexed for 30 s and incubated for 15 min at ambient temperature. Next, 45 µL of sodium hydroxide (2 M) was added to adjust the solution to near neutral conditions. After that, the fluorescence spectra were recorded over the wavelength range of 390–800 nm with an excitation at 360 nm, and the UV-Vis spectra were collected in the range of 390 to 580 nm and the absorbances at 425 nm were recorded.
Analysis of the Pickled Vegetable Samples
The samples were preprocessed according to the literature and slightly modified [23]. Three pickles including kimchi, pickled sea cabbage and pickled cucumber were purchased from a local supermarket. Briefly, 10 g of pickle sample was made into a homogenate sample and mixed with 12.5 mL of saturated borax (50 g/L) and about 50 mL of distilled water at about 70 °C. The mixture was heated in boiling water for 15 min. After cooling to room temperature, 5 mL of potassium ferrocyanide (106 g/L) and 5 mL of zinc acetate (220 g/L) were added and finalized to 100 mL. The supernatant was filtered with filter paper after 30 min. The filtrate was stored at 4℃ for the next measure. The samples were tested using the ratiometric fluorometric and the colourimetric method proposed in this study and detected by the National Food Safety Standard Method (GB 5009.33–2016) for comparison.
Results and Discussion
Characterization of N-CDs
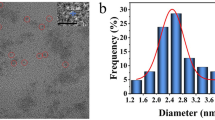
The morphology of N-CDs was analyzed by TEM. As revealed in Fig. 1A and B, the morphology of N-CDs are spherical with an average diameter of 3 nm. The high resolution transmission electron microscope (HRTEM) shows that the lattice spacing of N-CDs is around 0.2 nm, which is close to the (100) diffraction plane of sp2 graphitic carbon [12, 24]. Figure 1 C gives the FTIR of N-CDs. The strong absorption at 3434 cm− 1 is due to the stretching vibration of O-H, confirming the presence of the hydroxyl group. The weaker peaks at 3281 cm− 1 and 2920 cm− 1 correspond to the stretching vibrations of N-H and C-H, respectively [25, 26]. The characteristic absorption bands at 1714 cm− 1 and 1640 cm− 1 belong to C = O and C = N stretching vibrations, respectively [27, 28]. The absorption peak at 1540 cm− 1 is related to C = C, which is associated with the vibration of C = C in the benzene ring, proving the existence of aromatic structure in N-CDs and the formation of unsaturated C = C bond in the carbon core [29]. The peaks of 1350 cm− 1, 1280 cm− 1 and 1121 cm− 1 belong to the C = C, C-N and C-O groups, respectively [25]. The above results indicate that the surface of N-CDs is rich in functional groups, which confers good water solubility to N-CDs.
The elemental composition and structure of N-CDs were further studied by X-ray photoelectron spectroscopy (XPS). The full XPS spectrum of N-CDs (Fig. 1D) shows that the main peaks are 284.93, 399.19 and 530.95 eV, which belong to C 1s, N 1s, and O 1s, respectively. The high-resolution energy spectrum of C 1s (Fig. S1A) can be subdivided into four peaks of 284.48, 285.88, 287.18 and 288.38 eV, which belong to C-C, C-N, C-O and C = O, respectively. The high-resolution energy spectrum of N 1s (Fig. S1B) shows three peaks at 398.28 eV, 399.38 eV, and 401.98 eV, belonging to pyrrole N, graphite N, and N-(C)3, respectively. The two peaks in the high-resolution energy spectrum of O 1s (Fig. S1C) are caused by the C-O and C = O groups [30]. The XPS spectra are well consistent with FTIR spectra, indicating that N had been successfully doped into the carbon dots.
Optical Properties and Fluorescence Stability of N-CDs
The optical properties of N-CDs were analyzed by UV-Vis absorption and fluorescence spectra and the data is displayed in Fig. 2A and B. As shown by the UV absorption spectrum in Fig. 2A, the characteristic absorption centered at 360 nm is attributed to the n-π* transition of C = O and C = N in N-CDs and the acromion at 242 nm is caused by the π-π* transition in the aromatic sp2 domain [31]. The optimum fluorescence emission wavelength is observed at 455 nm under 360 nm excitation. The N-CDs solution is brown under visible light and fluoresces bright blue under ultraviolet lamps (the inset of Fig. 2A). As can be seen from Fig. 2B, the emission wavelength of N-CDs hardly changed while the excitation wavelength changes from 300 to 390 nm, indicating that N-CDs have no excitation dependent emission behavior. The fluorescence quantum yield of the N-CDs was calculated to be 39.7% by using quinine sulfate as a reference. Our N-CDs possess a higher fluorescence quantum yield compared to other reported carbon dots (Table S1). This is probably due to the fact that the internal electronic environment of the CDs was modified by doping the nitrogen atoms into the CDs, resulting in an effective improvement of the fluorescence properties of the CDs [32]. The aforementioned data suggest that N-CDs are ideal materials for fluorescence analysis.
(A) UV–Vis absorption, fluorescence excitation, and emission spectra of N-CDs. Images of the N-CDs in an aqueous solution under daylight (left) and UV irradiation (right) (inset). (B) Fluorescence spectra of N-CDs at different excitation wavelengths. The effect of pH (C), NaCl concentration (D), temperature (E) and storage time (F) on the fluorescent intensity of N-CDs
N-CDs, as a kind of fluorescent nanomaterial for detection applications, it is very important to study their fluorescence stability. According to Fig. 2C, the fluorescence intensity of N-CDs increased gradually as pH increased from 2.0 to 7.0 and when pH was above 7.0, the fluorescence intensity of N-CDs decreased triflingly. It is evident that acidic conditions had a great influence on the fluorescence intensity of N-CDs. This is probably due to the fact that the carboxyl groups on the surface of the N-CDs were protonated in acidic solutions, leading to the aggregation of N-CDs and resulting in fluorescence quenching [33]. Additionally, the impact of ionic concentration on the fluorescence intensity of N-CDs was investigated. From Fig. 2D, the fluorescence intensity of N-CDs was rarely impacted by NaCl concentrations as high as 1 M, which enables the N-CDs to work as a fluorescent probe in a solution with high ionic concentration. As indicated by Fig. 2E, the fluorescence intensity of N-CDs remained steady at temperatures ranging from 25℃ to 60℃, indicating that N-CDs tend to be highly stable throughout a broad temperature range. The fluorescence intensity of N-CDs solution kept in a refrigerator at 4℃ was measured every 10 days. The data in Fig. 2F suggests that the fluorescence intensity of the N-CDs remained substantially unaltered within two months, which means that the N-CDs have the potential to be stored in the fridge for prolonged use.
Construction of NO2 − Detection Platform
In this work, the feasibility of ratiometric fluorescent and colorimetric detection of NO2− was investigated. According to Fig. 3A, the fluorescence emission peak of N-CDs is at 455 nm. After the addition of OPD, there was no apparent variation in N-CDs’ fluorescence. When nitrite was introduced to the N-CDs solution, the fluorescence spectra of the N-CDs remained unchanged. Through the above data, we are certain that both OPD and nitrite don’t make prominent interference with the N-CDs’ fluorescence. However, when OPD, NO2− and N-CDs were mixed, the fluorescence of N-CDs decreased and a new fluorescence emission peak at 575 nm was observed. This is due to the fact that NO2− converted OPD into DAP (emitted at 575 nm) in acidic conditions and the fluorescence of the N-CDs was subsequently quenched by DAP. Thus, the ratio of fluorescence intensity of DAP to N-CDs changed with the increase of nitrite. Based on the above principle, a ratiometric fluorescent platform for nitrite detection was designed. On the other hand, in the absence of NO2− in the N-CDs/OPD system, there was no absorption and the solution was colorless. After the addition of NO2−, a new UV-Vis absorption was observed at 425 nm while the colour of the system emerged as yellow (Fig. 3B). These results indicated that it is possible to construct a colorimetric method for the detection of NO2−. The FL and UV-vis spectra of pure OPD, OPD + NO2− and N-CDs + DPA have provided in Fig. S2.
To create the optimal conditions for nitrite detection, the impacts of response time and pH on this method were studied in detail. Figure 3 C shows that the ratio of fluorescence intensity at 575 nm to 455 nm (F575/F455) gradually increased following the addition of NO2− and stabilized after 15 min. Consequently, 15 min is the best reaction time for NO2− detection. Given that NO2− is an effective oxidant in acidic conditions, it is critical to consider the impact of pH on the detecting system. According to Fig. 3D, F575/F455 consistently decreased in the pH range of 3.0–6.0, up to a maximum at pH 3. However, the N-CDs provided a low fluorescence intensity at pH 3.0 (Fig. 2C), while F575/F455 at pH 3.0 was close to pH 3.5. Based on the above considerations, the nitrite following detection experiments were all carried out at pH 3.5.
Ratiometric Fluorometric and Colorimetric Determination of NO2 −
The fluorescence intensity of the N-CDs/OPD system versus NO2− concentrations had been studied under the optimal detection conditions discussed above. As seen in Fig. 4A, the fluorescence intensity at 455 nm gradually decreases while the fluorescence intensity at 575 nm increases with NO2− increasing from 0.01 mM to 0.5 mM. Figure 4B displays the relationship between the NO2− concentration and the F575/F455 of the N-CDs/OPD system, which exhibited two good linear correlations in the range of 0.01–0.1 mM (Fig. 4C) and 0.1–0.5 mM (Fig. 4D) respectively. The linear regression equations are YF1=7.1127XF1 + 0.0040 (R2 = 0.9966) and YF1=9.2557XF2-0.2225 (R2 = 0.9996). Herein, YF refers to the fluorescence intensity of the N-CDs/OPD system in the presence of NO2− and XF refers to the NO2− concentration. The limit of detection (LOD) calculated according to 3σ/k (σ is the standard deviation) is 0.45 µM. The limit of quantitation (LOQ) (calculated according to 10σ/k) is 1.51 µM. Fig. 4E displayed the UV-Vis absorption spectra of the N-CDs/OPD system in the presence of NO2− up to 0.5 mM. The absorbance at 425 nm increased in proportion to the increase of NO2− and the solution changed from transparent to yellow can be observed by the naked eye (Inset in Fig. 4E). According to Fig. 4F, the NO2− concentration and the absorbance at 425 nm satisfied a good linear relationship in the range of 0-0.5 mM. The linear regression equation is YA=0.8753XA + 0.0423 (R2 = 0.9973). Herein, YA refers to the absorbance of the N-CDs/OPD system in the presence of NO2− and XA refers to the NO2− concentration. The LOD and LOQ were 3.03 µM and 10.10 µM, respectively. The data indicated that the ratiometric fluorescence method had a lower detection limit than the colorimetric detection method in this work. The detection limit of our ratiometric fluorescence method is one or even two orders of magnitude lower than those already reported. The detection limit of the colorimetric detection method is comparable to or even lower than other methods reported previously (Table S2).
(A) Fluorescence emission spectra of N-CDs/OPD system in the presence of NO2− at different concentrations (0.01–0.5 mM). (B) The relationship between the NO2− concentration and the F575/F455 of N-CDs/OPD system. Linear diagram of F575/F455 and the NO2− concentration in the range 0.01–0.1 mM (C) and 0.1–0.5 mM (D). (E) UV-Vis absorption spectra of N-CDs/OPD system upon the addition of NO2− at different concentrations (0–0.5 mM). The inset: the corresponding photographs of the N-CDs/OPD system with different concentrations of NO2− taken under daylight (from left to right: 0, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.08, 0.1, 0.2, 0.3, 0.4, 0.5 mM). (F) The linear plot of absorbance versus the concentration of NO2− (0-0.5 mM)
Selectivity and Interference
Selectivity and interference are key parameters for a sensing system. To research the selectivity of the ratiometric fluorescent platform, the fluorescence intensity ratio (F575/F455) of different cations and anions including Ca2+, K+, Na+, Mg2+, Ba2+, Co2+, Pb2+, Cu2+, HCO3−, CO32−, SO42−, HPO4−, H2PO4−, NO32−, CH3COO− and Cl− at a level of 0.5 mM were analyzed and the results are shown in Fig. 5A. As seen, only NO2− (0.1 mM) displayed a notable response to N-CDs/OPD. Furthermore, when nitrite was mixed with CDs/OPD system in the presence of the above mentioned ions at a level of 0.5 mM, the signal of nitrite variation was negligible, being less than ± 5%. Similarly, the colorimetric method also has pleasant selectivity and immunity to interference (Fig. 5B). The above data demonstrates the potential of the dual-mode fluorometric and colorimetric platform to detect nitrite.
Precision and Accuracy
The recovery experiments were used to assess the accuracy of the detection system. Samples including kimchi, pickled sea cabbage and pickled cucumber were spiked with 20, 50 and 80 µM of NO2− and determined according to the methods proposed in this paper. The data were presented in Table S3 and Table S4. For the ratiometric fluorescent mode detection, the recovery was between 96.84% and 104.48%, with an RSD ranging between 1.47% and 3.62%. In addition, the recovery of the colorimetric mode assay varied from 93.49 to 105.95%, with an RSD ranging between 1.41% and 5.99%, indicating good precision and accuracy.
Detection of the Nitrite Levels in Real Samples
To evaluate the feasibility of our methods in the testing of real samples, several pickles including kimchi, pickled sea cabbage and pickled cucumber were purchased from local market and tested by our methods. The results are illustrated in Table 1 and it shows that as determined by the ratiometric fluorescent method, the nitrite content in the kimchi, pickled sea cabbage and pickled cucumber was 8.23, 6.94 and 9.24 µg/g, respectively, which has no significant differences with the results tested by the national standard method based on the Student t-test (t>0.05) at a 95% confidence level. For colourimetric testing, the nitrite content in the kimchi, pickled sea cabbage and pickled cucumber was 7.80, 7.17 and 9.38 µg/g, respectively, which was consistent with the results tested by the national standard method. In addition, there were also no significant differences between the results of ratiometric fluorescent method and colourimetric method in this work. The nitrite content in the three pickled vegetable samples was below the national standard limit of 20 mg/kg (GB-2762-2012). Nitrite levels in pickles reported by other authors ranged from 5.8 to 11.65 µg/g [34, 35], which were comparable with our results.
a Mean value ± standard deviation (SD., n = 3).
b Ratiometric fluorescent method compared to national standard method.
c Colourimetric method compared to national standard method.
d Ratiometric fluorescent method compared to colourimetric method.
Conclusion
In summary, N-CDs were synthesized by taking citric acid and diethylenetriamine as the precursors, which possess a high fluorescence quantum yield of 39.7%. Based on N-CDs/OPD regent, dual-mode ratiometric fluorometric and colorimetric methods for nitrite detection was developed. Compare to the conventional single mode methods, the ratiometric fluorometric method overcame the drawback of weak interference immunity. The colourimetric method achieved the on-site visual detection. The dual-mode detection platform showed satisfied selectivity, precision and accuracy, which has no remarkable difference to the national standard method, and could be used as an alternative method for the determination of nitrite in pickles. In addition, this special dual-mode detection platform provide an enlightenment to other sensing applications such as •OH, H2O2, uric acid, cholesterol, and xanthine, etc.
Data Availability
All relevant data are presented in the manuscript.
References
Hou JC, Jiang CG, Zhong CL (2013) Nitrite level of pickled vegetables in Northeast China. Food Control 29(1):7–10. https://doi.org/10.1016/j.foodcont.2012.05.067
Wang QH, Yu LJ, Liu Y, Lin L, Lu RG, Zhu JP, He L, Lu ZL (2017) Methods for the detection and determination of nitrite and nitrate: a review. Talanta 165:709–720. https://doi.org/10.1016/j.talanta.2016.12.044
Choi SY, Chung MJ, Lee SJ, Shin JH, Sung NJ (2007) N-nitrosamine inhibition by strawberry, garlic, kale, the effects of nitrite-scavenging and N-nitrosamine formation by functional compounds in strawberry and garlic. Food Control 18(5):485–491. https://doi.org/10.1016/j.foodcont.2005.12.006
Adarsh N, Shanmugasundaram M, Ramaiah D (2013) Efficient reaction based colorimetric probe for sensitive detection, quantification, and on-site analysis of nitrite ions in natural water resources. Anal Chem 85(21):10008–10012. https://doi.org/10.1021/ac4031303
Wang X, Hou J, Shen X, He Q, Hou C, Huo D (2020) Fluorescence-based measurements for the determination of nitrite using a coumarin derivative sensor based on inner filter effect. Anal Methods 12(8):1107–1114. https://doi.org/10.1039/c9ay02431j
Zuo Y, Wang C, Van T (2006) Simultaneous determination of nitrite and nitrate in dew, rain, snow and lake water samples by ion-pair high-performance liquid chromatography. Talanta 70(2):281–285. https://doi.org/10.1016/j.talanta.2006.02.034
Yang S, Liu X, Zeng X, Xia B, Gu J, Luo S, Mai N, Wei W (2010) Fabrication of nano-copper/carbon nanotubes/chitosan film by one-step electrodeposition and its sensitive determination of nitrite. Sens Actuators B Chem 145(2):762–768. https://doi.org/10.1016/j.snb.2010.01.032
Kalaycıoğlu Z, Erim FB (2016) Simultaneous determination of nitrate and nitrite in fish products with improved sensitivity by sample stacking-capillary electrophoresis. Food Anal Methods 9:706–711. https://doi.org/10.1007/s12161-015-0241-4
Wang L, Li B, Zhang L, Zhang L, Zhao H (2012) Fabrication and characterization of a fluorescent sensor based on rh 6G-functionlized silica nanoparticles for nitrite ion detection. Sens Actuators B Chem 171:946–953. https://doi.org/10.1016/j.snb.2012.06.008
Liu H, Yang G, Abdel-Halim E, Zhu JJ (2013) Highly selective and ultrasensitive detection of nitrite based on fluorescent gold nanoclusters. Talanta 104:135–139. https://doi.org/10.1016/j.talanta.2012.11.020
Hao X, Liang Y, Zhen H, Sun X, Liu X, Li M, Shen A, Yang Y (2020) Fast and sensitive fluorescent detection of nitrite based on an amino-functionalized MOFs of UiO-66-NH2. J Solid State Chem 287:121323. https://doi.org/10.1016/j.jssc.2020.121323
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49(38):6726–6744. https://doi.org/10.1002/anie.200906623
Zan M, Rao L, Huang H, Xie W, Zhu D, Li L, Qie X, Guo SS, Zhao X-Z, Liu W (2018) A strong green fluorescent nanoprobe for highly sensitive and selective detection of nitrite ions based on phosphorus and nitrogen co-doped carbon quantum dots. Sens Actuators B Chem 262:555–561. https://doi.org/10.1016/j.snb.2017.12.177
Liu Y, Luo S, Wu P, Ma C, Wu X, Xu M, Li W, Liu S (2019) Hydrothermal synthesis of green fluorescent nitrogen doped carbon dots for the detection of nitrite and multicolor cellular imaging. Anal Chim Acta 1090:133–142. https://doi.org/10.1016/j.aca.2019.09.015
Jing J, Wen JL, Lin L, Yuan J, Yi FG, Shuang SM (2019) Orange luminescent carbon dots as fluorescent probe for detection of nitrite. Chin J Anal Chem 47(4):560–566. https://doi.org/10.1016/s1872-2040(19)61155-2
He H, Sun DW, Wu Z, Pu H, Wei Q (2022) On-off-on fluorescent nanosensing: materials, detection strategies and recent food applications. Trends Food Sci Technol 119:243–256. https://doi.org/10.1016/j.tifs.2021.11.029
Guo Y, Wang R, Wei C, Li Y, Fang T, Tao T (2023) Carbon quantum dots for fluorescent detection of nitrite: a review. Food Chem 135749. https://doi.org/10.1016/j.foodchem.2023.135749
Kong Y, Cheng Q, He Y, Ge Y, Zhou J, Song G (2020) A dual-modal fluorometric and colorimetric nanoprobe based on graphitic carbon nitrite quantum dots and fe (II)-bathophenanthroline complex for detection of nitrite in sausage and water. Food chem 312:126089. https://doi.org/10.1016/j.foodchem.2019.126089
Liu Y, Xue H, Liu J, Wang Q, Wang L (2018) Carbon quantum dot-based fluorometric nitrite assay by exploiting the oxidation of iron (II) to iron (III). Microchim Acta 185:1–7. https://doi.org/10.1007/s00604-018-2668-y
Li F, Liu J, Hu Y, Deng N, He J (2018) An ultrasensitive label-free colorimetric assay for glutathione based on Ag+ regulated autocatalytic oxidation of o-phenylenediamine. Talanta 186:330–336. https://doi.org/10.1016/j.talanta.2018.04.078
Wu C, Zhu L, Lu Q, Li H, Zhang Y, Yao S (2019) A dual-signal colorimetric and ratiometric fluorescent nanoprobe for enzymatic determination of uric acid by using silicon nanoparticles. Microchim Acta 186:1–8. https://doi.org/10.1007/s00604-019-3862-2
Wang L, Liu Y, Yang Z, Wang Y, Rao H, Yue G, Wu C, Lu C, Wang X (2020) A ratiometric fluorescence and colorimetric dual-mode assay for H2O2 and xanthine based on Fe, N co-doped carbon dots. Dyes Pigm 180:108486. https://doi.org/10.1016/j.dyepig.2020.108486
Chen Y, Zhao C, Yue G, Yang Z, Wang Y, Rao H, Zhang W, Jin B, Wang X (2020) A highly selective chromogenic probe for the detection of nitrite in food samples. Food Chem 317:126361
Liang Z, Kang M, Payne GF, Wang X, Sun R (2016) Probing energy and electron transfer mechanisms in fluorescence quenching of biomass carbon quantum dots. ACS Appl Mater Interfaces 8(27):17478–17488. https://doi.org/10.1021/acsami.6b04826
Yi Z, Li X, Zhang H, Ji X, Sun W, Yu Y, Liu Y, Huang J, Sarshar Z, Sain M (2021) High quantum yield photoluminescent N-doped carbon dots for switch sensing and imaging. Talanta 222:121663. https://doi.org/10.1016/j.talanta.2020.121663
Hu Q, Sun H, Zhou X, Gong X, Xiao L, Liu L, Yang ZQ (2020) Bright-yellow-emissive nitrogen-doped carbon nanodots as a fluorescent nanoprobe for the straightforward detection of glutathione in food samples. Food Chem 325:126946. https://doi.org/10.1016/j.foodchem.2020.126946
Tang X, Yu H, Bui B, Wang L, Xing C, Wang S, Chen M, Hu Z, Chen W (2021) Nitrogen-doped fluorescence carbon dots as multi-mechanism detection for iodide and curcumin in biological and food samples. Bioact Mater 6(6):1541–1554. https://doi.org/10.1016/j.bioactmat.2020.11.006
Ali HRH, Hassan AI, Hassan YF, El-Wekil MM (2021) Colorimetric and fluorometric nanoprobe for selective and sensitive recognition of hazardous colorant indigo carmine in beverages based on ion pairing with nitrogen doped carbon dots. Food Chem 349:129160. https://doi.org/10.1016/j.foodchem.2021.129160
Liu Y, Wu P, Wu X, Ma C, Luo S, Xu M, Li W, Liu S (2020) Nitrogen and copper (II) co-doped carbon dots for applications in ascorbic acid determination by non-oxidation reduction strategy and cellular imaging. Talanta 210:120649. https://doi.org/10.1016/j.talanta.2019.120649
Zhou M, Zhou Z, Gong A, Zhang Y, Li Q (2015) Synthesis of highly photoluminescent carbon dots via citric acid and tris for iron (III) ions sensors and bioimaging. Talanta 143:107–113. https://doi.org/10.1016/j.talanta.2015.04.015
Wu Y, Cao L, Zan M, Hou Z, Ge M, Dong WF, Li L (2021) Iron and nitrogen-co-doped carbon quantum dots for the sensitive and selective detection of hematin and ferric ions and cell imaging. Analyst 146(15):4954–4963. https://doi.org/10.1039/d1an00828e
Miao S, Liang K, Zhu J, Yang B, Zhao D, Kong B (2020) Hetero-atom-doped carbon dots: doping strategies, properties and applications. Nano Today 33:100879. https://doi.org/10.1016/j.nantod.2020.100879
Wang R, Wang X, Sun Y (2017) One-step synthesis of self-doped carbon dots with highly photoluminescence as multifunctional biosensors for detection of iron ions and pH. Sens Actuators B Chem 241:73–79. https://doi.org/10.1016/j.snb.2016.10.043
Zhang ML, Huang DK, Cao Z, Liu YQ, He JL, Xiong JF, Feng ZM, Yin YL (2015) Determination of trace nitrite in pickled food with a nano-composite electrode by electrodepositing ZnO and pt nanoparticles on MWCNTs substrate. LWT 64(2):663–670. https://doi.org/10.1021/acsfoodscitech.2c00278
Wang HM, Feng XN, Xia Y, Yin XB (2022) Dual-ligand Terbium Metal–Organic Framework for visual ratiometric fluorescence sensing of Nitrites in Pickles. ACS Food Sci Technol 2(12):1911–1920. https://doi.org/10.1021/acsfoodscitech.2c00278
Funding
This research was supported by Zhejiang Provincial Natural Science Foundation of China (LY22C200008) and the National Natural Science Foundation of China (31772085).
Author information
Authors and Affiliations
Contributions
Chendi Heng: Conceptualization, methodology, validation, analysis, investigation, data curation, writing—original draft preparation, and writing reviewing and editing. Bowen He: Data curation, validation, and investigation. Li Wang: Supervision, conceptualization, methodology, resources, validation, formal analysis, investigation, writing—original draft preparation, writing—reviewing and editing, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing of Interests
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heng, C., He, B. & Wang, L. A Dual-mode Ratiometric Fluorometric and Colorimetric Platform Based on Nitrogen-doped Carbon Dots and o-phenylenediamine for the Detection of Nitrite. J Fluoresc (2023). https://doi.org/10.1007/s10895-023-03432-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03432-8