Abstract
Based on DNA strand replacement reaction and aptamer-specific recognition, a simple dual-labeled DNA nanostructure is designed for the simultaneous detection of Ochratoxin A (OTA) and aflatoxin B1 (AFB1). C1 is labeled with Cy3 and Cy5, while C2 and C3 are labeled with BHQ2. The fluorescence intensity of DNA nanostructure composed of C1, C2 and C3 is weak because of fluorescence resonance energy transfer. When OTA Aptamer (OTA-Apt) and AFB1 Aptamer (AFB1-Apt) are added to the homogeneous system at the same time, C1 can be replaced with the help of toehold strand displacement, resulting in fluorescence enhancement. In the presence of both OTA and AFB1, the toehold strand displacement reaction is inhibited due to preferential binding between the target and their corresponding aptamers. The limit of detection of OTA was 0.007 ng/mL and that of AFB1 was 0.03 ng/mL. The recoveries of OTA and AFB1 were 96%-101% and 97%-101% in the corn sample, and 99%-101% and 92%-106% in the wine sample. Compared with other sensors, the preparation of this aptasensor needs simpler experimental steps and a shorter total-preparing time, confirming the convenient, rapid, and time-saving operation process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ochratoxin A (OTA) and aflatoxin B1 (AFB1) have strong hepatotoxicity, nephrotoxicity, immunotoxicity, and carcinogenic teratogenicity [1]. It has been reported that OTA and AFB1 can coexist in wheat and maize [2, 3]. When OTA and AFB1 coexist, toxins cause greater toxicity due to accumulation or synergy, and pose a greater threat to human health [4, 5]. Therefore, it is urgent to establish a rapid, safe, accurate, and simultaneous detection method of OTA and AFB1 to accurately assess the degree of food pollution.
Traditional analysis methods include high-performance liquid chromatography (HPLC) [6] and high-performance liquid chromatography-tandem mass spectrometry (HPLC–MS) [7], but these methods are limited by expensive instruments, professionals and complex operating processes. The liquid crystal-based biosensor has also attracted wide attention due to its great potential in on-site detection, but its practical application is also limited because the liquid crystal is not resistant to high temperatures [8, 9]. At the same time, based on antibodies specific to mycotoxins, enzyme-linked immunosorbent assay [10] and immunochromatography [11] were developed for the simultaneous detection of various mycotoxins. However, mycotoxins are small molecules, so the production process of antibodies is complex and the stability is low, and it is difficult to produce high-quality antibodies, which seriously hinders the practicability of immunoassay in the detection of multiple mycotoxins. The aptamer is a single-stranded oligonucleotide sequence with high affinity and specificity for targets. As a new class of recognition elements, they not only have the same specific recognition ability as antibodies, but also have some other advantages such as easy synthesis, good chemical stability, good repeatability, easy storage and transportation [12, 13]. In addition, aptamers can easily modify functional groups, fluorescent groups, biotins and enzymes, simplify the detection steps and make the sensor platform more flexible [14].
Due to the advantages of aptamer, it has been widely used in the fabrication of sensors. The colorimetric aptasensor is simple and intuitive, and has a good application in single target detection, but it has been greatly limited in the field of multivariate detection [15, 16]. Although the electrochemical aptasensor can be used for the detection of dual targets, the electrode surface modification is time-consuming and laborious, which significantly increases the preparation time of the aptasensor [17]. So far, fluorescent aptasensor has received extensive attention due to their simple operation, short analysis time, high sensitivity and good stability [18, 19].
Because of the easy modification, controllable self-assembly and good stability of DNA sequences, complex DNA nanostructures such as tweezers, walkers, gears and nanocages have been widely used in the biomolecular assembly, signal recognition and biosensors [20,21,22]. Although these complex nanostructures can be used to detect a variety of targets, the costs are prohibitive. Conversely, simple nanostructures can not only reduce the interference of other factors in the system, but also save costs.
In addition, enzyme-free DNA amplification technology has been widely concerned because of its low cost and high efficiency [23, 24]. In the enzyme-free amplification method, the strand displacement reaction refers to the displacement of one or more pre-hybridized strands, initiated by the toehold region and the single strand, and carried out by a rapid branch migration process [25]. The toehold region can first act as a recognition site to bind to a single strand, and then trigger a cascade of hybridization to form a partially or fully complementary DNA double strand [26]. This reaction allows for single-strand exponential amplification in less than 15 min without the presence of the enzyme [27], which provides an efficient way to control DNA hybridization and amplify target-binding signals. Thus, the strand displacement reaction is a very useful technique that provides straightforward solutions for the analysis of various objectives, such as nucleic acids and proteins. So far, the combination of simple DNA nanostructures and DNA strand displacement techniques is rare in the detection of mycotoxins, especially in the simultaneous detection of multiple mycotoxins.
In this study, a fluorescent aptasensor based on DNA dual-labeled nanostructure was constructed using simple DNA nanostructures for simultaneous detection of OTA and AFB1. In the beginning, C1 labeled by Cy3 and Cy5 can simultaneously hybridize with C2 labeled by BHQ2 (C2-BHQ2) and C3 labeled by BHQ2 (C3-BHQ2). And the fluorescence intensity of DNA nanostructure composed of C1, C2 and C3 is weak because of the fluorescence resonance energy transfer (FRET) between Cy3 and BHQ2, Cy5 and BHQ2. In the presence of OTA Aptamer (OTA-Apt) and AFB1 Aptamer (AFB1 -Apt), OTA-Apt and AFB1-Apt respectively combine with C2 and C3 by toehold-mediated chain replacement, so C1 labeled by Cy3 and Cy5 can be replaced, resulting in enhanced fluorescence intensity. In the presence of OTA and AFB1, owing to the preferential binding between OTA and OTA-Apt, and the preferential binding between AFB1 and AFB1-Apt, toehold displacement reaction is inhibited. Then C1 labeled by Cy3 and Cy5 can combine with C2-BHQ2 and C3-BHQ2, then the fluorescence intensity has a significant decrease.
Experimental Section
Materials
All HPLC-purified DNA sequences were synthesized by Shanghai Sangon. The detailed sequences are provided in Table 1. Among them, M1 and N1 are part of C1, which are complementary to C2 and C3, respectively. AFB1, OTA, zearalenone (ZEN) and deoxynivalenol (DON) were purchased from Sigma-Aldrich. Fumonisin B1 (FB1) was provided by Acros Organics. Wine and corn were obtained from a local supermarket in Zhengzhou, China. All the chemicals were of analytical grade and all aqueous solutions were prepared via ultra-pure water (18.25 MΩ).
Preparation of Dual-labeled DNA Nanostructure
Before the experiment, all containers were sterilized and the DNA was dissolved using 20 mM Tris buffer solution (20 mM Tris, 50 mM NaCl, 50 mM MgCl2, pH 8.0). The dual-labeled DNA nanostructure was prepared based on the complementary pairing via the denaturation annealing process. Briefly, 10 μL of C1, C2 and C3 at a concentration of 2 μM were stirred in the PCR tube. The mixtures were put into a PCR instrument and heated to 95 ℃ for 10 min. Then, the DNA cooled to 4 ℃ at a rate of 1 ℃/min. After 20 min at 4℃, the dual-labeled DNA nanostructure was prepared.
Simultaneous Determination of OTA and AFB1
The analytical methods of OTA and AFB1 based on dual-labeled DNA nanostructure are as follows: 10 μL of 2 μM OTA-Apt and AFB1-Apt, 10 μL of OTA and AFB1 at different concentrations were added to the prepared aptasensor, respectively. After that, the mixture was placed at 37℃ for 30 min and supplemented to 200 μL with Tris–HCl solution. And the excitation scanning was measured by a Hitachi F-7100 fluorescence spectrophotometer. The slit width of excitation and emission were set at 5 nm, the voltage was set at 950 V, and the excitation wavelength was set at 530 nm (Cy3) and 635 nm (Cy5), respectively. The selectivity of the aptasensor to OTA and AFB1 was evaluated by monitoring the relative fluorescent changes at 570 nm (Cy3) and 670 nm (Cy5) to 100 ng/mL other mycotoxins (FB1, DON, ZEN) and their mixtures (OTA, AFB1, FB1, ZEN and DON). All experiments were measured at least three times.
OTA and AFB1 Detection in Real Samples
The corn and wine were pretreated for OTA and AFB1 detection in real samples [19]. Firstly, corn was ground into powder (1.0 g), and 1 mL of OTA and AFB1 solution (OTA and AFB1 concentrations were 10 ng/mL, 100 ng/mL and 1000 ng/mL, respectively) was added. After drying at room temperature, the extraction solvent (9 mL, methanol: water, 7:3 [v/v]) was added and stirred with vibration for 30 min. The actual concentrations of toxins in real samples at this time were 1 ng/mL, 10 ng/mL and 100 ng/mL. After that, the solutions were centrifuged at 5000 rmp for 10 min, and the supernatants were filtered by a 0.22 μm filter, and then diluted to 100 mL with 20 mM Tris buffer. At this time, different concentrations of OTA and AFB1 (0.1 ng/mL, 1 ng/mL, 10 ng/mL) in corn samples were obtained. Furthermore, 5.0 mL wine was mixed with different concentrations of OTA and AFB1 (OTA and AFB1 concentrations were 10 ng/mL, 100 ng/mL and 1000 ng/mL, respectively) solution and filtered with a 0.22 μm filter membrane. Then the solution was diluted to 100 mL with 20 mM Tris buffer. In the end, different concentrations of OTA and AFB1 (0.1 ng/mL, 1 ng/mL, 10 ng/mL) were obtained from wine samples. The spiked samples can be used for real sample detection.
Results and Discussion
Design of the Fluorescent Aptasensor
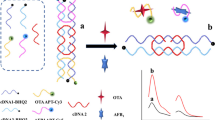
The principle of the proposed fluorescent aptasensor was displayed in Scheme 1. C1 is labeled with Cy3 and Cy5, while C2 and C3 are labeled with BHQ2. When the self-assembly of the dual DNA labeled nanostructure is completed, the distance of Cy3 and BHQ2, Cy5 and BHQ2 are extremely close, resulting in the suppression of their fluorescence signal. When OTA-Apt and AFB1-Apt are added to the homogeneous system, OTA-Apt and AFB1-Apt can respectively combine with C2 and C3 by toehold-mediated chain replacement. At this time, Cy3 and Cy5 are far away from BHQ2, and the fluorescence signal is recovered. In the presence of OTA and AFB1, OTA and AFB1 preferentially combine with their respective aptamers to form complexes due to the affinity between the aptamer and the target. This phenomenon inhibits toehold-mediated chain replacement reaction. Then the C2 and C3 in the DNA dual-labeled nanostructure cannot be replaced, and the fluorescence signals of Cy3 and Cy5 are inhibited.
Schematic illustration of the formation of fluorescent aptasensor based on dual-labeled DNA nanostructure. The two-dimensional structure of OTA aptamer (A) and AFB1 aptamer (B) at 37 ℃ were predicated by NUPACK. (C) The working principle of the fluorescent aptasensor for the simultaneous detection of OTA and AFB1 was based on a dual-labeled DNA nanostructure
To simulate the reaction among DNA strands, the secondary structures of C1, C2, C3 OTA Apt and AFB1 Apt at 37℃were shown in Fig. 1A, which are predicted by the UNPACK online website (http://www.nupack.org/). The free energy and secondary structures of dual-strand DNA are also shown in Fig. 1B, C. Since the free energy of the dual-strand DNA formed by C1 and C2 ((a) in Fig. 1B), M1 and C2 ((b) in Fig. 1B) are higher than that of the dual-strand DNA formed by C2 and OTA-Apt ((c) in Fig. 1B), C2 will react with OTA-Apt preferentially, thus making C1 fall off. Similarly, because the free energy of C1 and C3 ((a) in Fig. 1C), N1 and C3 ((b) in Fig. 1C) forming dual chains are higher than that of C3 and AFB1 -Apt ((c) in Fig. 1C) forming double chains, C3 will preferentially react with AFB1-Apt, resulting in C1 shedding. Therefore, theoretically speaking, the addition of OTA-Apt and AFB1-Apt will cause C1 be replaced, thus destroying the nanostructures formed by C1, C2 and C3.
A The secondary structures of a C1, b C2, c C3, d OTA-Apt and e AFB1-Apt at 37℃; B the free energy and secondary structures for formed double strands of a C1 + C2, b M1 + C2, c OTA-Apt + C2 at 37℃; C the free energy and secondary structures for formed double strands of a C1 + C3, b N1 + C3, c AFB1-Apt + C3 at 37℃
To study the feasibility of this fluorescent aptasensor, the effect of OTA and AFB1 on fluorescence intensity was investigated under optimized conditions. Figure 2A showed a high fluorescence intensity due to the weak FRET between BHQ2-Cy3/Cy5, with little change in fluorescence intensity at 570 nm and 670 nm with the addition of target-free buffer solution. In the presence of OTA, the fluorescence intensity was decreased by 57% at 570 nm and remained unchanged at 670 nm (Fig. 2B). Similarly, in the presence of AFB1, the fluorescence intensity was reduced by half at 670 nm and remained constant at 570 nm (Fig. 2C). When OTA and AFB1 were present simultaneously, the fluorescence intensity of the aptasensor decreased significantly at 570 nm and 670 nm due to the separation of aptamer from DNA nanostructure by target (Fig. 2D). The above results indicated that the fluorescent aptasensor can be used for the simultaneous detection of OTA and AFB1.
The construction of the aptasensor was characterized by 3.5% agarose gel electrophoresis. As shown in Fig. 3A, all DNA nanostructures and strands can be displayed in the corresponding lanes. The assembled dual-labeled DNA nanostructure showed the lowest migration in lane 1, demonstrating the successful formation of the aptasensor. Owning 67 bases, C1 showed the slower move in lane 2. Since C2 and C3 were short chains with only 26 and 23 bases, respectively, the migration rate was fast and ran at the front end. At the same time, because they were single chains and have few bases, there were few dyes bound and only weak light. The above gel electrophoresis results indicated that the self-assembly of dual-labeled DNA nanostructure was successful.
The agarose gel electrophoresis characterization of A C1 + C2 + C3 (lane 1), C1 (lane 2), C2 (lane 3), C3 (lane 4) and B C1 + C2 + C3 (lane 1), C1 + C2 + C3 + OTA aptamer + AFB1 aptamer (lane 2), C1 + C2 + C3 + OTA aptamer + AFB1 aptamer + OTA + AFB1 (lane 3); the concentrations of all ssDNA were 10 μM
In Fig. 3B, lane 1 represents the dual-labeled DNA nanostructure. In the presence of OTA-aptamer and AFB1-aptamer, the gel electrophoresis result was shown in lane 2 of Fig. 3B. At this time, there are still a few dual-labeled DNA nanostructure in the system, so the migration rate of the first band is the same as that of lane 1. However, the second band in lane 2 was relatively fast than lane 1, which was due to the fact that OTA aptamer and AFB1 aptamer form double chains with C2 and C3, respectively. Lane 3 represents the result when OTA and AFB1 are present at the same time, the first band of lane 3 migrated at the same rate as lane 1. This is because OTA and AFB1 form complexes with aptamers respectively, resulting in the presence of a large number of double-labeled DNA nanostructures in the system.
In conclusion, the above electrophoresis results indicated that the aptasensor was successfully prepared and can be successfully used for OTA and AFB1 detection.
Optimization of Detection Conditions
As shown in Fig. 4, the incubation time for C1, C2, and C3 and the reaction time of OTA and AFB1 were optimized. With the increase of incubation time for C1, C2 and C3, the quenching effect of BHQ2 on Cy3 and Cy5 on C1 increased, and the fluorescence signal gradually decreased. When the incubation time reached 80 min, it no longer decreases, so 80 min was optimal at this time. With the increase in reaction time, the fluorescent intensity gradually increased and then became stabilized. When the reaction time reached 30 min, the maximum change indicated the optimal reaction time.
The fluorescent intensity of Cy3 (A) and Cy5 (B) at different incubation times for C1, C2, and C3; (C) the fluorescent intensity after adding OTA at different reaction times (10 min, 20 min, 30 min, 40 min, 50 min); (D) the fluorescent intensity after adding AFB1 at different reaction time (10 min, 20 min, 30 min, 40 min, 50 min) [COTA = CAFB1 = 5 ng/mL] (n = 3)
Simultaneous detection of OTA and AFB1 using fluorescent aptasensors
As depicted in Fig. 5A, the fluorescence intensity at 570 nm decreased with increasing concentrations of OTA in the range of 0.01 to 500 ng/mL. The logarithm of OTA concentration displayed an excellent linear relationship with F0-F (\({F}_{0}\) and \(F\) are the emission intensities of Cy3 in the absence and presence of OTA) (Fig. 5B). The corresponding linear equation was calculated to be y = 645.3x + 1516, with a correlation coefficient of R2 = 0.998 in the range of 0.01 to 100 ng/mL. The LOD of OTA was calculated to be 0.007 ng/mL. In addition, the presence of AFB1 reduced the fluorescence intensity at 670 nm (Fig. 5C). In the range of 0.1–100 ng/mL, F0-F increased with the increase of AFB1 concentration, and the linear equation was y = 146.3x + 261, R2 = 0.996 (Fig. 5D). The LOD was 0.03 ng/mL (S/N = 3). To further highlight the performance of the fluorescence aptasensor, Table 2 summarizes the comparison of different analytical methods for the detection of OTA and AFB1. The proposed fluorescence aptasensor can realize the simultaneous detection of OTA and AFB1, and has a wider detection range and lower LOD compared with other analytical methods. Also, as compared with other aptasensors, the experimental steps are simpler and the total preparing time is shorter, confirming the convenient, rapid and time-saving operation process.
Representative fluorescence emission spectra of aptasensor at different concentrations of OTA (0.01–500 ng/mL) (A) and AFB1 (0.1–500 ng/mL) (C). The corresponding calibration plot of relative fluorescence intensity toward OTA (B) and AFB1 (D) concentrations (n = 3). Inset: The linear relationship of relative fluorescence intensity toward the logarithm of OTA and AFB1 concentration
Furthermore, the selectivity of the aptasensor was evaluated by comparing the fluorescence changes of different mycotoxins under the same conditions. Figure 6 showed that the F0-F significantly increased in the existence of OTA, AFB1 and the corresponding mixtures. However, the F0-F changed little when the interfering mycotoxins including FB1, ZEN and DON existed. Moreover, the F0-F between the target and the mixture was similar, indicating that there was no interaction between OTA and AFB1. This was mainly ascribed to the high affinity between the aptamer and its target. These results demonstrate that the fluorescent aptasensor has a broad application prospect in the simultaneous detection of OTA and AFB1 in food.
Practical detections for OTA and AFB1
In practical applications, this fluorescent aptasensor was applied to determine the concentrations of OTA and AFB1 in corn and wine samples. The recoveries of OTA and AFB1 in the corn sample ranged from 96 to 101% and 97% to 101% respectively, and those in the wine sample were 99% to 101% and 92% to 106% (Table 3). All of these results indicated that the fluorescent aptasensor detection system has obvious advantages over other methods, which can be used as an alternative method for the practical application of OTA and AFB1.
Conclusions
In summary, a fluorescent aptasensor based on the dual-labeled DNA nanostructure was successfully developed for the simultaneous detection of OTA and AFB1. The stable dual-labeled DNA nanostructure is assembled from C1, C2 and C3, which can realize the detection of OTA and AFB1 by comparing the fluorescence changes before and after the addition of the targets. In addition, with the help of toehold strand displacement, the fluorescence resonance energy transfer is effectively blocked, resulting in a change in the fluorescence signal. Moreover, due to the high selectivity of the OTA and AFB1 aptamers, the aptasensor presents an excellent specificity. The aptasensor can also be applied to the analysis of OTA and AFB1 in the complicated matrix (corn and wine samples). For the preparation of the aptasensor, the experimental steps are simpler and the total preparation time is shorter, confirming the convenient, rapid and time-saving operation process. In conclusion, the rational design of this fluorescent aptasensor provides a promising method for the simultaneous detection of multiple mycotoxins and promotes the application of aptamer in sensors.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sun S, Zhao R, Xie Y, Liu Y (2019) Photocatalytic degradation of aflatoxin B1 by activated carbon supported TiO2 catalyst. Food Control 100:183–188. https://doi.org/10.1016/j.foodcont.2019.01.014
Tittlemier SA, Cramer B, Dall’Asta C, Iha MH, Lattanzio VMT, Malone RJ, Maragos C, Solfrizzo M, Stranska-Zachariasova M, Stroka J (2019) Developments in mycotoxin analysis: an update for 2017–2018. World Mycotoxin J 12(1):3–29. https://doi.org/10.3920/wmj2018.2398
Kolpin DW, Schenzel J, Meyer MT, Phillips PJ, Hubbard LE, Scott T-M, Bucheli TD (2014) Mycotoxins: Diffuse and point source contributions of natural contaminants of emerging concern to streams. Sci Total Environ 470:669–676. https://doi.org/10.1016/j.scitotenv.2013.09.062
Van Pamel E, Verbeken A, Vlaemynck G, De Boever J, Daeseleire E (2011) Ultrahigh-Performance Liquid Chromatographic-Tandem Mass Spectrometric Multimycotoxin Method for Quantitating 26 Mycotoxins in Maize Silage. J Agric Food Chem 59(18):9747–9755. https://doi.org/10.1021/jf202614h
Sun S, Zhao R, Xie Y, Liu Y (2021) Reduction of aflatoxin B1 by magnetic graphene oxide/TiO2 nanocomposite and its effect on quality of corn oil. Food Chem. https://doi.org/10.1016/j.foodchem.2020.128521
Liu C, Cao Y, Zhao T, Wang X, Fang G, Wang S (2021) A Novel Multi-purpose MIP for SPE-HPLC and QCM Detection of Carbaryl Residues in Foods. Food Anal Methods 14(2):331–343. https://doi.org/10.1007/s12161-020-01875-4
Xue X, Selvaraj JN, Zhao L, Dong H, Liu F, Liu Y, Li Y (2014) Simultaneous Determination of Aflatoxins and Ochratoxin A in Bee Pollen by Low-Temperature Fat Precipitation and Immunoaffinity Column Cleanup Coupled with LC-MS/MS. Food Anal Methods 7(3):690–696. https://doi.org/10.1007/s12161-013-9723-4
Khoshbin Z, Abnous K, Taghdisi SM, Verdian A, Sameiyan E, Ramezani M, Alibolandi M (2022) An ultra-sensitive dual-responsive aptasensor with combination of liquid crystal and intercalating dye molecules: A food toxin case study. Food Chem. https://doi.org/10.1016/j.foodchem.2022.132265
Khoshbin Z, Abnous K, Taghdisi SM, Verdian A (2021) Liquid crystal-based biosensors as lab-on-chip tools: Promising for future on-site detection test kits. TrAC, Trends Anal Chem 142. https://doi.org/10.1016/j.trac.2021.116325
Zhao F, Tian Y, Shen Q, Liu R, Shi R, Wang H, Yang Z (2019) A novel nanobody and mimotope based immunoassay for rapid analysis of aflatoxin B1. Talanta 195:55–61. https://doi.org/10.1016/j.talanta.2018.11.013
Wang Y-K, Yan Y-X, Li S-Q, Wang H-a, Ji W-H, Sun J-H (2013) Simultaneous Quantitative Determination of Multiple Mycotoxins in Cereal and Feedstuff Samples by a Suspension Array Immunoassay. J Agric Food Chem 61(46):10948–10953. https://doi.org/10.1021/jf4036029
Seok Y, Byun J-Y, Shim W-B, Kim M-G (2015) A structure-switchable aptasensor for aflatoxin B1 detection based on assembly of an aptamer/split DNAzyme. Anal Chim Acta 886:182–187. https://doi.org/10.1016/j.aca.2015.05.041
Zhu C, Liu D, Li Y, Shen X, Ma S, Liu Y, You T (2020) Ratiometric electrochemical aptasensor for ultrasensitive detection of Ochratoxin A based on a dual signal amplification strategy: Engineering the binding of methylene blue to DNA. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2019.111814
Iliuk AB, Hu L, Tao WA (2011) Aptamer in Bioanalytical Applications. Anal Chem 83(12):4440–4452. https://doi.org/10.1021/ac201057w
Yang C, Wang Y, Marty J-L, Yang X (2011) Aptamer-based colorimetric biosensing of Ochratoxin A using unmodified gold nanoparticles indicator. Biosens Bioelectron 26(5):2724–2727. https://doi.org/10.1016/j.bios.2010.09.032
Khoshbin Z, Moeenfard M, Abnous K, Taghdisi SM (2023) Nano-gold mediated aptasensor for colorimetric monitoring of acrylamide: Smartphone readout strategy for on-site food control. Food Chem. https://doi.org/10.1016/j.foodchem.2022.133983
Wei M, Xin L, Feng S, Liu Y (2020) Simultaneous electrochemical determination of ochratoxin A and fumonisin B1 with an aptasensor based on the use of a Y-shaped DNA structure on gold nanorods. Microchim Acta 187(2). https://doi.org/10.1007/s00604-019-4089-y
Ren W, Pang J, Ma R, Liang X, Wei M, Suo Z, He B, Liu Y (2022) A signal on-off fluorescence sensor based on the self-assembly DNA tetrahedron for simultaneous detection of ochratoxin A and aflatoxin B1. Anal Chim Acta. https://doi.org/10.1016/j.aca.2022.339566
Suo Z, Liang X, Jin H, He B, Wei M (2021) A signal-enhancement fluorescent aptasensor based on the stable dual cross DNA nanostructure for simultaneous detection of OTA and AFB. Anal Bioanal Chem 413(30):7587–7595. https://doi.org/10.1007/s00216-021-03723-8
Huang R, He N, Li Z (2018) Recent progresses in DNA nanostructure-based biosensors for detection of tumor markers. Biosen Bioelectron 109:27–34. https://doi.org/10.1016/j.bios.2018.02.053
Gong X, Zhou W, Li D, Chai Y, Xiang Y, Yuan R (2015) RNA-regulated molecular tweezers for sensitive fluorescent detection of microRNA from cancer cells. Biosen Bioelectron 71:98–102. https://doi.org/10.1016/j.bios.2015.04.003
Xiong Z, Wang Q, Xie Y, Li N, Yun W, Yang L (2021) Simultaneous detection of aflatoxin B1 and ochratoxin A in food samples by dual DNA tweezers nanomachine. Food Chem. https://doi.org/10.1016/j.foodchem.2020.128122
Han B, Fang C, Sha L, Jalalah M, Al-Assiri MS, Harraz FA, Cao Y (2021) Cascade strand displacement reaction-assisted aptamer-based highly sensitive detection of ochratoxin A. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127827
Park Y, Lee CY, Kang S, Kim H, Park KS, Park HG (2018) Universal, colorimetric microRNA detection strategy based on target-catalyzed toehold-mediated strand displacement reaction. Nanotechnology 29(8). https://doi.org/10.1088/1361-6528/aaa3a3
Song X, Ding Q, Pu Y, Zhang J, Sun R, Yin L, Wei W, Liu S (2021) Application of the Dimeric G-Quadruplex and toehold-mediated strand displacement reaction for fluorescence biosensing of ochratoxin A. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2021.113537
Yin J, Liu Y, Wang S, Deng J, Lin X, Gao J (2018) Engineering a universal and label-free evaluation method for mycotoxins detection based on strand displacement amplification and G-quadruplex signal amplification. Sens Actuators B 256:573–579. https://doi.org/10.1016/j.snb.2017.10.083
Bader A, Cockroft SL (2020) Conformational enhancement of fidelity in toehold-sequestered DNA nanodevices. Chem Commun 56(38):5135–5138. https://doi.org/10.1039/d0cc00882f
Zhu X, Kou F, Xu H, Han Y, Yang G, Huang X, Chen W, Chi Y, Lin Z (2018) Label-free ochratoxin A electrochemical aptasensor based on target-induced noncovalent assembly of peroxidase-like graphitic carbon nitride nanosheet. Sens Actuators B 270:263–269. https://doi.org/10.1016/j.snb.2018.05.048
Bonel L, Vidal JC, Duato P, Castillo JR (2011) An electrochemical competitive biosensor for ochratoxin A based on a DNA biotinylated aptamer. Biosens Bioelectron 26(7):3254–3259. https://doi.org/10.1016/j.bios.2010.12.036
Wang Y, Hu X, Pei Y, Sun Y, Wang F, Song C, Yin M, Deng R, Li Z, Zhang G (2015) Selection of phage-displayed minotopes of ochratoxin A and its detection in cereal by ELISA. Anal Methods 7(5):1849–1854. https://doi.org/10.1039/c4ay02290d
Lai W, Guo J, Wu Q, Chen Y, Cai Q, Wu L, Wang S, Song J, Tang D (2020) A novel colorimetric immunoassay based on enzyme-regulated instant generation of Turnbull’s blue for the sensitive determination of ochratoxin A. Analyst 145(6):2420–2424. https://doi.org/10.1039/c9an02447f
Huang L, Chen K, Zhang W, Zhu W, Liu X, Wang J, Wang R, Hu N, Suo Y, Wang J (2018) ssDNA-tailorable oxidase-mimicking activity of spinel MnCo2O4 for sensitive biomolecular detection in food sample. Sens Actuators B 269:79–87. https://doi.org/10.1016/j.snb.2018.04.150
Liu R, Li W, Cai T, Deng Y, Ding Z, Liu Y, Zhu X, Wang X, Liu J, Liang B, Zheng T, Li J (2018) TiO2 Nanolayer-Enhanced Fluorescence for Simultaneous Multiplex Mycotoxin Detection by Aptamer Microarrays on a Porous Silicon Surface. ACS Appl Mater Interfaces 10(17):14447–14453. https://doi.org/10.1021/acsami.8b01431
Wu K, Ma C, Zhao H, Chen M, Deng Z (2019) Sensitive aptamer-based fluorescene assay for ochratoxin A based on RNase H signal amplification. Food Chem 277:273–278. https://doi.org/10.1016/j.foodchem.2018.10.130
Goud KY, Catanante G, Hayat A, Satyanarayana M, Gobi KV, Marty JL (2016) Disposable and portable electrochemical aptasensor for label free detection of aflatoxin B1 in alcoholic beverages. Sens Actuators B 235:466–473. https://doi.org/10.1016/j.snb.2016.05.112
Goud KY, Hayat A, Catanante G, Satyanarayana M, Gobi KV, Marty JL (2017) An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim Acta 244:96–103. https://doi.org/10.1016/j.electacta.2017.05.089
Migliorini FL, dos Santos DM, Soares AC, Mattoso LHC, Oliveira Jr ON, Correa DS (2020) Design of a low-cost and disposable paper-based immunosensor for the rapid and sensitive detection of aflatoxin B1. Chemosensors 8(3). https://doi.org/10.3390/chemosensors8030087
Lerdsri J, Chananchana W, Upan J, Sridara T, Jakmunee J (2020) Label-free colorimetric aptasensor for rapid detection of aflatoxin B1 by utilizing cationic perylene probe and localized surface plasmon resonance of gold nanoparticles. Sens Actuators B. https://doi.org/10.1016/j.snb.2020.128356
Guo M, Hou Q, Waterhouse GIN, Hou J, Ai S, Li X (2019) A simple aptamer-based fluorescent aflatoxin B1 sensor using humic acid as quencher. Talanta. https://doi.org/10.1016/j.talanta.2019.120131
Wei M, Zhao F, Xie Y (2020) A novel gold nanostars-based fluorescent aptasensor for aflatoxin B1 detection. Talanta. https://doi.org/10.1016/j.talanta.2019.120599
Funding
This study was funded by the Key Scientific and Technological Project of Henan Province (212102310001), the Special Project for Collaborative Innovation of Zhengzhou (21ZZXTCX15), and the Innovative Funds Plan of Henan University of Technology (2021ZKCJ13).
Author information
Authors and Affiliations
Contributions
Luyang Zhao: Investigation, Methodology, Validation and Writing-Original Draft. Xiujun Liang: Validation and Writing-Original Draft. Yong Liu: Supervision, Resources. Min Wei: Conceptualization, Methodology, Resources, Supervision and Funding acquisition. Huali Jin: Methodology, Resources, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, L., Liang, X., Liu, Y. et al. A Novel Fluorescent Aptasensor Based on Dual-labeled DNA Nanostructure for Simultaneous Detection of Ochratoxin A and Aflatoxin B1. J Fluoresc 33, 1641–1650 (2023). https://doi.org/10.1007/s10895-022-03071-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03071-5