Abstract
A fast and reproducible method for the simultaneous determination of nitrate and nitrite ions in canned fish samples by capillary zone electrophoresis has been developed. The sensitivity of the method was increased by applying a sample stacking technique. Optimal separation conditions were selected as 30 mmol L−1 formic acid and 30 mmol L−1 sodium sulfate at a pH of 4.0. The separation of nitrate and nitrite ions was achieved within 2.5 min. The limits of detection obtained at a signal-to-noise ratio of 3 for nitrate and nitrite were 0.55 and 0.82 μmol L−1, while the relative standard deviations of intra-day corrected peak areas were 0.99 and 2.74 %, respectively. Recovery values ranged between 88.7 and 104 % for both ions. The method was successfully applied to canned fish samples, namely tuna, mackerel and sardine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrate and nitrite are natural constituents of many foodstuffs. Vegetables and drinking water are the main sources of nitrate and nitrite in the human diet. Furthermore, nitrate and nitrite are official food additives within European Union (EU) used in cured meats. Nitrate itself is not toxic, but under specific conditions can be reduced to nitrite, which is toxic. Nitrate is reduced to nitrite by microorganisms, and nitrite produces the carcinogenic N-nitroso compound with the amine ingredients of food in specific conditions. Nitrite can also result in methemoglobinemia (blue baby syndrome) which is dangerous for babies younger than 6 months. Due to the growing concern on health effects of N-nitroso compounds, a good number of reports on the nitrate and nitrite contents of several vegetables and cured meats all over the world have been produced (De González et al. 2012; Hsu et al. 2009; Iammarino et al. 2013; Pereira et al. 2012; Sungur and Atan 2013). However, reports on the nitrate and nitrite contents of fish products are rare. Nitrate exists in fresh fish and, although nitrate and nitrite are not added directly to salted and canned fish products, nitrite forms either through impure sea or rock salt used to salt fish or by bacterial reduction of nitrate (Al Bulushi et al. 2009; Prester 2011). On the other hand, the formation of biogenic amines in fish products are great concerns due to the adverse health effects of these amines, known generally as histamine poisoning (Al Bulushi et al. 2009; Prester 2011). A huge amount of reports on the biogenic amine contents of salted fish exists in the literature (Başkan et al. 2010; Erim 2013; Koral et al. 2013, Köse et al. 2012; Park et al. 2010; Zhai et al. 2012). It was reported that consumption of fish products containing amine contents may lead to the formation of N-nitroso compounds in the human stomach, if adequate levels of nitrite are also ingested at the same time (Sen et al. 2001), which can lead to stomach cancer (Mirvish 1982). Consequently, nitrate and nitrite contents of fish products should be monitored in order to estimate their dietary intakes.
Capillary zone electrophoresis (CZE) is a separation method known for easy method development, low sample consumption, fast analysis times, high separation efficiency and simple experimental apparatus. In the present study, a fast CZE method was developed for the simultaneous determination of nitrate and nitrite ions in canned fish samples. By now, CZE methods have been successfully used in order to find the level of nitrate and nitrite as additives in cured meat products (Öztekin et al. 2002; Pereira et al. 2012). However, naturally occurring nitrite ion levels in vegetables and baby foods have been found under the detection limits of capillary electrophoretic methods (Della Betta et al. 2014; Merusi et al. 2010; Öztekin et al. 2002). In this study, the sample stacking technology is for the first time optimized for fish matrix and the resulting detection limits make possible the detection of nitrate and, more importantly, nitrite contents in fish samples, whereas nitrite contents of foodstuff are generally under detection limits of many analytical techniques if nitrite is not an additive. Since nitrate and nitrite are not used as additive in canned fish samples investigated, the sensitivity of the method in the present study was increased by developing an on-line sample concentration method. The simultaneous detection of nitrate and nitrite were achieved in 2.5 min.
The developed method was applied to control the nitrate and nitrite contents of commercially canned tuna, mackerel and salted sardine that are frequently consumed by children and adults in Turkey.
Materials and Methods
Materials
Analytical grade sodium nitrite, potassium nitrate, formic acid, sodium sulfate and sodium hydroxide were purchased from Merck (Darmstadt, Germany); hexane and acetonitrile were obtained from J. T. Baker (Deventer, Holland). Solutions were prepared using ultrapure water from a Milli-Q water system (Purelab Option Q).
Food Samples
Canned fish: tuna, mackerel and sardine were purchased from local markets in Istanbul. Fish extracts were stored in refrigerator at 4 °C and analysed within 48 h. All fish samples contain sodium chloride but not nitrate or nitrite as ingredients.
Standard Solutions
The nitrate and nitrite stock solutions were prepared separately for each analyte at 10 mmol L−1 levels in deionized water and stored at 4 °C. The stock solutions were gradually diluted to the working concentration levels with deionized water.
Preparation of Fish Samples
Fish samples were homogenized in a hand blender for 3 min, and 0.5 g of the blended portions was extracted twice with 5 mL of deionized water. The suspensions were stirred for 10 min at 75 °C and then ultrasonicated for 15 min. The supernatants were combined after being filtered through a Whatman 41 filter paper and defatted twice with 10 mL portions of hexane using a separating funnel.
Acetonitrile (ACN) was added to extract, and the final extract was diluted to 10 mL with deionized water. The resulting solution containing 7.5 % (v/v) ACN was filtered from 0.45 μm microfilter and directly injected. Fish samples were analysed in duplicate.
Instrumentation and Separation Conditions
All experiments were performed with an Agilent 1600 capillary electrophoresis system (Waldbronn, Germany). Separations were carried out in uncoated fused silica capillaries with 50 μm i.d. (Polymicro Technology, Phoenix, AZ, USA). The total length of capillary was 65 cm and the length to detector was 50 cm.
The optimized separation electrolyte consisted of 30 mmol L−1 formic acid buffer at pH 4.0 containing 30 mmol L−1 sodium sulfate. Samples were injected at 50 mbar for 160 s. The separation voltage was −25 kV, and measurements were performed at 25 °C. The UV detection was carried out at 210 nm. Data collection and processing were carried out using Agilent ChemStation software. Before use, new capillaries were conditioned with 1 mol L−1 sodium hydroxide and water for 30 min. The capillary was flushed successively with 0.1 mol L−1 sodium hydroxide, water and running electrolyte between runs for 2 min each.
Results and Discussion
Optimization of the Separation pH
In capillary electrophoresis, all ions injected from the anodic side, regardless of their charges are carried by strong electroosmotic flow (EOF) toward the cathode, i.e. detector side. However, small anions like nitrate and nitrite are exceptions and they cannot be easily swept due to their high electrophoretic mobilities. In order to provide the fast migration of small anions, EOF is reduced working with low pH buffers and sample injection is made from the cathodic side changing the polarity. With this way, small anions having high mobilities can migrate across reduced EOF. The second way is to reverse the direction of EOF by using either an electroosmotic modifier in buffer or by coating the capillary with a positively charged polymer. In this way small anions co-migrate with the EOF. Both methods have been successfully applied in the literature for the separation of small ions (Alonso and Prego 2000; Della Betta et al. 2014; Nutku and Erim 1999; Öztekin et al. 2002; Tanaka et al. 2004). In the present study, formic acid/formate buffer was selected as the low pH separation medium, EOF was reduced in that way and the resolution of nitrite and nitrate anions were checked at pHs between 3.5 and 4.2. Since nitric acid is a strong acid, even at small pHs, it is fully dissociated. So the nitrate ion is not affected from pH changes in this pH range. However, nitrous acid is a weak acid having 3.39 pKa value, and nitrite ion is partially protonated, consequently mobility of nitrite ion change with pH. The optimal resolution was obtained as changing the pH and consequently changing the mobility of nitrite ion. The best conditions in terms of migration times and resolution were selected as 30 mmol L−1 formic acid at pH 4.0. Sample was injected at 50 mbar for 6 s which is the commonly used small injection volume in CE. In the preliminary experiments with fish samples, only nitrate contents could be detected with these separation conditions. Consequently, a sample stacking method was developed in order to increase the detection sensitivity of both ions for the analyses of real samples.
Optimization of Sample Stacking Conditions
In order to increase detection sensitivities of both ions, injection volume of the sample solution can be increased. However, large volume injection will cause broadened peaks. In order to obtain sharp analyte peaks, analytes in the sample zone were stacked, i.e. concentrated. For this purpose, the conductivity of the separation buffer (30 mmol L−1 formic acid at pH 4.0) was increased by the addition of sodium sulfate. Larger conductivity of separation buffer than conductivity of sample zone means that the electrical field in the separation buffer is lower than the electrical field of the sample zone. When the high voltage is applied, nitrite and nitrate ions in the low conductivity sample zone experience a higher field strength than those in the high-conductivity separation buffer. Thus, both ions move quickly through the sample matrix and slow down when they reach the boundary of separation electrolyte. Consequently, sample ions stack as a narrow zone at the boundary of the sample and buffer solutions. When this concentrated zone reaches the detector, sharp peaks are observed and the detection sensitivities of ions increase. Since, in our system, the migration of nitrite and nitrate is opposite to the direction of the weak EOF in the capillary, during the stacking process, EOF serves pumping the sample matrix out of capillary.
We added acetonitrile to fish samples to remove proteins. The same amount of acetonitrile was added to standard samples during stacking pre-experiments. Since acetonitrile has low conductivity, the presence of acetonitrile in the sample zone also improved the stacking effect.
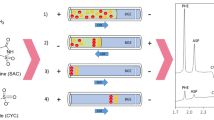
Stacking conditions were optimized by changing the sodium sulfate concentration in the separation buffer and changing the injection time of the sample zone. Finally, the sodium sulfate concentration was fixed at 30 mmol L−1 in 30 mmol L−1 formic acid solution at pH 4.0. Injection times were changed between 60 and 280 s at 50 mbar. When the injection time exceeded 160 s, peak broadening was observed for both anions. The optimum injection condition was selected as 160 s at 50 mbar (Fig. 1). Excellent separation efficiency was gained when the sample solution contained 7.5 % (v/v) ACN. The effect of the concentration of acetonitrile was also studied, but no improvement in peak heights and shapes was observed by increasing ACN content.
Comparison of the sample stacking experiment and small-volume injection of standard sample containing 100 μmol L−1 NO3ˉand NO2ˉ. Injection: a 50 mbar, 160 s; b 50 mbar, 6 s. Running potential, −25 kV; capillary, 65 cm (50 cm to detector) × 50 μm i.d.; 210 nm, buffer: 30 mmol L−1 formic acid, 30 mmol L−1 Na2SO4, pH 4.0. Peaks: 1 NO3ˉ, 2 NO2ˉ
When detection limits of both ions are compared between small-volume injection and sample stacking modes, it was observed that the stacking mode resulted in a 30-fold improvement in detection sensitivity for both anions as seen in Fig. 1. The improvement factor was ordinarily obtained by dividing the limit of detection (LOD) obtained with small-volume injection by the LOD obtained with sample stacking mode. Since stacking techniques are based on the difference between conductivities of the sample injected and the buffer inside the capillary, the improvement of detection sensitivity strictly depends on the sample matrix. Therefore, the comparison in the improvement of stacking methods can only be made for the same type of real samples. Our study is the first sample stacking method in capillary electrophoresis employed for fish matrix. The sensitivities that are obtained with our method make possible the detection of nitrite and nitrate contents of fish products.
Method Validation
Calibration curves were constructed by the addition of ACN as the final concentration 7.5 % ACN (v/v) to the nitrate and nitrite standards. The calibration curves show linear dynamic ranges from 4 to 24 μmol L−1 and 4 to 18 μmol L−1 with correlation coefficients of 0.998 and 0.999 for nitrate and nitrite ions, respectively. The limit of detections, LOD (S/N = 3), were found to be 0.55 and 0.82 μmol L−1, and the limit of quantifications, LOQ (S/N = 10), were 1.82 and 2.75 μmol L−1 for nitrate and nitrite, respectively. The precision of the method was performed in terms of inter-day and intra-day repeatability as RSD%. Intra-day analyses were determined by injecting the anions seven times in the same day. Inter-day repeatability was calculated by injecting both anions on three different days, seven injections in each day. The relative standard deviation was smaller than 2.80 for intra-day analysis and inter-day analysis for both anions as seen in Table 1.
Recoveries of the anions were calculated by using the standard addition method. Three different fish samples, namely tuna, mackerel and sardine chosen were fortified with the analyte ions, each at three different concentrations corresponding to 50, 100 and 200 % of the real sample concentrations. The percentage of recovery was calculated with the formula (1):
where C1 is the concentration determined in fortified sample, C2 is the concentration determined in unfortified sample and C3 is the concentration of added standard.
Good results of mean recoveries were obtained, with values ranging between 88.7 and 104 % as is apparent from Table 2.
Determination of Nitrite and Nitrate Concentration in Fish Samples
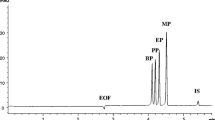
Nitrate and nitrite concentrations were determined in nine canned fish samples using the optimized CE sample stacking method. Quantification of anions was performed by using an external standard calibration method. Sample solutions were prepared as described in the experimental part. One representative electropherogram of tuna, mackerel and sardine sample is given in Fig. 2. Peaks are seen clearly, and there are no unknown peaks in this region for all fish samples. As a result of the deproteinization step before injection, proteins likely to adsorb onto the capillary wall were removed, so that efficient results of samples were obtained. The nitrate and nitrite contents of fish samples are given in Table 3. As seen from Table 3, except for mackerel and one tuna sample, all fish samples contain nitrite. It seems that there is a correlation between nitrate and nitrite contents of fish samples, except for mackerel samples. Nitrite contents of samples are more or less half of the nitrate contents. Since, nitrate content of one tuna sample (Tuna 6) is comparatively low, its nitrite content is probably under detection limit. However, even for nitrate contents of mackerel samples as high as those of tuna samples, nitrite was not detected in mackerels. There are very few reports in the literature on the nitrate and nitrite contents of marine fish products. We could not find any report on canned fish products. Jastrzębska (2011) investigated nitrite contents of prawns, squids and different mixes of seafood together with their some phosphate compounds by capillary isotachophoresis within 15 min. The detection limits were 0.40 and 1.15 mg L−1 for nitrite and nitrate, respectively. Nitrite was not detected in all fish samples investigated. Iammarino et al. (2013) investigated nitrate and nitrite contents of clams and mussels by ion chromatography within 20 min. LOD values are 1.5 and 3.2 mg kg−1 for nitrite and nitrate. No nitrite and nitrate residues were detected in all clam samples. In mussel samples, nitrite was not detected and nitrate contents were found as between 9.9 and 205.3 mg kg−1. Karl (1998) reported nitrate contents of marine fishes, crustaceans and squids with a colorimetric method. Nitrite was detected in none of the samples. Low concentrations of less than 1.84 mg NO3/kg edible part were found in most of the marine fish samples, but some elevated levels were measured in farmed fresh water prawns. Monser et al. (2002) applied their flow injection method to one mackerel and one sardine samples. The detection limits were 0.01 μg mL−1 for nitrite and 0.025 μg mL−1 for nitrate. The reported amounts were 36 and 6.7 μg mL−1 nitrate and 34.3 and 30.2 μg mL−1 nitrite for sardine and mackerel, respectively.
Conclusion
A capillary electrophoresis and sample stacking method was developed for the simultaneous analysis of nitrate and nitrite ions. The method yields very short analysis time, good accuracy and reproducibility. By the on-line sample stacking application, the sensitivity of the method enabled monitoring both nitrate and nitrite ions in canned fish samples.
References
Al Bulushi I, Poole S, Deeth HC, Dykes GA (2009) Biogenic amines in fish: roles in toxication, spoilage, and nitrosamine formation: a review. Crit Rev Food Sci Nutr 49:369–377
Alonso MCB, Prego R (2000) Determination of silicate, simultaneously with other nutrients (nitrite, nitrate and phosphate), in river waters by capillary electrophoresis. Anal Chim Acta 416:21–27
Başkan S, Tezcan F, Köse S, Öztekin N, Erim FB (2010) Non-ionic micellar electrokinetic chromatography with laser-induced fluorescence: a new method tested with biogenic amines in brined and dry-salted fish. Electrophoresis 31:2174–2179
De González MTN, Osburn WN, Hardin MD, Longnecker M, Garg HK, Bryan NS, Keeton JT (2012) Survey of residual nitrite and nitrate in conventional and organic/natural/uncured/indirectly cured meats available at retail in the United States. J Agric Food Chem 60:3981–3990
Della Betta F, Vitali L, Fett R, Costa ACO (2014) Development and validation of a sub-minute capillary zone electrophoresis method for determination of nitrate and nitrite in baby foods. Talanta 122:23–29
Erim FB (2013) Recent analytical approaches to the analysis of biogenic amines in food samples. Trac-Trends Anal Chem 52:239–247
Hsu J, Arcot J, Lee NA (2009) Nitrate and nitrite quantification from cured meat and vegetables and their estimated dietary intake in Australians. Food Chem 115:334–339
Iammarino M, Di Taranto A, Cristino M (2013) Endogenous levels of nitrites and nitrates in wide consumption foodstuffs: results of five years of official controls and monitoring. Food Chem 140:763–771
Jastrzębska A (2011) Capillary isotachophoresis as rapid method for determination of orthophosphates, pyrophosphates, tripolyphosphates and nitrites in food samples. J Food Compos Anal 24:1049–1056
Karl H (1998) Nitrate and nitrite content in edible part of fishes, crustaceans and molluscans. Deutsche Lebensmittel Rundschau 94:17–20
Koral S, Tufan B, Ščavničar A, Kočar D, Pompe M, Köse S (2013) Investigation of the contents of biogenic amines and some food safety parameters of various commercially salted fish products. Food Control 32:597–606
Köse S, Koral S, Tufan B, Pompe M, Ščavničar A, Kočar D (2012) Biogenic amine contents of commercially processed traditional fish products originating from European countries and Turkey. Eur Food Res Technol 235:669–683
Merusi C, Corradini C, Cavazza A, Borromei C, Salvadeo P (2010) Determination of nitrates, nitrites and oxalates in food products by capillary electrophoresis with pH-dependent electroosmotic flow reversal. Food Chem 120:615–620
Mirvish SS (1982) In vivo formation of N-nitroso compounds: Formation from nitrite and nitrogen dioxide, and relation to gastric cancer. In: Magee PN (ed) Nitrosamines and Human Cancer, Banbury Report 12 (pp. 227-241). Cold Spring Harbour Laboratory, USA
Monser L, Sadok S, Greenway GM, Shah I, Uglow RF (2002) A simple simultaneous flow injection method based on phosphomolybdenum chemistry for nitrate and nitrite determinations in water and fish samples. Talanta 57:511–518
Nutku MS, Erim FB (1999) Polyethyleneimine-coated capillary electrophoresis capillaries for the analysis of organic acids with an application to beverages. J Microcolumn Sep 11:541–543
Öztekin N, Nutku MS, Erim FB (2002) Simultaneous determination of nitrite and nitrate in meat products and vegetables by capillary electrophoresis. Food Chem 76:103–106
Park JS, Lee CH, Kwon EY, Lee HJ, Kim JY, Kim SH (2010) Monitoring the contents of biogenic amines in fish and fish products consumed in Korea. Food Control 21:1219–1226
Pereira EA, Petruci JFS, Cardoso AA (2012) Determination of nitrite and nitrate in Brazilian meats using high shear homogenization. Food Anal Methods 5:637–642
Prester L (2011) Biogenic amines in fish, fish products and shellfish: a review. Food Addit Contam 28:1547–1560
Sen NP, Seaman SW, Baddoo PA, Burgess C, Weber D (2001) Formation of N-Nitroso-N-methylurea in various samples of smoked/dried fish, fish sauce, seafoods, and ethnic fermented/pickled vegetables following incubation with nitrite under acidic conditions. J Agric Food Chem 49:2096–2103
Sungur S, Atan MM (2013) Determination of nitrate, nitrite and perchlorate anions in meat, milk and their products consumed in Hatay region in Turkey. Food Addit Contam B Surveill 6:6–10
Tanaka Y, Naruishi N, Fukuya H, Sakata J, Saito K, Wakida S (2004) Simultaneous determination of nitrite, nitrate, thiocyanate and uric acid in human saliva by capillary zone electrophoresis and its application to the study of daily variations. J Chromatogr A 1051:193–197
Zhai H, Yang X, Li L, Xia G, Cen J, Huang H, Hao S (2012) Biogenic amines in commercial fish and fish products sold in southern China. Food Control 25:303–308
Acknowledgments
We thank the Research Foundation of Istanbul Technical University for financial support.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
F. Bedia Erim declares that she has no conflict of interest. Zeynep Kalaycıoğlu declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalaycıoğlu, Z., Erim, F.B. Simultaneous Determination of Nitrate and Nitrite in Fish Products with Improved Sensitivity by Sample Stacking-Capillary Electrophoresis. Food Anal. Methods 9, 706–711 (2016). https://doi.org/10.1007/s12161-015-0241-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0241-4