Abstract
Colorimetric and ratiometric fluorescent probe for cations gain very well attention by the chemist, biologist and environmentalist. Metals has two sides, first is biolgical active for living creature and toxic nature for the ecosystem. From last three decades the scientists are contiously trying to find out the best solution for the detection of cations at micro as well as nanomolar levels. In the present review we discussed the colorimetric and ratiometric fluorescent probe synthesized by the authors in almost half decade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sensing of cations is gaing more attention by many scientists, including chemists, biologists, and environmentalists. Metals are involved in many vital biological course of action such as transmission, muscle contraction, cell activity, etc. [1,2,3,4,5]. Metals play a crucial role in cell functions. It is involved in electron transfer processes in DNA and RNA synthesis by facile redox chemistry and its high affinity for oxygen [6,7,8,9,10,11,12,13].
Several methods such as F-AAS, AAS, ICP emission spectrometry, spectrophotometry, voltammetry and electrochemical stripping analysis have been developed for detecting metal ions [14,15,16,17,18,19,20]. Conventional process has good accuracy but needed expensive instrument and tedious process for detection, and very less applicability in situ analysis [21,22,23,24,25,26]. UV–visible and fluorescence spectroscopy is most favorable modes over other common technique for the detection of environmental significant samples because its concentration is very less in nature [27,28,29,30,31].
Among various photophysical pathways like Charge Transfer (CT), Electron Transfer (ET), Förster Resonance Energy Transfer (FRET), are generally utilized for reporting the binding induced phenomena [32,33,34,35,36,37,38,39]. In last few decates, many authors sythesise the sensor for the sensing of metal ions and susesfully reported its biological application.
Need for Chemosensors
In the last few decades, the awareness regarding the significance and toxic effect of cation is well understood by the scientist in the field of chemistry, biology and environment [40,41,42]. Various heavy metal ions uses are banned by various international agencies, because of its toxic nature, nonbiodegradable and hence can accumulate in the environment and foodchain [43,44,45,46,47,48,49].
Today, scientist and chemist are trying to develop a cation chemical sensor which can be used for analysis of environmental sample and industrial sample. On the other hand the biosensor is used in medicine and biological application.
Chemosensors for Cations
1 was developed by pyridine-2-hydrozine which shows 7.63-fold fluorescent enhancement for Zn2+ in CH3CN/HEPES(v/v 1:1) [50]. 1:1 complexation formation supported using HRMS. The association constant 1.83 × 105M−1 and LOD for Zn2+ ion 2.2 × 10− 6M and effectively useful in the cell-imaging of Zn2+. 2-((benzylimino)-methyl)-naphthalen-1-ol (2) shows discraminating emmision for Cu2+ and Zn2+ under ACN [51]. The binding constant for Cu2+ and Zn2+ (6.55 ± 0.8) × 103 M− 1 and (4.32 ± 0.8) × 104M−1 respectively and free energy change calculated − 26.44 kJmol− 1 for Zn2+ and − 21.77 kJmol− 1 for Cu2+, negative free energy indictates the thermodynamic feasibility. Probe 3 [52] selective for Au3+ by discriminant colour transform from yellow-to-pink in PBS 4% ethanol pH = 7.4 (Fig. 1). The LOD was determined to be 8.44 × 10− 6M and cell imaging response rates of 3 to Au3+ in differentiated adipocytes are greater than HeLa cells, because lipid droplets in adipocytes act as surfactants.

Left: Au3+ ion-induced (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0 equiv) changes in the fluorescence spectrum of 3 (20 µM) in PBS buffer containing 4% ethanol at pH 7.4 in the presence of CTAC (50 µM). Right: Fluorescence response of 3 (20 µM) to various metal ions (5 equiv) in PBS buffer containing 4% ethanol at pH 7.4 in the presence of CTAC (50 µM). All the fluorescence data were obtained after 60 min incubation of 3 with metal ions (λex = 364 nm, slit: 3 nm/3 nm). ‘Reprinted from reference number 52 with permission of Elsevier publication’
4a and 4b [53] absorption data shows a yellow solution to orange-red solution on Hg2+ (λabs.max = 506→532 nm) addition with “naked eye” detection at concentrations 10− 6M. Cellular neurobiological studies were then undertaken extremely low cell toxicity is also evident with this probe. 5a and 5b detect Hg2+ with change of colour from colourless to pink, visible to the naked eye [54]. The quantum yield for 5a and 5b was (Φ = 0.004 and 0.0034), 5a.Hg2+ and 5b.Hg2+ was (Φ = 0.68 and 0.46) and LOD of 5a and 5b 50 × 10− 9M and 10 × 10− 8M respectively. Rhodamine-naphthalimide derivative sensor 6 was used for effective sensing of Cu2+ with large red shift (115 nm) [55]. The detection limit was 3.88 × 10− 7M for Cu2+ and Cytotoxicity testing in MCF-7 cells shows that 6 is almost nontoxic to living cells (Fig. 2).


A cyano-rhodamine moiety 7 easily undergoes selective Pd2+ induced ‘C–CN’ bond breaking to produce the pink coloration of rhodamine with an 88-fold enhancement of quantum yield [56]. Sensor 7 biological applicability shown by the cell imaging of Pd2+ on HeLa-cells and LOD of the Pd2+ is found to be 0.57 µM. Cyclen based receptor 8 was developed for the recoginition of Zn(II) with enhancement in fluoresecnec spectra [57]. In MeOH, 8 is poorly fluorescent (ϕ ≈ 0.4%) with addition of Zn2+, the fluorescence increases. Stiochiometric was 1:1 and 2:1 for Zn:8 complexes. Sikdar and its coauthors [58] synthesised a rhodamine-B-based 9 for detection of Cu2+ ion aqueous media and inside living cells. The 1:1 complex is confirmed, bindng constant is found at 2.5 × 104 and LOD at 1.22 µM. Pyrene-based 10 (ϕ ≈ 0.001) was synthesized as Fe3+ sensor in biological environment (ϕ ≈ 0.0.041) [59]. The 1:1 stiochiometry 10.Fe3+ is estimated by Job’s method and ESI-MS and association constant found to be 1.27 × 104M−1.


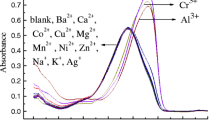
Wang et al. [60] synthesized 11 for Zn2+ sensing, with red fluorescence in methanol (λem ≈ 630 nm, Фfl.≈0.8). A probe 12 based on 2,2′,6′,2′′-terpyridine for the detection of Zn2+ (green) and Cd2+ (blue) [61]. The association constants (log Ka) of the probe 12 for Zn2+ and Cd2+ are found to be 3.75 and 3.62 respectively. The detection limit of 12 for Zn2+ and Cd2+ found to be 1.63 and 2.81 ppb respectively. Probe 13 shows colorimetric colour [62] and significant enhancment in absorbance (Fig. 3) change on the micromolar level addition of Cu2+ and the LOD at 7.27 × 10− 7M− 1.

Change in color of 10 µM 13 in CH3OH/HEPES (20 mM, pH7.2, 1:1,v/v) semiaqueous solution with 5-time cation from left to right: Cu2+, Hg2+, Ag+, Al3+, Fe3+, Cr3+, Co2+, Ni2+, Pb2+, Na+, K+, Ba2+, ca.2+, Cd2+, Zn2+, Mg2+ and only 13. ‘Reprinted from reference number 62 with permission of Elsevier publication’
Chawla et al.. synthesized 14 a calix[4]arene derivatives used for the detection of Cu2+ [63]. Analysis of the data obtained from Job’s-plot revealed the 1:1 molecular complexation. A peak at m/z 1170.5413 in ESI-MS data showed the formation of 1:1 complex [8 + Cu2++ClO4]+. The detection limit was found to be 6.02 × 10− 6M and association constant, value 1.655 × 106M − 1. Kuwar et al.. synthesized sensor 15 characterized by X-ray crystallography and explore its sensing ability towards Cu2+ [64]. 1:1 stoichiometry formed between 15 and Cu2+ and association constant calculated by B-H and S-P at (43000 ± 11)M− 1. Probe 16 based on 1,8-naphthalimide and rhodamine shows green-to-orange transformation with addition of Cr3+ in CH3CN–HEPES buffer solution. The LOD found to be at 0.14 nM and 1:2 complexation. 16 is successfully applied to detect Cr3+ in cell lysate and blood serum [65]. Chereddy et al.. synthesized the naphthalimide based probe (17) which demonstrated high emission intensity upon Fe3+ addition [66]. The binding constants and LOD were found to be 1.04 × 105M−1 and 3.0 × 10− 8M, respectively. The 17 is stable, nontoxic and ‘turn-on’ for the imaging of intracellular Fe3+ ions.


A TACN (1,4,7-triazacyclononane) derivatives of 18a and 18b which has ability to recognize the zinc ion [67]. The fluorescence quantum yield of 18a-Zn (ϕ = 0.070) is 2.6-fold greater than free 5 (ϕ = 0.027), and more than twice that of 18b-Zn (ϕ = 0.032) with 1:1 complexation. A 5-diethylamino-2-(quinolin-8-yliminomethyl)-Phenol probe (19) which is capable of detecting multiple ions, Mg2+ and Zn2+, fluorescence and Co2+ by UV/Vis [68]. The LOD for Mg2+ and Zn2+ was 70 nM and1.85 µM. The binding constant for the complex of Mg2+, Zn2+ and Co2+ was calculated to be 8.17 × 106M−2, 1.03 × 106M−2 and 1.78 × 1022M−2 respectively. Hu et al.. [69] developed a sensor 20 for the effective sensing of Hg2+ with limit of detection very low at 9.56 × 10− 9M and furthermore it is used as test kit for Hg2+ sensing. In 1H-NMR titration experiments chemical shifts of NH appearing at a low-field of the probe 20 at 13.21 ppm, and led to the sensor 20 of the conjugate rigid plane structure.

Fegade et al. developed probe 21 for selective detection of Cu2+ [70]. DFT indicates 21:Cu2+ showed the ICT, lesser the band-gap between the HOMO-LUMO of 21 which due to change in spectra (Fig. 4). The LOD was calculated at 50 nM and Kb at 11667M− 1. Rhodamine-B derivative probe 22 is colorimetric and fluorescent naked eye sensor for Hg2+ [71]. Quantum yield of 22 was < 0.05%, quantum yield of 22-Hg2+ was found to be 14.6%. The stoichiometric ratio is 1:3 and LOD found to be 0.75 ppb. A PET fluorescence chemodosimeter 23 was designed for Cu2+ detection [72]. Upon addition of Cu2+, 23 solution changed from pink to green and acts as a colorimetric chemodosimeter. The 1:1 stoichiometry estimated by Job’s plot and the LOD at 2.3 × 10− 7M. Roy et al.. [73] synthesized probe N,N/-bis(salicylidene)trans1,2–diaminocyclohexane (24) has chemoselective Zn2+ sensor. The quantum yield increases drastically from 3.6 × 10− 3 for 24 compared to 1.8 × 10− 1 for 24–Zn2+ complex. 1:1 complexation estimated by continous variation method and the Ka was at 3.7 × 104M−1. Rhodamine-B derivative sensor 25 was developed, which exhibits effective sensing for Hg2+ in ethanol solution [74]. 1:1 stoichiometry estimated by Job’s plot and LOD at 3.1 × 10− 6M. The filter paper in the present Cu2+ or Hg2+ showed a pink color change, only Hg2+ induced a strong yellow color change under UV lamp.



Probe 26, can selectively detect Co2+ in CH3OH/H2O (70:30,v/v) solution [75]. DFT study shows the tautomeric form of 26 found to be less stable by 11.37kcalmol− 1 and 26.Co2+ lowering of energy by -358.88kcalmol− 1 which indicates stable Complex. 1:1 complexation was determined using continous variation method and Ks at 50000M− 1. 27 exhibited a remarkable selectivity for Zn2+ and its absorbance at 256 nm gradually increased with addition of Zn2+. Fluorescence emission of 27 exhibits at 408 nm with Φ = 0.069 and with the addition of Zn2+ the 81 nm red-shift from 408 to 489 nm with Φ = 0.138. DFT shows the HOMO–LUMO energy gap for the 27–Zn complex (3.34 eV) is smaller than 27 (4.20 eV) [76]. Zhang et al. [77] developed chemosensor (28) for selective detection of Hg2+. In the UV-vis spectrum of 28 shows broad absorption from 405 to 490 nm vanished when Hg2+ were added. 28 emitted very weakly (λem = 428 nm; λex = 345 nm), demonstrating that the predictable fluorescence of the naphthalene units was quenched via transformation in ICT state. A probe, (E)-3-(3-(4-([2,2’:6’,2’’-terpyridin]-4’-yl)phenyl)acryloyl)-7-(diethylamino)-2H-chromen-2-one (29), used for sensing of Zn2+ with low LOD at 10 nM and also shows imaging of Zn2+ in cells as applications in cell-imaging [78]. DFT shows when Zn2+ coordinated with the 29, the electrons are located on the coumarin part in the ground state, but are rearranged to the part from carbonyl to the terpyridine group when excited, showing ICT effect. 30 had a high affinity and selectivity towards Zn2+ and shows detection of Zn2+ in intracellular environment of HeLa cell [79]. The detection limit for Zn2 at 35 nM and binding constant Ka was about (2.1 ± 0.3) × 105M−1 (Fig. 5).


A Control cells devoid of receptor 30 and Zn2+, B HeLa cells with receptor 30, C Fluorescence image with only receptor 30, D Fluorescence image with receptor 30 and Zn2+, E In higher magnification uptake of Zn2+ is visible [arrow head]. F Fluorescence image with only Zn. ‘Reprinted from reference number 79 with permission of Elsevier publication’
Mian Wang et al. synthesized “turn-on” chemosensor (31) for Pd2+ [80] with LOD calculated at 2.4 nM. 1:1. Job-plot and HRMS provided evidence for the formation of a 1:1complex of 31-Pd2+ (Figs. 6 and 7). Probe 32 demonstrated high selectivity by discriminating Al3+ over other metal ions and LOD at 21.6 nM [81]. Furthermore, to check its biocompatibility sensing of Al3+ has been done in live cells. Azodye–rhodamine based probe (33) has been designed [82] for selective detection of Pd2+ with 40-times increses in fluorescence by red-shift. The 1:1 stoichiometry determined by Job’s plot and LOD 0.45 µM at pH 7.4. Furthermore, the probe 33 used to image Pd2+ in living cells. A reversible fluorescent-colorimetric imino-pyridyl bis-Schiff base receptor 34 (N1E,N4E)-N1,N4-bis(pyridine-4-ylmethylene)benzene-1,4-diamine for the detection of Al3+ in aqueous medium [83]. The LOD at 0.903 µM for Al3+ is very low than the limit recommended by WHO (7.41 µM). 1H-NMR titration and MASS shows that the formation of 1:2 complexation. Zhiyuan Zhang et al. synthesised probe 35 and used for the effective detection of Cr4+[84]. The sensing mechanism was explored by reversibility and LC/MS, and the results suggested that the recognition was based on the oxidation of the primary alcohol in the structure of the sensor by the Cr4+ sources.


Yan-Cheng Wu et al. synthesized three bisbenzimidazole derivatives 36 (Fig. 8) as dual-functional fluorescent and visual sensors for effective sensing of Ag(I) and Fe(III) with a response time of 10 s and steadily work in wide pH 4–13 range. [85]. Jitendra Bhosale et al. explored the selctive sensing of Zn2+ cation by pyrrole-based derivative 37 [86]. The sensing behaviour has been supported by UV-vis absorption and DFT calculations indicating the formation of a 1:1 complex between the pyrrole based receptor 37 and Zn2+. The asprepared 37 was further used for cell-imaging. A phthalazine based sensor 38 was designed for sensitive detection of Co2+ in CH3CN–H2O with LOD at 25 nM. Sensor 38 show that change in colour from yellow to green with red-shifted from 383 nm to 435 nm in the presence of Co2+ [87]. The sensor 38 has capability to monitoring Co2+ in cell-imaging. Rahul Patil et al. developed probe 3-((2-(1H-benzo[d]imidazol-2-yl)phenylimino)methyl)benzene-1,2-diol (39) for selective sensing of Hg2+ with LOD low at 0.20 mM [88]. The emission spectrum of probe 39 was quenched on complexation with Hg2+ ion. Chemosensor, 2-((E)-(-2-aminophenylimino)methyl)-6-isopropyl-3-methylphenol (40), has been designed and confirmed by the single crystal X-ray [89]. The binding constant values for Ni2+ and Cu2+ were calculated to be 25000 and 30000M− 1 and detection limits of Ni2+ and Cu2+ were 100 and 50 nM.


Fluorescent receptor (41) was designed for the detection of Cu2+ and Zn2+ with 1:1 complexation and LOD at 5 nM and 15 nM for Cu2+ and Zn2+ ions, respectively. [90]. Furthermore, it is used as INHIBIT type logic gate at molecular level (Fig. 9). Di Zhou et al. synthesized highly selective fluorescent chemosensor for aluminum(III) ions in DMF [91]. The outcome of 1H-NMR titration, HRMS and density functional theory shows that 42-Al3+ form a 1:1 complexation. The Ka was calculated at 2.67 × 106 and the LOD was found at 10− 6M. Jie Cui et al. designed a fluorescent probe 43 which has selective recoginition ability for Pd2+ which have 21.3 nM LOD [92]. A selective fluorescent sensor 44 based on a pyrazoline derivate was synthesized and applied for the detection of Al3+ ion with 1:1 complexation via fluorescent quenching [93]. The Ka value obtained at 1.75 × 105M−1 and LOD found to be 2.27 × 10− 7M.


Lianqing Li et al. successfully developed a rhodamine derivative chemodosimeter which shows naked-eye fluorescent in the presence of Pd2+ [94] (Fig. 10). The detection limit of 45 at 10− 7M level and H-G ratio was found at 1:2 according via continuous variation jobs method. Rhodamine functionalized fluorogenic Schiff base 46 was synthesized [95] It exhibited highly selective colorimetric and “off-on” fluorescence response towards Al3+. Kb and LOD of Al3+ to 46 are calculated at 1.0 × 104M−1 and 1.4 × 10− 7M, respectively. Berberine (47), an important medicinal herb which effectively utilized as a sensing probe for silver ion [96]. The 47 is found to be selective towards Ag + with a detection limit of 0.1 × 10− 4 molL− 1. The effective quenching of 47 uponbinding with Ag+ ion is attributed to suppression of intramolecular charge transfer (ICT). Quinoline-base sensor 48 showed a highly selective fluorescent enhancement towards Mg2+[97]. The association constant of a 1:1 complex was determined as 1.91 × 107M−1 and the detection limit was determined as 19.1 ppb. Ahmadreza Bekhradnia et al. reported nitro-3-carboxamide coumarin derivatives (49), fopr selective sensing of Cu2+ in (HEPES:DMSO) 9:1,v/v) solution [98]. The emmision of 6-nitro-N-[2-(dimethylamino)ethyl]-2-oxo-2H-chromene-3-carboxamide enlarged on the adding up Cu2+ with stronger excitation at k = 320 nm than for the other cations tested.



A dye 50 (Fig. 11) is found to be highly specific in detecting of Al3 + ion with sensitivity of 0.19 mM [99]. Moreover, the dye shows moderately cytotoxicity and can be employed for the detection of intracellular concentration of Al3+ ions in living cells. The energy gap between HOMO and LUMO in the probe 50 and 50–Al3+ complexes are 2.6691 eV and 2.1089 eV respectively (Fig. 12). Xu Zheng et al. synthesized a bis(pyridine-2-ylmethyl)amine derivative 51 displays significant colorimetric and fluorescent changes upon binding of Cu2+ [100]. 51 has potential candidate for the Cu2+ sensing in aqueous solution and mammalian cells. Qi Huang et al. developed a ‘‘off–on’’ fluorescent sensor for the detection of Al3+ with a high sensitivity and detection limits of 0.23 and 1.90 lM [101]. The sensor 52 was used for the effective sensing of Al(III) furthermore it can be used as a bioimaging reagent for imaging of Al3+ in living cells.
A dicyanoisophorone-based turn-on chemodosimeter 53 has been synthesized to detect Cu2+ with significant color change [102]. The LOD of chemodosimeter 53 was calculated as low as 0.2 µM for Cu2+. The probe 53 was also successfully applied to fluorescence imaging of Cu2+ in HeLa cells. Enze Wang et al. synthesized 54 by condensation of 5-Hydroxymethylfurfural and rhodamine B hydrazide which indicate high selective and reversible colorimetric chemosensor for Cu2+ [103]. The high absorbance at 565 nm, molecular fraction close to 0.33, which exhibit the 1:2 complexation (Fig. 13).


Jing-Can Qin et al. synthesized 55a and 55b fluorescent probes for Al3+, upon addition of Al3+, they exhibit a large fluorescence enhancement by PET process [104]. More importantly, the lowest detection limits of the sensors for Al3+ were determined as 4 × 10− 8M and 8 × 10− 8M (Fig. 14). Vinod Kumar Gupta et al. synthesized probes 56a and 56b which displayed excellent “off-on” fluoregenic selectivity with Zn(II) [105]. The results revealed that the sensors provided colorimetric and fluoregenic sensing excellent response with low limit of detection, under neutral conditions. Hak-Soo Kim et al. designed 57 which displayed “OFF–ON–OFF” dual responce for the sensing of Cu2+ and Al3+ [106]. LOD of the 57 for Cu2+ and Al3+ were 4.726 × 10− 7 and 4.43 × 10− 7M, respectively. The 1:1 complexation was anticipated between 57and Cu2+/Al3+ by the 1H NMR binding studies.

Kandasamy Ponnuvel et al. designed chemosensor 58 which shows extremely good sensing ability towards Zn2+ ions (Fig. 15) [107]. 1:1 complexation formation is supported by Job’s plot and its can be employed for fluorescent imaging of Zn2+ (Fig. 16). Jiao Geng et al. developed 4,4,-n-butyl-5,5-(pyridin-4-yl)-2,2,-bithiazol (59) and it exhibits high senstivity toward Fe3+ with LOD at 0.6 µM. The Ka of [Fe592] is determined at 2.76 × 103M−2[108]. The living cell and zebrafish imaging experiments demonstrated its applicability in biological systems. Fluorenyl-diformyl phenol Schiff (60) base shows maximum intensity upon addition of Al3+ at 600 nm and the LOD is 6.22 × 10− 9M. [109]. Applicability for DSSC device fabrication of probe shows photovoltaic efficiency 0.021%. Probe N,N–bis((2–hydroxynaphthalen–1–yl)methylene)malonohydrazide (61), exhibited selective detection of Al3+ ions with a binding constant KB=5.74 × 109M−1 and detection limit 5.78 × 10− 8M [110]. The (612Al3+) complex mechanism was studied by DFT and cell-imaging study shows that the aluminium ion in cells can be detected by 61.

Chemosensor 62 displayed 16-time increases in fluorescence for selective detection of Mg2+ with limit of detections for Mg2+ at 10− 8M in DMSO:H2O (1:5v/v) medium [111]. Sensor 62 has ability to detect Mg2+ in cells (Fig. 17). The thioethers 63a and 63b have shown excellent selective recognition toward Hg2+ with detection limit 6.93 × 10− 7M and 4.79 × 10− 7M respectively [112]. Moreover, ferrocenyl-based sulphone 63c and 63d which exhibited more selective recognition toward Cu2+ and the detection limit values can reach 5.22 × 10− 7M and 4.97 × 10− 7M (Fig. 18). A pyridylvinyl-rhodamine-naphthalimide fluorescent probe 64, was synthesized which shows recognition ability towards Fe3+ and Hg2+ [113]. The 64 represented dual-channel behavior, with detection limit at 2.72 × 10− 8M and 9.08 × 10− 8M, and the Kd were calculated to be 4.95 × 10− 7M3/2 and 6.68 × 10− 8M3/2, respectively. The probe 65 only showed an enhancement and quenching (naked-eye colour change) in fluorescence for Hg2+ and Cu2+ respectively [114]. Additionally, the probe was effectively used in cell imaging, indicating its promising application in living cells. A rhodamine derivative (66) designed for the detection of Cd2+ with LOD of 1.025 × 10− 8M [115]. New emmision peak produce at 590 nm due to recognition of Cd2+ ions with 66 in a 1:1 cmplexation with a Kb of 4.2524 × 104M−1. 66 has exhibited extremely superior results in Cells imaging.


A Schiff-base 67 was synthesized and used for detection of Zn2+ with on the CHEF and PET mechanisms [116]. The UV–vis spectra of the Zn2+ complex exhibit four clear isosbestic points which may be assigned to the ONON moieties binding to Zn2+ and complex crystal characterized by X-ray crystallography (Fig. 19). Ujjal Ghosh et al. synthesized meta-di-4-methylpyridyl benzene 68 probe for Hg2+ detection [117]. The probe shows a dual fluorescence emission at long wavelength region (350, 425 nm) and theoretical calculation and NMR titration suggest that the probe binds Hg2+ through the coordination with two pyridyl nitrogens. A schiff base (69) based on 4,5- diazafluorene used for selective sensing of Al3+ ions and it shows 1312-time fluorescence enhancement when the Al3+ added in the 69 [118]. The LOD found to be at 3.7 × 10− 8M. A sensor (70) designed for the effective detection of Cu2+ through the PET mechanism [119]. The sensor showed “off–on” fluorescence response with a 120-fold increase toward Cu2+, and its limits of detection were 0.26 mM and 0.17 mM for UV-vis and fluorescence measurements, respectively.


Chemosensors (71) which shows excellent sensitivity for Hg(II) with LOD at 1.06µmolL− 1 [120]. 1:1 complexation is confirmed by 1H-NMR titration (Fig. 20) and interference study (Figs. 21) shows that the good capability of chemosensor for detction of Hg2+ in presence of other ions. Rui Yan et al. fabricated a biomimetic chemosensor, 72 used for the detection of zinc(II) and cell imaging [121]. Moreover, cytotoxicity and bio-imaging tests were conducted to study the potential bio-application of the chemosensor. Xianjiao Meng et al. synthesized chemosensor, ethyl(E)-2-((2-((2-(7-(diethylamino)- 2-oxo-2H-chromene-3-carbonyl)hydrazono)methyl)quinolin-8-yl)oxy)acetate (73), which showed an “on–off” fluorescence response to Pb2+ with a 1:1 complexation and LOD determined to be 0.5µmM [122]. A “off-on-off” probe (74) displayed the selective detection towards Hg2+ with Ka estimated to be 4.66 × 106 and LOD calculated as 2.64 × 10− 8M [123]. Furthermore, the HOMO-LUMO energy gaps of the complex are lower than the free probe (Figs. 22 and 23).

Somnath Khanra et al. synthesized imine and azine derivatives 75a, 75b, 75c and 75d, shows fluorescence turns ON for Zn2+ detection at nano-molar level. The LOD of 75a, 75b, 75c and 75d for Zn2+ are 32.66 nM, 36.16 nM, 15.20 nM and 33.50 nM respectively [124]. Pravat Ghorai et al. represented economic probe (76) for efficient detection of both Zn(II) and Cu (II) with the formation 1:1 complexation confirmed by Job’s Plot. The LOD values of both the ions are 2.29 × 10− 9M and 3.67 × 10− 9M, respectively. The probe used for Candida albicans cell fluorescence imaging [125]. Yingying Zhang et al. reported water-soluble probe 77 for the detection of Hg2+ in real water samples and showing changed from pale yellow to pink. Probe was confirmed to have low cytotoxicity and excellent cell membrane permeability [126]. Serkan Erdemir et al. developed Triphenylamine appended rhodamine (78) (Fig. 24) was built as a selective fluorescent probe for Al3+ and Hg2+ ions with 1:1 complexation confirmed by by job plot analysis. The LOD of 78 for sensing Al3+ and Hg2+ are down to 71.8 nM and 0.48 µM, respectively [127].


Kalyani Rout et al. fabricated a triazole-based probe (79) which have good sensing ability for Cu2+ and Pb2+ ions with the naked eye colour change. The 79 shows its potential application in real samples, living cells and building of molecular logic gate [128]. Yunfan Yang et al. developed fluorescence probe 80 for detecting Hg2+ and OCl− ions. The FMO and overlap between hole and electron analyses confirmed that the interaction between 80 and Hg2+ impeded the ESICT behavior [129]. Vishaka V. H et al. demonstrated a biocompatible fluorescent receptor (81) for detection of Fe3+ upto 8.2 nM LOD. Receptor imaging Fe3+ in cells is a significant increase towards biosensing and cytotoxicity studies also proved the nontoxic nature of this receptor [130]. Jae Min Jung et al. synthesized naphthol-based chemosensor 82 for the Zn2+ sensing through π→π* transition with a unique fluorescence enhancement (Fig. 25). We confirmed the sensing properties of 82 toward CN − and Zn2+ with theoretical calculation [131].

Yaping Zhang et al. synthesized a fluorescent receptor 83 by attaching a diarylethene molecule to a functional group for the detection of Al3+ and Zn2+ at detection limit is very low. Moreover, based on the properties of 83, we designed a logic circuit, and that also can be used for water sample testing [132]. Jeya Shree Ganesan et al. synthesized pyrazole bearing imidazole derivative 84 for the selective detection of Al3+/Fe3 + ions with 1:1 binding stoichiometry confirmed by Job’s plot. The LOD of 84 with Al3+/Fe3 + was calculated as 2.12 × 10− 7M and 1.73 × 10− 6M, respectively [133]. Pinkesh G. Sutariya et al. reported calix[4]arene conjugate bearing 1-aminoanthraquinone with amide linkage (85) recognize three metals La3+, Cu2+ and Br− with detection limit 0.88 nM for La3+, 0.19 nM for Cu2+ and 0.15 nM for Br− (Fig. 26) [134]. Akshay Krishna T G et al. developed isatin appended Schiff’s base probes (86a-86c) and characterized by spectroscopic techniques. The sensing ability of probe towards Hg2+ ions was established through UV-Visible techniques and achieved detection limit at ppm levels [135].



Suman Srivastava et al. synthesized a fluorescent probe 87 for the selective sensing for Fe3+ and Hg2+. “The LOD of 87 toward Fe3+ and Hg2+ has been found at 4.0 ppb and 1.0 ppb, respectively and also shows fluorescence signalling both in vitro and in vivo” [136]. Awad I. Said et al. developed rhodamine-pyrazole based probe 88 which has ability to detect the Cu2+, Fe3+, Al3+, Hg2+ and Ni2+ discriminately. Furthermore, the probe exhibited a high potential for logical operations and INHIBIT logic gates [137]. Jessica C. Berrones-Reyes et al. developed (S,E)-11-amino-8-((8-hydroxybenzylidene)amino)-11-oxopentanoic acid (89) receptor for the Zn2+ ions detection with LOD of 1.20 mM [138]. Barbara Panunzi et al. reported a complex of pyridyl/phenolic/benzothiazole functionalized ligand (90) with Zn(CH3COO). The structural and photoluminescence properties of the complex were investigated by X-ray diffraction and DFT study and LOD at 375 nM (Fig. 27) [139].


Shengling Li et al. reported highly selective probe 91 for the recognition of Cu2+ and HSO3− with detection limit of the sensor 91 was 0.36 mM to Cu2+ and 1.4 mM to HSO3− (Fig. 28) [140]. Yun-Qiong Gu et al. developed sensor 92, based on pyrazolopyrimidine for the simultaneous detection of Ni2+ and Cu2+ ions with LOD at 8.9 nM for Ni2+ and 8.7 nM for Cu2+ and fluorescence imaging in T-24 cells was investigated because of the low cytotoxicity of 92 [141]. Jianwei Xu et al. synthesized Ferrocene–based naphthalene receptors 93a and 93b and behaved as naked-eye receptor for Cu2+ and with low detection limit. Furthermore, 93a and 93b were nontoxicity and receptor 93a exhibited certain antibacterial activity (Fig. 29) [142]. Qiang Zhang et al. fabricated multi-response probe 94 for the detecting Al3+, Cu2+ and Mg2+ in ethanol. The optimized structure of the sensor 94 and its sensing mechanism for Al3+, Cu2+ and Mg2+ were confirmed by the calculations of TD-DFT methods Fig. 30 [143].

Xiaowei Mao et al. reported 1,2-bis-(2-pyren-1-ylmethylamino-ethoxy)ethane (95) brought to the surface of GNs via π − π stacking, which shows Mn2+ sensing with 641 LOD of 4.6 × 10− 5M (Fig. 31). These sensing capabilities of 95 in living cell make it applicable in intracellular tracking, intracellular imaging, etc. [144]. Jianping Guan et al. developed a luminophor, 96, which showed a sensitive fluorescence response to Fe2+ with low detection limits of 115.2 nM. Sensing mechanism indicates that fluorescence-quenched due to Fe2+ chelate with the oxygen and nitrogen of 96 (Figs. 32) [145]. Gyeong Jin Park et al. synthesized a colorimetric probe 97 for Co2+ detection with changing its color from yellow to orange. Furthermore 97 could be used as a practical, visible colorimetric test kit for Co2+ [146]. R. P. Cox et al. developed Crown ethers (98a and 98b) for sensing of cations, via changes in absorption/emission and with a 1:1 addition of Na+ or K+, providing clear colourimetric readout [147].


Minoo Bagheri et al. developed fluorescent MOF sensor, 99 for the ca.2+ sensing concentrations similar to that of blood plasma. The two dimensional signal transduction produce by 99 can reduce interfering responses from the environment and thus generate outstanding sensitivity (Fig. 33) [148]. Jing-Ru Zhou et al. synthesized tripodal amide based probe (100), for the colorimetric sensing for cobalt(II) ions by an obvious color change from colorless to yellow [149]. Xiaopeng Yang et al. reported turn on NIR-fluorescent probe 101 based ICT for detection of Fe2+ with excellent sensitivity (DL = 4.5 µM) (Fig. 34), rapid response (15 min) and “naked-eye colorimetric sensor” [150]. Diana Pendin et al. synthesized a fluorescent ca2+ sensor 102, shows ratiometric ca2+ indicator. 102 binds ca2+ with a dissociation constant of &1.5 mm in vitro [151].


Minuk Yang et al. synthesized sensor with pyridyl and carbohydrazide (103) gives visibly blue colour in the presence of Fe2+ and yellow when exposed to Co2+ and Cu2+. The binding constants of the sensor are: Fe2+: 1.0 × 109M−2, Co2+: 2 × 109M−2, and Cu2+: 3.0 × 109M−2 [152]. Meng-Xia Huang et al. synthesized fluorescence probe (104) showed a outstanding fluorescence enhancement toward Cd2+ with a LOD of 29.3 nM. The binding stoichiometry between 104 and Cd2+ was 2:1 as confirmed by the Job’s Plot and 1H NMR titration experiment (Fig. 35) [153]. Suman Swami et al. reported sensor (105a to 105c) for excellent selectivity and sensitivity towards detection of Mn2+ and Zn2+ ions using UV-visible titration, fluorescent titration. Job’s plot methods revealed that sensor interact with Mn2+ and Zn2+ ion in 1:1 and 1:2 binding stoichiometry respectively [154].


Azzurra Sargenti et al. presented fluorescent sensor, 106b for the quantitative assessment of total intracellular Mg content. The 106b accurately quantify the intracellular total Mg in much smaller samples than 106a, also displaying an increased stable intracellular staining [155]. Bing Zhao et al. developed probe containing 1H-phenanthro[9,10-d]imidazole (107) moieties linked to double ethylenediamino units for the detection of Ag+ with LOD calculated at1.01 × 107M. The 1:1 binding stoichiometry of L–Ag + complex was confirmed by Job’s plot and ESI-MS (Fig. 36) [156]. Juhye Kang et al. reported sensor of bispicolylamine (108) covalently attached to coumarin for the sensing of Mg2+. The formation of a 1:2 complex between the sensor and Mg2+ ions was confirmed based on NMR as well as Job’s plot [157].


Concluding Remarks
The significance and allied adverse consequences of the cationic presence in biological and environmental systems have recognized. Therefore, attention has been given towards the developing of cationic sensors. The sensors were classified into several categories according to their main moiety, such as rhodamine, anthracene, pyrene, imine, quinoline, benzimidazole, BODIPY and nanoparticles based sensing systems. In this review, a huge number of diverse approaches for the development of sensors for cationic ions have been discussed and successfully applied to monitor environmental metal concentrations with high accuracy, precision, reproducibility with a low detection limit is a challenging area from a present day perspective.
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- RNA:
-
Ribonucleic acid
- F-AAS:
-
Flame Atomic absorption spectrometry
- ICP:
-
inductively coupled plasma
- CT:
-
Charge Transfer
- LOD:
-
Limit of detection
- Kb :
-
Bindig constant
- ET:
-
Electron Transfer
- FRET:
-
Förster Resonance Energy Transfer
- Ka :
-
Association constant
- Ks :
-
Stability constant
- CH3CN:
-
Acetonitrile
- CH3OH:
-
Methanol
- C2H5OH:
-
Ethanol
- HRMS:
-
High rsolution mass spectroscopy
- PBS buffer:
-
Phosphate-buffered saline buffer
- µM:
-
micro molar
- DFT:
-
Density functional theory
- mM:
-
Mili molar
- ICT:
-
Intramolecular charge transfer
- DMSO:
-
Dimethyl sulfoxide
- B-H:
-
Benesi-Hildebrand
- S-P:
-
Scatchard-plot
- PET:
-
Photo electron effect
- HOMO:
-
Highest occupied molecule orbital
- LUMO:
-
Lowest unoccupied molecular orbital
- FMO:
-
Frontier molecular orbitals
- GNs:
-
Graphene nanosheets
- CAN:
-
Acetonitrile
- CTAC:
-
Cetyltrimethylammonium chloride
- DSSC:
-
Dye-sensitized solar cell
- CHEF:
-
Chelation-enhanced fluorescence
References
Atood JL, Davies JED, MacNicol DD, Vogetle F (1996) Comprehensive supramolecular chemistry, ed. Elsevier Exeter
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40
Lehn JM (1995) In: Supramolcular chemistry-concept and perspective. VCH, Weinheim
Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rich TE (1997) Signalling recognition event with fluorescent sensors and switches. Coord Chem Rev 97:1515–1566
Atwood JL, Holman KT, Steed JW (1996) Chem Commun. 1401–1407
Sahoo SK, Sharma D, Bera RK, Crisponi G, Callan JF (2012) Iron(III) selective molecular and supramolecular fluorescent probes. Chem Soc Rev 41:195–7227. https://doi.org/10.1039/c2cs35152h
Lehninger AL (1976) Biochemistry. Worth Publishers, New York
Lee JD (2008) Concise of Inorganic Chemistry. Wiley, India
Zheng YJ, Orbulescu J, Ji XJ, Andreopoulos FM, Pham SM, Leblanc RM (2003) J Am Chem Soc 125:2680–2686
Quang DT, Kim JS (2010) Chem Rev 110:6280–6301
Zhou Y, Kim HN, Yoon J (2010) Bioorg Med Chem Lett 20:125
Kaim W, Schwederski B (1995) Bioinorganic Chemistry
Jacobs A (1977) Blood 50:433–439
Udhayakumari D, Naha, S, Velmathi S (2016) Colorimetric and Fluorescent chemosensors for Cu2+. A comprehensive review from the year 2013-15, Anal. Methods. https://doi.org/10.1039/C6AY02416E
Zhang YM, Qu WJ, Gao GY, Shi BB, Wu GY, Wei TB, Lin Q, Yao H (2014) A highly selective dual-channel chemosensor for mercury ions: utilization of the mechanism of intramolecular charge transfer blocking. New J Chem 38:5075–5080
Goswami S, Chakraborty S, Paul S, Halder S, Panjac S, Mukhopadhyay SK (2014) A new pyrene based highly sensitive fluorescence probe for copper(II) and fluoride with living cell application. Org Biomol Chem 12:3037–3044
Fegade U, Singh A, Krishna Chaitanya G, Singh N, Attarde S, Kuwar A (2014) Highly selective and sensitive receptor for Fe3+ probing. Spectrochim Acta Part A Mol Biomol Spectrosc 121:569–574
Huang C-B, Li H-R, Luoc Y, Xu L (2014) A naphthalimide-based bifunctional fluorescent probe for the differential detection of Hg2 + and Cu2 + in aqueous solution. Dalton Trans 43:8102–8108
Zhou J-R, Lui D-P, He Y, Kong X-J, Zhang Z-M, Ren Y-P, Long L-S, Huang R-B, Zheng L-S (2014) A highly selective colorimetric chemosensor for cobalt(II) ions based on a tripodal amide ligand. Dalton Trans 43:11579–11586
Liu J, Wu K, Li S, Song T, Han Y, Li X (2013) A highly sensitive and selective fluorescent chemosensor for Pb2+ ions in an aqueous solution. Dalton Trans 42:3854–3859
Chen G, Guo Z, Zeng G, Tang L (2015) Fluorescent and Colorimetric Sensors for Environmental Mercury Detection. Analyst. https://doi.org/10.1039/C5AN00389J
Zhou Y, Zhang J, Zhang L, Zhang Q, Ma T, Niu J (2013) Dyes Pigm 97:148
Udhayakumari D, Suganya S, Velmathi S (2013) J Lumin 141:48
Ping X, Cuicui P, Yingjie Z, Xiangxue K, Juanjuan S, Maoyou X, Zhiqiang S (2012) Tunable fluorescent pH sensor based on water soluble perylenetetracarboxylic acid/Fe3+. Luminescence 27:307–309
Chen X, Hong H, Han R, Zhang D, Ye Y, Zhao YF (2012) A new bis(rhodamine)-based fluorescent chemosensor for Fe3+. J Fluoresc 22:789–794
Jung H, Singh N, Jang DO (2008) Highly Fe3+ selective ratiometric fluorescent probe based on imine-linked benzimidazole. Tetrahedron Lett 49:2960–2964
Saleem M, Lee KH (2015) Optical sensor: a promising strategy for environmental and biomedical monitoring of ionic species. RSC Adv. https://doi.org/10.1039/C5RA11388A
Tang L, Dai X, Zhong K, Wen X, Wu D (2014) A phenylbenzothiazole derived fluorescent sensor for Zn(II) recognition in aqueous solution through “Turn-On” excited-state intramolecular proton transfer emission. J Fluoresc 24:1487–1493
Sivaraman G, Sathiyaraja V, Chellappa D (2014) Turn-on fluorogenic and chromogenic detection of Fe(III) and its application in living cell imaging. J Lumin 145:480–485
Zhao L, Chen X, Guo F, Gou B, Yang C, Xia W (2014) Luminescent properties and logic nature of a crown Schiff base responding to sodium ion and zinc ion. J Lumin 145:486–491
Dudina NA, Antina EV, Guseva GB, Vyugin AI (2014) The high sensitive and selective “Off-On” fluorescent Zn2+ sensor based on the Bis(2,4,7,8,9-pentamethyldipyrrolylmethene-3-yl)methane. J Fluoresc 24:13–17
Mahato P, Saha S, Das P, Agarwal H, Das A (2014) An overview of the recent developments on Hg2 + recognition. RSC Adv. https://doi.org/10.1039/C4RA03594A
Fegade U, Saini A, Sahoo SK, Singh N, Bendre R, Kuwar A (2014) 2,2’-(Hydrazine-1,2-diylidenedimethylylidene) bis(6-isopropyl-3-methylphenol) based selective dual-channel chemosensor for Cu2 + in semiaqueous media. RSC Adv 4:39639–39644
Patil R, Moirangthem A, Butcher R, Singh N, Basu A, Tayade K, Fegade U, Hundiwale D (2014) Anil Kuwar, Al3+ selective colorimetric and fluorescent red shifting chemosensor: application in living cell imaging. Dalton Trans 43:2895–2899
Ye Z, Xiao Y, Song B, Yuan J (2014) Design and synthesis of a new terbium complex-based luminescent probe for time-resolved luminescence sensing of zinc ions. J Fluoresc 24:1537–1544
Safin DA, Babashkina MG, Garcia Y (2013) Crown ether-containing Schiff base as a highly efficient “turn-on” fluorescent sensor for determination and separation of Zn2+ in water. Dalton Trans 42:1969–1972
Li P, Zhou X, Huang R, Yang L, Tang X, Dou W, Zhao Q, Liu W (2014) A highly fluorescent chemosensor for Zn2+ and the recognition research on distinguishing Zn2+ from Cd2+. Dalton Trans 43:706–713
Patil S, Patil R, Fegade U, Bondhopadhyay B, Pete U, Sahoo SK, Singh N, Basu A, Bendre R, Kuwar A (2015) A novel phthalazine based highly selective chromogenic and fluorogenic chemosensor for Co2+ in semi-aqueous medium: application in cancer cell imaging. Photochem Photobiol Sci 14:439–443
Fegade U, Sahoo SK, Attarde S, Singh N (2014) Anil Kuwar, Colorimetric and fluorescent “On-Off” chemosensor for Cu2+ in semi-aqueous medium. Sensors Actuators B 202:924–928
Li M-M, Huang S-Y, Ye H, Ge F, Miao J-Y, Zhao B-X (2013) A new pyrazoline-based fluorescent probe for Cu2+ in live cells. J Fluoresc 23:799–806
Liu S, Zhang L, Gao J, Zhou J (2013) Synthesis and analytical application of a novel fluorescent Hg2+ Probe 3′, 6′-Bis(Diethylamino)-2-((2,4-Dimethoxybenzylidene) Amino)Spiro[Isoindoline-1,9′-Xanthene]-3-Thione. J Fluoresc 23:989–996
Zhang D, Wang M, Wang C, Li M, Ye Y, Zhao Y (2013) Two highly sensitive and selective colorimetric “Off-On” rhodamine-based fluorescent chemosensor for Hg(II) in aqueous media. J Fluoresc 23:1045–1052
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41:3210–3244
The European Parliament and the Council of the European Union. Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment, 2002/95/EC
de Vries W (2007) Ro¨ mkens and G. Schu¨ tze. Rev Environ Contam Toxicol 191:91
World Health Organization (2004) Guidelines for drinking-water quality (3rd ed) vol 1, Geneva, 188
Fu Y, Mu L, Zeng X, Zhao J-L, Redshaw C, Ni X-L, Yamato T (2013) An NBD-armed thiacalix[4]arene-derived colorimetric and fluorometric chemosensor for Ag+: a metal–ligand receptor of anions. Dalton Trans 42:3552–3560
Li M, Zhang D, Liu Y, Ding P, Ye Y, Zhao Y (2014) A Novel Colorimetric and Off–On Fluorescent Chemosensor for Cr3 + in Aqueous Solution and its Application in Live Cell Imaging. J Fluoresc 24:119–127
Huang S, Du P, Min C, Liao Y, Sun H, Jiang Y (2013) Poly(1-amino-5-chloroanthraquinone): Highly Selective and Ultrasensitive Fluorescent Chemosensor For Ferric Ion. J Fluoresc 23:621–627
Shu-Yan Jiao, Ling-Ling Peng, Kun Li, Yong-Mei Xie, Mei-Zhen Ao, Xin Wang, Xiao-Qi Yu (2013) A BINOL-based ratiometric fluorescent sensor for Zn2+ and in situ generated ensemble for selective recognition of histidine in aqueous solution, Analyst, 138, 5762–5768
Ganguly A, Paul BK, Ghosh S, Kar S, Guchhait N (2013) Selective fluorescence sensing of Cu(II) and Zn(II) using a new Schiff base-derived model compound: naked eye detection and spectral deciphering of the mechanism of sensory action. Analyst 138:6532–6541
Choi JY, Kim G-H, Guo Z, Lee HY, Swamy KMK (2013) Jaeyoung Pai, Seunghoon Shin, Injae Shin, Juyoung Yoon, Highly selective ratiometric fluorescent probe for Au3+ and its application to bioimaging. Biosensors Bioelectronics 49:438–441
Singh AP, Murale DP, Ha Y, Liew H, Lee KM, Segev A, Suh Y-H, Churchill DG (2013) A novel, selective, and extremely responsive thienyl based dual fluorogenic probe for tandem superoxide and Hg2+ chemosensing. Dalton Trans 42:3285–3290
Vandana Bhalla V, Vij R, Tejpal G, Singh, Kumar M (2013) Solvent dependent competition between fluorescence resonance energy transfer and through bond energy transfer in rhodamine appended hexaphenylbenzene derivatives for sensing of Hg2+ ions. Dalton Trans 42:4456–4463
Jiangli F, Zhan P, Wen MH, Tang SJ, Wang J, Sun S, Song F, Peng X (2013) A fluorescent ratiometric chemodosimeter for Cu2+ based on TBET and its application in living cells. Org Lett 15(3):492–495
Goswami S, Manna A, Maity AK, Paul S, Das AK, Das MK, Saha P, Quahc CK, Func H-K (2013) Selective detection and bio-imaging of Pd2+ with novel ‘C–CN’ bond cleavage of cyano-rhodamine, cyanation with diaminomaleonitrile. Dalton Trans 42:12844–12848
Nouri H, Cadiou C, Lawson-Daku LM, Hauser A, Chevreux S, Déchamps-Olivier I, Lachaud F, Ternane R, Trabelsi-Ayadi M, Chuburua F, Lemercier G (2013) A modified cyclen azaxanthone ligand as a new fluorescent probe for Zn2+. Dalton Trans 42:12157–12164
Sikdar A, Roy S, Haldar K, Sarkar S, Panja SS (2013) Rhodamine-based Cu2+ selective fluorosensor: synthesis, mechanism, and application in living cells. J Fluoresc 23:495–501
Chung PK, Liu S-R, Wang H-F, Wu S-P (2013) A pyrene-based highly selective turn-on fluorescent chemosensor for Iron(III) ions and its application in living cell imaging. J Fluoresc 23:1139–1145
Wang J, Chu Q, Liu X, Wesdemiotis C, Pang Y (2013) Large fluorescence response by alcohol from a Bis(benzoxazole) – Zinc(II) complex: the role of excited state intramolecular proton transfer. J Phys Chem B 117:4127–4133
Tan Y, Gao J, Yu J, Wang Z, Cui YuY, Yang Qian G (2013) A newfluorescent probe for distinguishing Zn2+ and Cd2+ with high sensitivity and selectivity. Dalton Trans 42:11465–11470
Ge F, Ye H, Luo J-Z, Wang S, Sun Y-J, Zhao B-X, Miao J-Y (2013) A new fluorescent and colorimetric chemosensor for Cu(II) based on rhodamine hydrazone and ferrocene unit. Sensors Actuators B Chemical 181:215–220
Har Mohindra Chawla (2013) Preeti Goel, Richa Shukla, new calix[4]arene based oxalylamido receptors for recognition of copper(II). Tetrahedron Lett 54:2766–2769
Kuwar A, Fegade U, Tayade K, Patil U, Puschmann H, Gite V, Dalal D, Bendre R (2013) Bis(2-Hydroxy-3-Isopropyl-6-Methyl-Benzaldehyde) Ethylenediamine: A novel cation sensor. J Fluoresc 23:859–864
Fangzhi Hu, Zheng B, Wang D, Liu M, Du J, Xiao D (2014) A novel dual-switch fluorescent probe for Cr(III) ion based on PET–FRET processes. Analyst 139:3607–3613
Chereddy NR, Niladri Raju MV, Nagaraju P, Krishnaswamy VR, Korrapati PS, Bangal PR, Rao VJ (2014) A naphthalimide based PET probe with Fe3+ selective detection ability: theoretical and experimental study. Analyst 139:6352–6356
Mikata Y, Nodomi Y, Kizu A, Konno H (2014) Quinoline-attached triazacyclononane (TACN) derivatives as fluorescent zinc sensors. Dalton Trans 43:1684–1690
Li Y, Wu J, Jin X, Wang J, Han S, Wu W, Xu J, Liu W, Yao X, Tang Yu (2014) A bimodal multianalyte simple molecule chemosensor for Mg2+, Zn2+, and Co2+. Dalton Trans 43:1881–1887
Hu JH, Li JB, Qi J, Chen JJ (2015) Highly selective and effective mercury(II) fluorescent sensors. New J Chem 39:843–848
Fegade U, Sahoo SK, Attarde S, Singh N (2014) Anil Kuwar, Colorimetric and fluorescent “On-Off” chemosensor for Cu2+ in semi-aqueous medium. Sensors Actuators B 202:924–928
Hava Ozay R, Kagit M, Yildirim S, Yesilot O, Ozay (2014) Novel hexapodal triazole linked to a cyclophosphazene core rhodamine-based chemosensor for selective determination of Hg2+ ions. J Fluoresc 24:1593–1601
Liang L, Zhao L, Zeng X (2014) A highly selective turn-on fluorescent chemodosimeter for Cu2+ through a Cu2+ promoted redox reaction. J Fluoresc 24:1671–1677
Nayan R, Pramanik HAR, Paul PC, Singh ST (2014) A sensitive schiff-base fluorescent chemosensor for the selective detection of Zn2+. J Fluoresc 24:1099–1106
Li LQ, Yuan L, Liu ZH (2014) A highly selective turn on fluorescence sensor for Hg2+ based on rhodamine derivative. J Fluoresc 24:1357–1361
Patil S, Fegade U, Sahoo SK, Singh A, Marek J, Singh N, Bendre R, Kuwar A (2014) Highly sensitive ratiometric chemosensor for selective ’Naked-Eye’ nanomolar detection of Co2+ in semi- aqueous media. Chemphyschem 5:2230–2235. https://doi.org/10.1002/cphc.201402076
Dong Z, Le X, Zhou P, Dong C, Ma J (2014) An ‘‘off–on–off’’ fluorescent probe for the sequential detection of Zn2+ and hydrogen sulfide in aqueous solution. New J Chem 38:1802–1808
Zhang YM, Qu WJ, Gao GY, Shi BB, Wu GY, Wei TB, Lin Q, Yao H (2014) A highly selective dual-channel chemosensor for mercury ions: utilization of the mechanism of intramolecular charge transfer blocking. New J Chem 38:5075–5080
Tan Y, Liu M, Gao J, Yu J, Cui Y, Yang Yu, Qian G (2014) A newfluorescent probe for Zn2+ with red emission and its application in bioimaging. Dalton Trans 43:8048–8053
Fegade U, Sharma H, Bondhopadhyay B, Basu A, Attarde S, Singh N, Kuwar A (2014) Turn-on” fluorescent dipodal chemosensor for nano-molar detection of Zn2+: Application in living cells imaging. Talanta 125:418–424
Wang M, Liu X, Lu H, Wang H, Qin Z (2015) Highly selective and reversible chemosensor for Pd2 + detected by fluorescence, colorimetry, and paper T. ACS Appl Mater Interfaces 7:1284–1289
Gui S, Huang Y, Hu F, Jin Y, Zhang G, Yan L, Zhang D, Zhao R (2015) Fluorescence turn-on chemosensor for highly selective and sensitive detection and bioimaging of Al3 + in living cells based on ion-induced aggregation. Anal Chem 87(3):1470–1474
Manna AKumarMSaikatK, Maiti K, Mondal S, Maji R, Mandal D (2015) Sukhendu Mandal, Md. Raihan Uddin, Shyamaprosad Goswami, Ching Kheng Quahd, Hoong-Kun Fund, An azodye–rhodamine-based fluorescent and colorimetric probe specific for the detection of Pd2 + in aqueous ethanolic solution: synthesis, XRD characterization, computational studies and imaging in live cells. Analyst 140:1229–1236
Ghorai A, Chandra Mondal J, Goutam R, Patra K (2015) A reversible fluorescent-colorimetric imino-pyridyl bis-Schiff base sensor for expeditious detection of Al3 + and HSO3 – in aqueous media. Dalton Trans 44:13261–13271
Zhang Z, Sha C, Liu A, Zhang Z, Xu D (2015) Highly selective detection of Cr(VI) in WaterMatrix by a simple 1,8-Naphthalimide-based turn-on fluorescent sensor. J Fluoresc 25:335–340
Wu Y-C, Jiang K, Luo S-H, Cao L, Wu H-Q (2019) Zhao-YangWang, Novel dual-functional fluorescent sensors based on bis(5,6-dimethylbenzimidazole) derivatives for distinguishing of Ag + and Fe3 + in semi-aqueous medium. Spectrochim Acta Part A Mol Biomol Spectrosc 206:632–641
Bhosale J, Fegade U, Bondhopadhyay B, Kaur S, Singh N, Basu A, Dabur R, Bendre R, Kuwar A (2015) Pyrrole-coupled salicylimine-based fluorescence “turn on” probe for highly selective recognition of Zn2 + ions in mixed aqueous media: Application in living cell imaging. J Mol Recognit 28:369–375
Patil S, Patil R, Fegade U, Bondhopadhyay B, Pete U, Sahoo SK, Singh N, Basu A (2015) Ratnamala Bendre and Anil Kuwar, A novel phthalazine based highly selective chromogenic and fluorogenic chemosensor for Co2 + in semi-aqueous medium: application in cancer cell imaging. Photochem Photobiol Sci 14:439–443
Patil R, Fegade U, Kaur R, Sahoo SK (2015) Narinder Singh & Anil Kuwar, Highly sensitive and selective determination of Hg2 + by using 3-((2-(1H-benzo[d]imidazol-2- yl)phenylimino)methyl)benzene-1,2-diol as fluorescent chemosensor and its application in real water sample. Supramol Chem 27:527–532
Pawar S, Fegade U, Bhardwaj VK, Singh N, Bendre R, Kuwar A (2015) 2-((E)-(2-aminophenylimino)methyl)-6-isopropyl-3-methylphenol based fluorescent receptor for dual Ni2 + and Cu2 + recognition: Nanomolar detection. Polyhedron 87:79–85
Fegade UA, Sahoo SK, Singh A, Singh N, Attarde SB, Kuwar AS (2015) A chemosensor showing discriminating fluorescent response for highly selective and nanomolar detection of Cu2 + and Zn2 + and its application in molecular logic gate. Anal Chim Acta 872:63–69
Zhou D, Sun C, Chen C, Cui X, Li W (2015) Research of a highly selective fluorescent chemosensor for aluminum(III) ions based on photoinduced electron transfer. J Mol Struct 1079:315–320
Cui J, Li D-P, Shen S-L, Liu J-T, Zhao B-X (2015) A simple and effective fluorescent probe based on rhodamine B for determining Pd2 + ions in aqueous solution. RSC Adv 5:3875–3880
Shengli Hu, Song J, Wua G, Cheng C, Gao Q (2015) A new pyrazoline-based fluorescent sensor for Al3 + in aqueous solution. Spectrochim Acta Part A Mol Biomol Spectrosc 136:1188–1194
Li L, Liu Z (2015) A colorimetric and fluorescent turn on chemodosimeter for Pd2 + detection. Spectrochim Acta Part A Mol Biomol Spectrosc 138:954–957
Gupta VK, Mergu N, Kumawat LK, Singh AK (2015) A reversible fluorescence “off-on-off” sensor for sequential detection of Aluminum and Acetate/Fluoride ions. Talanta 144:80–89
Affrose A, Parveen DS, Kumar BS, Pitchumani K (2015) Selective sensing of silver ion using berberine, a naturally occurring plant alkaloid. Sensors Actuators B 206:170–175
Kao M-H, Chen T-Y, Cai Y-R, Hu C-H, Liu Y-W, Jhong Y, Wu A-T (2016) A turn-on Schiff-base fluorescence sensor for Mg2þ ion and its practical application. J Lumin 169:156–160
Bekhradnia A, Domehri E, Khosravi M (2016) Novel coumarin-based fluorescent probe for selective detection of Cu(II). Spectrochim Acta Part A Mol Biomol Spectrosc 152:18–22
Sahana S, Bose S, Mukhopadhyay SK, Bharadwaj PK (2016) A highly selective and sensitive turn-on fluorescence chemosensor based on a rhodamine–adenine conjugate for Al3 + in aqueous medium: Bioimaging and DFT studies. J Lumin 169:334–341
Zheng X, Lee KH, Liu H, Park S-Y, Yoon SS, Lee JY, Kim Y-G (2016) A bis(pyridine-2-ylmethyl)amine-based selective and sensitive colorimetric and fluorescent chemosensor for Cu2+. Sensors Actuators B 222:28–34
Huang Q, Zhang Q, Wang E, Zhou Y, Qiao H, Pang L, Yu F (2016) A new ‘‘off–on’’ fluorescent probe for Al3 + in aqueous solution based on rhodamine B and its application to bioimaging. Spectrochim Acta Part A Mol Biomol Spectrosc 152:70–76
An R, Zhang D, Chen Y, Cui Y-z (2016) A “turn-on” fluorescent and colorimetric sensor for selective detection of Cu2 + in aqueous media and living cells. Sensors Actuators B 222:48–54
Wang E, Zhou Y, Huang Q, Pang L, Qiao H, Yu F, Gao B, Zhang J, Min Y, Ma T (2016) 5-Hydroxymethylfurfural modified rhodamine B dual-function derivative: Highly sensitive and selective optical detection of pH and Cu2+. Spectrochim Acta Part A Mol Biomol Spectrosc 152:327–335
Qin J-C, Cheng Xiao-ying, Fang R, Wang M-f, Yang Z-y, Li T-r, Li Y (2016) Two Schiff-base fluorescent sensors for selective sensing of aluminum (III): Experimental and computational studies. Spectrochim Acta Part A Mol Biomol Spectrosc 152:352–357
Gupta VK, Singh AK, Kumawat LK, Mergu N (2016) An easily accessible switch-on optical chemosensor for the detection of noxious metal ions Ni(II), Zn(II), Fe(III) and UO2(II). Sensors Actuators B 222:468–482
Kim H-S, Angupillai S, Son Y-A (2016) A dual chemosensor for both Cu2 + and Al3+: A potential Cu2 + and Al3 + switched YES logic function with an INHIBIT logic gate and a novel solid sensor for detection and extraction of Al3 + ions from aqueous solution. Sensors Actuators B 222:447–458
Ponnuvel K, Padmini V, Sribalan R (2016) A new tetrazole based turn-on fluorescence chemosensor for Zn2 + ions and its application in bioimaging. Sensors Actuators B 222:605–611
Geng J, Liu Y, Li J, Yin G, Huang W, Wang R, Quan Y (2016) A ratiometric fluorescent probe for ferric ion based on a 2,2,-bithiazole derivative and its biological applications. Sensors Actuators B 222:612–617
Panda U, Roy S, Mallick D, Layek A, Ray PP, Sinh C (2017) Aggregation induced emission enhancement (AIEE) of fluorenyl appended Schiff base: A turn on fluorescent probe for Al3+, and its photovoltaic effect. J Lumin 181:56–62
Singh DP, Dwivedi R, Singh AK, Koch B, Singh P (2017) Vinod Prasad Singh, A dihydrazone based “turn–on” fluorescent probe for selective determination of Al3 + ions in aqueous ethanol. Sensors Actuators B 238:128–137
Gharami S, Sarkar D, Ghosh P, Acharyya S, Aich K, Murmu N, Mondal TK (2017) A coumarin based azo-phenol ligand as efficient fluorescent “OFF-ON-OFF” chemosensor for sequential detection of Mg2 + and F–: Application in live cell imaging and as molecular logic gate. Sensors Actuators B 253:317–325
Lui Y, Hu J, Teng Q, Zhang H (2017) Ferrocenyl-based thioethers and sulphones as optical, and electrochemical sensors for the differential detection of Hg2 + and Cu2 + ions. Sensors Actuators B 238:166–174
Liu J, Qian Y (2017) A novel pyridylvinyl naphthalimide-rhodamine dye: synthesis, naked-eye visible and ratiometric chemodosimeter for Hg2+/Fe3+. J Lumin 187:33–39
Gao Y, Zhang C, Peng S, Chen H (2017) A fluorescent and colorimetric probe enables simultaneous differential detection of Hg2 + and Cu2 + by two different mechanisms. Sensors Actuators B 238:455–461
Maniyazagan M, Mariadasse R, Jeyakanthan J, Lokanath NK, Naveen S, Premkumar K, Muthuraja P, Manisankar P, Stalin T (2017) Rhodamine based “turn–on” molecular switch FRET–sensor for cadmium and sulfide ions and live cell imaging study. Sensors Actuators B 238:565–577
Dong W-K, Akogun SF, Zhang Y, Sun Y-X, Dong X-Y (2017) A reversible “turn-on” fluorescent sensor for selective detection of Zn2+. Sensors Actuators B 238:723–734
Ghosh U, Bag SS, Mukherjee C (2017) Bis-pyridobenzene as a fluorescence light-up sensor for Hg2 + Ion in water. Sensors Actuators B 238:903–907
Li H, Wang J, Zhang ShuJiang, Gong ChenLiang, Wang F (2018) A novel off-on fluorescent chemosensor for Al3 + derived from a 4,5-diazafluorene Schiff base derivative. RSC Adv 8:31889–31894
Shuai Wang H, Ding Y, Wang C, Tu FY, Liu G, Pu S (2018) An ‘‘off–on–off’’ sensor for sequential detection of Cu2 + and hydrogen sulfide based on a naphthalimide–rhodamine B derivative and its application in dual-channel cell imaging. RSC Adv 8:33121–33128
Xu K, Li Y, Si Y, He Y, Ma J, He J, Hou H, Li K (2018) A “turn-on” fluorescent chemosensor for the detection of Hg(II) in buffer free aqueous solution with excellent selectivity. J Lumin 204:182–188
Rui Y, Wang Z, Du Z, Wang H, Cheng Xu, Xiong J (2018) A biomimetic fluorescent chemosensor for highly sensitive zinc(II) detection and its application for cell imaging. RSC Adv 8:33361–33367
Meng X, Cao D, Hu Z, Han X, Li Z, Ma W (2018) A highly sensitive and selective chemosensor for Pb2 + based on quinoline–coumarin. RSC Adv 8:33947–33951
Kumar GGangatharanV, Kesavan MP, Tamilselvi A, Rajagopal G, Raja JD, Sakthipandi K, Rajesh J, Sivaraman G (2018) A reversible fluorescent chemosensor for the rapid detection of Hg2 + in an aqueous solution: Its logic gates behavior. Sensors Actuators B Chem 273:305–315
Khanra S, Ta S, Ghosh M, Chatterjee S, Das D (2019) Subtle structural variation in azine/imine derivatives controls Zn2 + sensitivity: ESIPT-CHEF combination for nano-molar detection of Zn2 + with DFT support. RSC Adv 9:21302–21310
Ghorai P, Banerjee S, Nag D, Mukhopadhyay SK, Saha A (2019) Design and synthesis of a novel fluorescentcolorimetric chemosensor for selective detection of Zn(II) and Cu(II) ions with applications in live cell imaging and molecular logic gate. J Lumin 205:197–209
Zhang Y, Zhang C, Wu Y, Zhao B, Wang L, Song B (2019) A novel water-soluble naked-eye probe with a large Stokes shift for selective optical sensing of Hg2 + and its application in water samples and living cells. RSC Adv 9:23382–23389
Serkan E (2019) Fluorometric dual sensing of Hg2 + and Al3 + by novel triphenylamine appended rhodamine derivative in aqueous media. Sensors Actuators B Chem 290:558–564
Rout K, Manna AK, Sahu M, Mondal J, Singh SK, Patra GK (2019) Triazole-based novel bis Schiff base colorimetric and fluorescent turn-on dual chemosensor for Cu2+ and Pb2+: application to living cell imaging and molecular logic gates. RSC Adv 9:25919–25931
Yang Y, Yu Z, Shi W, Ma F, Li Y (2019) Colorimetric fluorescence probe detecting Hg2 + and OCl– based on intramolecular charge transfer and excited-state intramolecular roton transfer mechanisms. J Lumin 209:102–108
Vishaka VH, Saxena M, Geetha Balakrishna R, Latiyanbc S, Jainc S (2019) Remarkably selective biocompatible turn-on fluorescent probe for detection of Fe3 + in human blood samples and cells. RSC Adv 9:27439–27448
Jung JM, Yun D, Lee H, Kim K-T, Kim C (2019) Selective chemosensor capable of sensing both CN – and Zn2+: Its application to zebrafish. Sensors Actuators B Chem 297:126814
Zhang Y, Li H, Gao W, Pu S (2019) Dual recognition of Al3 + and Zn2 + ions by a novel probe based on diarylethene and its application. RSC Adv 9:27476–27483
Ganesan JS, Sepperumal M, Balasubramaniem A, Ayyanar S (2019) A novel pyrazole bearing imidazole frame as ratiometric fluorescent chemosensor for Al3+/Fe3 + ions and its application in HeLa cell imaging. Spectrochim Acta Part A :117993
Sutariya PG, Soni H, Gandhi SA (2019) Alok Pandya, Novel tritopic calix[4]arene CHEF-PET fluorescence paper based probe for La3+, Cu2+, and Br–: Its computational investigation and application to real samples. J Lumin 212:171–179
Akshay Krishna TG, Tekuri V, Mohan M, Trivedi DR (2019) Selective colorimetric chemosensor for the detection of Hg2 + and arsenite ions using Isatin based Schiff’s bases; DFT Studies and applications in test strips. Sensors Actuators B Chem 284:271–280
Srivastava S, Thakur N, Singh A, Shukla P, Maikhuri VK, Garg N, Prasad A, Pandey R (2019) Development of a fused imidazo[1,2-a]pyridine based fluorescent probe for Fe3 + and Hg2 + in aqueous media and HeLa cells. RSC Adv 9:29856–29863
Said AI, Georgiev NI, Bojinov VB (2019) A smart chemosensor: Discriminative multidetection and various logic operations in aqueous solution at biological pH. Spectrochim Acta Part A Mol Biomol Spectrosc 223:117304
Berrones-Reyes JC, Munoz-FloresBM, Cant´on-Di´az AM, Treto-Su´arez MA (2019) Dayan P´aez-Hern´andez, Eduardo Schott, Ximena Zarate, Vıctor M. Jim´enez-P´erez, Quantum chemical elucidation of the turn-on luminescence mechanism in two new Schiff bases as selective chemosensors of Zn2+: synthesis, theory and bioimaging applications. RSC Adv 9:30778–30789
Panunzi B, Diana R, Concilio S, Sessa L, Tuzi A, Piotto S, Caruso U (2019) Fluorescence pH-dependent sensing of Zn(II) by a tripodal ligand. A comparative X-ray and DFT study. J Lumin 212:200–206
Li S, Cao D, Hu Z, Li Z, Meng X, Hana X, Ma W (2019) A chemosensor with a paddle structure based on a BODIPY chromophore for sequential recognition of Cu2 + and HSO3-. RSC Adv 9:34652–34657
Gu Y-Q, Shen W-Y, Mi Y, Jing Y-F, Yuan J-M, Yu P, Zhuc X-M (2019) Dual-response detection of Ni2 + and Cu2 + ions by a pyrazolopyrimidine-based fluorescent sensor and the application of this sensor in bioimaging. RSC Adv 9:35671–35676
Xu J, Yang Y, Baigude H, Zhao H (2019) New ferrocene–triazole derivatives for multisignaling detection of Cu2 + in aqueous medium and their antibacterial activity, Spectrochimica Acta Part A :117880
Zhang Q, Ma R, Li Z, Liu Z (2019) A multi-responsive crown ether-based colorimetric/fluorescent chemosensor for highly selective detection of Al3+, Cu2 + and Mg2+. Spectrochim Acta Part A Mol Biomol Spectrosc 94:117857
Mao X, Su H, Tian D, Li H, Yang R (2013) Bipyrene-functionalized graphene as a “Turn-on” fluorescence sensor for manganese(II) ions in living cells. ACS Appl Mater Interfaces 5:592–597
Guan J, Tu Q, Chen L, Yuan M-S, Wang J (2019) A benzothiazole-rhodol based luminophor: ESIPT-induced AIE and an application for detecting Fe2 + ion. Spectrochim Acta Part A Mol Biomol Spectrosc 220:117114
Park GJ, Na YJ, Jo HY, Lee SA, Kim C (2014) A colorimetric organic chemo-sensor for Co2 + in a fully aqueous environment. Dalton Trans 43:6618–6622
Cox RP, Sandanayake S, Scarborough DLA, Izgorodina EI, Langford SJ, Bell TDM (2019) Investigation of cation binding and sensing by new crown ether core substituted naphthalene diimide systems. New J Chem 43:2011–2018
Minoo, Bagheri, Masoomi MY (2020) Sensitive ratiometric fluorescent metal-organic framework (MOF) sensor for calcium signaling in human blood ionic concentrations media. ACS Appl Mater Interfaces 12(4):4625–4631
Zhou J-R, Liu D-P, He Y, Kong X-J, Zhang Z-M, Ren Y-P, Long L-S, Huang R-B, Zheng L-S (2014) Lan-Sun Zheng, A highly selective colorimetric chemosensor for cobalt(II) ions based on a tripodal amide ligand. Dalton Trans 43:11579–11586
Yang X, Wang Y, Liu R, Zhang Y, Tang J, Yang E-b, Zhang D, Zhao Y, Ye Y (2019) A novel ICT-based two photon and NIR fluorescent probe for labile Fe2 + detection and cell imaging in living cells. Sensors Actuators B Chem 288:217–224
Pendin D, Norante R, Nadai AD, Gherardi G, Vajente N, Basso E, Kaludercic N, Mammucari C, Paradisi C, Pozzan T, Mattarei A (2019) A Synthetic Fluorescent Mitochondria-Targeted Sensor for Ratiometric Imaging of Calcium in Live Cells. Angew Chem Int Ed 58:9917–9922
Yang M, Chae JB, Kim C, Harrison RG (2019) A visible chemosensor based on carbohydrazide for Fe(II), Co(II) and Cu(II) in aqueous solution. Photochem Photobiol Sci 18:1249–1258
Huang M-X, Lv C-H, Huang Q-D, Lai J-P, Sun H (2019) A novel and fast responsive turn-on fluorescent probe for the highly selective detection of Cd2 + based on photo-induced electron transfer. RSC Adv 9:36011–36019
Swami S, Agarwala A, Behera D, Shrivastava R (2018) Diaminomaleonitrile based chromo-fluorescent receptor molecule for selective sensing of Mn(II) and Zn(II) ions. Sensors Actuators B Chem 260:1012–1017
Sargenti A, Farruggia G, Malucelli E, Cappadone C, Merolle L, Marraccini C, Andreani G, Prodi L, Zaccheroni N (2014) Massimo Sgarzi, Claudio Trombini, Marco Lombardo, Stefano Iottia, A novel fluorescent chemosensor allows the assessment of intracellular total magnesium in small samples. Analyst 139:1201–1207
Zhao B, Xu Yu, Fang Y, Wang L, Deng Q (2015) Synthesis and fluorescence properties of phenanthro[9,10-d] imidazole derivative for Ag + in aqueous media. Tetrahedron Lett 56:2460–2465
Juhye Kang HK, Kang H, Kim J, Lee EJ, Song K-D, Jeong C, Kim J, Kim (2013) Fluorescent chemosensor based on bispicolylamine for selective detection of magnesium ions. Supramol Chem 25:65–68
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patil, N.S., Dhake, R.B., Ahamed, M.I. et al. A Mini Review on Organic Chemosensors for Cation Recognition (2013-19). J Fluoresc 30, 1295–1330 (2020). https://doi.org/10.1007/s10895-020-02554-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02554-7