Abstract
Four boron-dipyrrine (BODIPY) based dyes with π-extended substituents in 8-position of dipyrrin ligand have been synthesized and characterized. Photophysical properties of the obtained compounds have been investigated in different individual solvents. Deposits of solvent polarity and viscosity were evaluated. BODIPY with 8-biphenyl substituent was found to be the fluorescent molecular rotor in contrast to more extended substituents. The complex nature of solvent-solute interactions leads to the poor applicability of standard multiparameter approaches to BODIPY solvatochromic properties. Fluorescence intensity was found to increase in case of solvent polarity growth, it is not typical for BODIPY. Taking that into account the BODIPY with π-extended substituents could be used for fluorescence viscosity measurements, and as the fluorescent media polarity indicators in analytical chemistry and biochemistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increased interest for fluorescent small molecules has inspired the development of a large variety of fluorescence-based spectroscopic and imaging techniques. Nowadays, boron-dipyrrine (BODIPY, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) based compounds are one of the most commonly used fluorescent sensors and labels, due to high photo-, chemical stability and fluorescence quantum yields [1, 2]. Among the BODIPY complexes the 8-substituted ones are of much interest due to the realization of electronic energy transfer between BODIPY core and the substituent. Here, one dye takes the role of the energy donor (i.e., the absorbing chromophore) and the other operates as the acceptor (i.e., the emitting fluorophore). In many biotechnology applications, the donor and acceptor subunits are connected by aliphatic tethers, which implies a through-space energy-transfer process (i.e., Forster mechanism) [3, 4]. A potential way to design next-generation molecules suitable for biological fluorescence labelling is to connect a donor component that has strong absorbance at the excitation wavelength to an acceptor that displays a high fluorescence quantum yield. An added requirement is that some type of internal barrier has to be inserted to prevent the donor–acceptor structure acting as a single “supermolecule”. This barrier can be imposed by twisting the molecular axis such that the donor and acceptor units are no longer coplanar, since this has the effect of preventing the system from forming an extended LUMO. BODIPY based donor-acceptor systems with charge-transfer ability are investigated using computer modeling methods [5]. This approach could be referred as fluorescent molecular rotors properties manifestation [6]. While being exited one part of those molecules are able to rotate towards the other, causing the intramolecular charge transfer (this effect is called twisted intramolecular charge transfer state, or TICT state) [7]. Such molecular rotors main feature is a strong dependence between its quantum yield and solvent dynamic viscosity. In this case, low viscosity mixtures may cause low relative fluorescence quantum yields due to high probability of non-radiative transition [8].
Taking that into account we investigated several BODIPY complexes in a range of solvents with the temperature variation to understand the influence of external effects on photohysical properties of BODIPY with bulky substituent in 8-position.
Experimental
Chemicals and Reagents
Reagents were purchased from Chimmed, Aldrich, and Sigma-Aldrich Co. and used without further purification unless otherwise noted. Solvents (Chimmed, Russia) were all of analytical grade purified by standard techniques [9]. The residual water content (<0.02 %) was determined by the Karl Fischer method [10]. Rheological characteristics of the solvents was changed under the temperature variations [11]. Properties of the solvents under investigation could be find in the Table 1.
Instrumentation and Methods
NMR spectra were recorded on AVANCE500 (Bruker) at 500 MHz for 1H NMR. Mass spectra (MS) were measured with AXIMA Confidence (Shimadzu) MALDI-TOF mass spectrometer. UV–Vis electronic absorption spectra (EAS) were recorded in the range 350–800 nm on an SF-104 spectrophotometer (Aquilon, Russia) controlled with a PC through the software package UVWin 5.1.0. The accuracy of the measurements was ±0.03 on the scale of optical density; wavelength accuracy was ±0.05 nm. The fluorescence spectra were obtained with a Cary Eclipse fluorescence spectrometer (Varian-Agilent, US-Australia) controlled with a PC using the software package Cary Eclipse Scan Application 1.1. The fluorescence spectra were measured in the wavelength range 500–900 nm and the excitation wavelength was 480 nm. The slit widths of excitation and emission ranged from 2.5 to 5 nm. Investigations were carried out in quartz cuvettes with a thickness of the absorbing layer of 2 and 10 mm. All experiments were performed in a temperature-controlled cell with Peltier PTC-2 module at fixed temperatures of 283 to 343 K.
Fluorescence quantum yield (φ) was defined as follows:
where φ st is the rhodamine 6G standard quantum yield in ethanol (φ = 0.95, [12]), A x and A st are the integrated area under the corrected fluorescence spectra, B x and B st are the absorbance (optical density) at the excitation wavelength, n x and n st are the refractive indices of solvents used for two solutions.
Radiative decay constant (k fl) was calculated in accordance with equation:
where n – refractive index of solvent, υ – wavenumber of absorption band maximum (cm−1), Δυ1/2 – half-width of the absorption band (cm−1), ε – extinction coefficient in the absorption band.
Nonradiative (k nr) decay constant and fluorescence lifetime (τ) were calculated from experimentally measured fluorescence quantum yield φ and radiative decay constant k fl according to the following equations:
Quantum chemical calculations were performed using software package HyperChem 8.0.3 [13]. Semi empirical PM3 method was used both for geometry pre-optimization and the potential energy surface (PES) calculations due to the most adequate reproduction of X-ray diffraction data and quantum chemical calculations performed for BODIPY. Exact geometric and energy parameters of the molecules were obtained using density functional theory method in version B3LYP6-31G (d, p).
Approaches to Solvatochromic Effect Numerical Interpretation
A general model which describes the properties of solvent and solvation does not exist. As a result, several empirical solvent scales have been proposed to characterize and rank empirically the polarity of the solvent. One of the most popular solvent polarity scale is the one parameter solvent polarity scale ET(30) or the normalized ET N, they are widely used to measure empirically the polarity of many systems. It is also frequently used as a parameter of linear solvation energy relationships for the correlation analysis of solvent effect on chemical and physical properties [14–16].
A more correct description should be carried out using a multiparameter solvent scales, taking into account solvent polarity, polarizability, proton and electron donating properties, with the linear regression method:
where Х –physical or chemical characteristic depending on the solvent (ν abs, ν fl and Δν); Х о – value of the physical or chemical property in the gas phase, or “standard” (inert) solvent; A, B and C – independent but complementary solvent parameters (polarity, polarizability proton or electron donating and other properties), describing various mechanisms of interaction of the solvent with the solute; а, b and c – coefficients characterizing the contribution of the parameters A, B and C of the solvent in the property studied. Application of the linear regression analysis method allows determining the contributions of the individual parameters of the solvents in the photophysical characteristics of the solute, quantitatively taking into account their impact. The most commonly used parameter sets are the parameters proposed by Kamlet and Taft – the scale of polarity / polarizability of the solvent (π*), scale of proton-donor properties of the solvent (α) and electron-donor properties scale (β) [17], as well as a set of parameters of Catalan - the scale of polarity / polarizability of the solvent (SPP N), acidity solvent scale (SA) and basic solvents scale (SB) [18]. The values of each parameter vary from 0 to 1. X o parameter calculated as the free term of the linear equation was taken as the property of the investigated molecules in the gas phase.
Synthetic Procedures
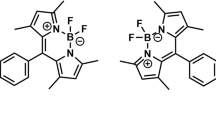
Complexes of BODIPY, differing by the nature of the substituent in the 8- position of the dipyrrin ligand, were synthesized and investigated (Fig. 1). 4,4-difluoro-8-phenyl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene (1), 4,4-difluoro-8-naphthyl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene (2), 4,4-difluoro-8-antryl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene (3), 4,4-difluoro-8-pyrryl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene ( 4 ), 4,4-difluoro-8-biphenyl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene ( 5 ) were synthesized by the methods described above [19]. The reagents were obtained from Sigma-Aldrich and were readily used in the synthetic procedures.
-
1)
4,4-difluoro-8-phenyl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene was synthesized according to the general synthesis procedure using benzaldehyde as a precursor. The reaction product is dark red crystals (yield =75 %). 1H NMR (500 MHz, CCl4): δ 1.10 (T, 6H), 2.25 (S, 6H), 2.45 (D, 4H), 2.73 (S, 6H), 7.16 (S, 5H). MALDI-TOF: calculated ([C23H27BF2N2]+) m/z = 380.29, found m/z = 379.99. Anal. Calculated for: C23H27BF2N2: C, 72.62; H, 7.16; N, 7.37. Found: C, 72.3; H, 7.05; N, 7.33.
-
2)
4,4-difluoro-8-naphthyl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene was synthesized according to the general synthesis procedure using 1-naphthaldehyde as a precursor. The reaction product is dark red crystals (yield =15.6 %). 1H NMR (500 MHz, CCl4): δ 1.16(T,6H), 1.76 (Q, 6H), 2.23 (Q, 4H), 2.75 (S,6H), 7.19 (S, 3H), 7.46 (Q, 2H), 7.97 (Q, 2H). MALDI-TOF: calculated ([C27H29BF2N2]+) m/z = 430.35, found m/z = 429.92. Anal. Calculated for: C27H29BF2N2: C, 75.36; H, 6.79; N, 6.51. Found: C, 75.13; H, 6.32; N, 6.50.
-
3)
4,4-difluoro-8-antryl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene was synthesized according to the general synthesis procedure using 9-anthracenecarboxaldehyde as a precursor. The reaction product is dark green crystals (yield =19.4 %). 1H NMR (500 MHz, CCl4): δ 1.16(T,6H), 1.80 (Q, 6H), 2.25 (Q, 4H), 2.73 (S,6H), 7.15 (S, 3H), 7.46 (Q, 4H), 7.93 (Q, 2H). MALDI-TOF: calculated ([C31H31BF2N2]+) m/z = 480.41, found m/z = 479.95. Anal. Calculated for: C31H31BF2N2: C, 77.51; H, 6.50; N, 5.83. Found: C, 77.50; H, 6.28; N, 5.79.
-
4)
4,4-difluoro-8-pyrryl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene was synthesized according to the general synthesis procedure using 1-pyrenecarboxaldehyde as a precursor. The reaction product is bright green crystals (yield =25.8 %). 1H NMR (500 MHz, CCl4): δ 1.17 (T, 6H), 2.07 (Q, 6H), 2.23 (S, 4H), 2.76 (S, 6H), 6.53 (S, 2H), 7.30 (D, 3H), 7.41 (S, 2H), 7.90 (S, 2H) MALDI-TOF: calculated ([C33H31BF2N2]+) m/z = 504.43, found m/z = 504.01. Anal. Calculated for: C33H31BF2N2: C, 78.58; H, 6.19; N, 7.53. Found: C, 78.32; H, 6.15; N, 7.51.
-
5)
4,4-difluoro-8-biphenyl-1,3,5,7-tetramethyl-2,6-diethyl-4-boron-3a,4a-diaza-s-indacene was synthesized according to the general synthesis procedure using biphenyl-4-carboxaldehyde as a precursor. The reaction product is bright green crystals (yield =24.3 %). 1H NMR (500 MHz, CCl4): δ 1.15 (T, 6H), 1.78 (Q, 6H), 2.27 (Q, 4H), 2.75 (S, 6H), 6.56 (S, 2H), 7.34 (D, 4H), 7,46 (S, 2H), 8.01 (S, 1H). MALDI-TOF: calculated ([C29H31BF2N2]+) m/z = 456.39, found m/z = 458.13. Anal. Calculated for: C29H31BF2N2: C, 76.32; H, 6.85; N, 6.14. Found: C, 76.28; H, 6.75; N, 6.19.
Result and Discussion
Spectral Properties and Photophysical Characteristics
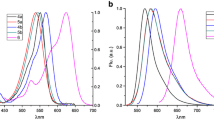
The spectroscopic properties of BODIPY 1–4 were evaluated in the range of solvents, and the results obtained are summarized in Table 2 and shown in Fig. 2.
Electronic absorption spectra of investigated complexes are characterized by intensive absorption maxima between 528 and 541 nm corresponding to an electronic transition S0 – S1. Fluorescence peaks are between 542 and 551 nm. Increasing of the π-conjugation in 8-position substituent leads to the bathochromic shifts in absorption and fluorescence spectra. Highest “red” shift was observed for 8-biphenyl substituted compound 5. Bathochromic shifts in the absorption spectra show that when the benzene moiety at the 8-position was changed from benzene to naphthalene or anthracene then to pyrene and biphenyl the electronic coupling between the donor and fluorophore in the ground-state configuration is increased. The same situation was observed for the fluorescence spectra indicating the same interactions in the molecules exited-state. Stokes Shifts are ranged from 7.5 up to 15.47 nm, strongest solvent influence is observed for compound 5.
The photophysical properties of investigated BODIPY were studied in a range of solvents of different nature. All investigations were held in the very diluted solutions of compounds (c = 1·10−6 M) to prevent compounds association, which could be erroneously be considered as solvatochromic effect [20]. Investigated compounds demonstrate negative solvatochromic effect: absorption and fluorescence maxima are batochromicaly shifted in non-polar solvents.
The average values of photophysical parameters for investigated dyes allowed us to interpret the substituted influence on complexes properties (Fig. 3.).
Increasing in the number of condensed benzene rings increases the values of quantum yield. Lowest quantum yield among investigated complexes was found for the biphenyl-substituted compound 5. Compounds with two or more phenyl rings displays smaller fluorescence in comparison with 8-phenyl substituted 1 [21]. This effect is caused primarily by changes in the values of radiative decay constants. This indicating a significant difference in the stability of the complexes of the compounds with a solvent at the excited state. Dye 5 could form the most stable dye-solvent complexes. This fact could be confirmed by the highest values of bathochromic shifts for this compound.
Spectral properties of investigated dyes were tested in 0.1 M solvent of trifluoroacetic acid in cyclohexane to determine the influence of strong proton donating media. The partial destruction of BODIPY to protonated ligand with absorption peak at about 487 nm was observed for all investigated compound. BODIPY absorption and fluorescence peaks were batochromicaly shifted on 1–2 nm. Thus, the presence of proton-donor agents causes the stronger polarization of BODIPY π-system. The preferable mechanism could be proposed as interaction of acid molecules with F-atoms of BODIPY via donor-acceptor interactions.
Temperature Dependent Spectral Changes
Fluorescent molecular rotor properties of investigated compounds were tested in benzene solutions using temperature variation as a route for viscosity changing (Fig. 4). Small (5–8 %) decrease in absorption intensity under temperature increase from 20 up to 60 °C was found for all compounds under investigation. Fluorescent changes are very small for 2, 3 and 4; intensity decrease are more pronounced for 5 and 1. Such difference could be explained by steric effect of the 8-substituent. In case of small phenyl or biphenyl substituent rotation is not hampered, at the same time naphthalene, antracene and pyrene substituents could not rotate. Experimental results were confirmed by computer modeling of rotation barriers for investigated dyes (Fig. 5). Barriers values are increased in the following order: 1 ≈ 5 < 2 ≈ 4 < 3, as the linear dimensions of the substituent increased. The lowest energy position corresponds to the perpendicular arrangement of the substituent respectively to dipyrrin core. Phenyl moieties are not coplanar for compound 5 (Dihedral angle is equal to 140°) in the optimized geometry state, but they could rotate freely to each other. For all compounds substituent rotation lead to drastic BODIPY core planarity lose, due to steric effect of substituent interaction with methyl groups in pyrrole β-positions (see Fig. 5).
Solvent Parameters Scales
Solvatochromic effects are usually described with the use of different solvents scales and parameters. And on the first step, taking into account the influence of solvent viscosity on spectral properties we used dynamic viscosity to determine its influence on the spectral characteristics (Fig. 6).
But, as we can see on Fig. 6. the dependency is not clear, so the nature of the solvent, ability for nonspecific interactions and solvent polarity should be taking into account. In case of the solvents of the same chemical group the dependency became clear.
When considering the change in the physical characteristics of the homologous series of alcohols an influence of the solvent polarity on the photophysical characteristics of the compounds were detected. The values of the ratio of fluorescence and absorption intensities are increasing under the polarity increasing for compounds 2, 3, 4 (Fig. 7). Fluorescence quantum yield values changes are more complex due to the influence of solvent polarity on the vibrational component of spectra and it’s broadening via solvent variation.
This dependency is very uncommon for BODIPY dyes, because polar solvents are usually considered as the fluorescence quenchers for the dyes. Closely connected results were obtained earlier by Sunahara et al. [22], and BODIPY were proposed for polarity evaluation in proteins, membranes and receptors. Spectral properties variations are more complex for compound 5 due to its properties of fluorescent molecular rotor. Fluorescence intensity is increasing up to 3.5 cP, on higher viscosities values the observed quantum yields are decreasing (Fig. 8).
The contribution of different solvent parameters has been also studied by the method of linear regression. The Catalan solvent parameters set – SPP, SB, SA as well as Kamlet-Taft parameters – π, a, b, were used characterizing the contribution of polarity, acid and basic properties of the solvent [23, 24]. For all compounds this approach of the solvatochromic effects description shows low efficiency (Table 3). In case of Catalan scales set we also tried the four parameter set which includes an additional dipolarity parameter (SdP, [25]), the obtained results are similar with classical approach and the accuracy of approximation are closely the same. It should be noted that increasing the number of condensed rings in 8-position substituent decrease the accuracy of the obtained data. Thus, the electron transfer from the substituent to BODIPY core plays essential role in compounds photophysical properties.
To investigate the reasons that account for the difference in optical properties of compounds in aggregated states, theoretical calculation was conducted (Table 4). The substituents on 8-position of the BODIPY core contribute very little to the conjugation on HOMO and LUMO orbitals, but electronic density mainly localized on the substituent π-system for the LUMO + 1 in case of all investigated compounds and on HOMO-1 for “non-rotor” dyes. HOMO-LUMO energy gap are very close for all investigated BODIPY (2.89–2.91 eV) indicating their similar characteristics of EAS. Dipole moment changes in the range of 4.084 up to 4.539 Debye. Increasing the substituent length lead to dipole moment increasing while the substituent broadening decreasing dipole moment.
Conclusions
Four BODIPY based dyes were synthesized and characterized. Spectral and photophysical properties were evaluated in a range of organic solvents. Structural and solvent influence on practically significant characteristics were determined. 8-biphenyl substituted BODIPY was found to be the fluorescent molecular rotor demonstrating the fluorescence changes under the temperature variations unlike the BODIPY with larger substituents. Fluorescence intensity is increasing in case of solvent polarity growth, it is not typical for BODIPY and that fact could be explained by the high hydrophobicity of compounds under investigation. Taking that into account the BODIPY with bulky substituents could be used for fluorescence viscosity measurements, and as the fluorescent media polarity indicators in analytical chemistry and biochemistry.
References
Rumyantsev EV, Aleshin SN, Desoki A, Marfina YS, Antina EV (2013) Kinetic resistance of Borofluoride complexes of Dipyrrolylmethenes to acids. Russ J Inorg Chem 58:596–601
Marfin YS, Rumyantsev EV, Yutanova SL, Antina EV (2013) Spectral and photophysical properties, photo and heat resistance of Dipyrrolylmethene Borofluoride complex and its hybrid material with Polymethylmethacrylate. Russ J Gen Chem 83:381–385
Ziessel R, Goze C, Ulrich G, Cosario M, Retailleau P, Harriman A, Rostron JP (2005) Intramolecular energy transfer in pyrene–Bodipy molecular dyads and triads. Chem Eur J 11:7366–7378
Karmakar A, Chaudhuria T, Mula S, Chattopadhyay S (2015) Charge transfer in the electron donor–acceptor complexes of a Meso-phenol BODIPY dye with Chloranils and fullerenes. Spectrochim Acta A Mol Biomol Spectrosc 137:1258–1264
Spiegel JD, Kleinschmidt M, Larbig A, Tatchen J, Marian CM (2015) Quantum-Chemical Studies on Excitation Energy Transfer Processes in BODIPY-based Donor-Acceptor Systems. Journal of Chemical Theory and Computation. Accepted manuscript 11:4316–4327
Bahaidarah E, Harriman A, Stachelek P, Rihn S, Heyerb E, Ziessel R (2014) Fluorescent molecular rotors based on the BODIPY motif: effect of remote substituents. Photochem Photobiol Sci 10:1397–1401
H. Rongrong, E. Lager, A. Aguilar-Aguilar, J. Liu, J.W.Y Lam, H.H.Y. Sung, I.D. Williams. Twisted intramolecular charge transfer and aggregation-induced emission of BODIPY derivatives. J Phys Chem C 113 (2009) 15845–15853.
Haidekker M, Theodorakis E (2010) Environment-sensitive behavior of fluorescent molecular rotors. J Biol Eng 4:11–25
Weissberger A, Proskauer ES, Riddick JA, Toops EE (1955) Organic solvents. Physical properties and methods of purification. Interscience Publishers Inc, New York
Bruttel P, Schlink R (2003) Water Determination by Karl Fischer Titration. Meteorol Monogr 8:2003–2009
Krestov GA, Afanas'yev VN, Yefremova LS (1988) (in Russian) Physical and Chemical Properties of Binary Solvents: Handbook. Khimiya, Leningrad
Wolfbeis OS (2008) Springer Series on Fluorescence. Standardization and Quality Assurance in Fluorescence Measurements Techniques. doi:10.1007/978-3-540-75207-3
HyperChem. Computational Chemistry. Hypercube Inc. (1996) Publication HC50–00-03-00. Hypercube, Canada
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94:2319–2358
Guzow K, Ceszlak A, Kozarzewska M, Wiczk W (2011) Influence of substituents on the nitrogen atom of 3-[2-(4-aminophenyl)benzoxazol-5- yl]alanine derivatives on their photophysical properties - solvatochromic studies. Photochem Photobiol Sci 10:1610–1621
Yu.S. Marfin, E.V. Rumyantsev, Ya.S. Fadeev, E.V. Antina. Relationship between the spectral properties of solutions of Borofluoride complex of alkylated Dipyrromethene and the physicochemical parameters of solvents. Russ J Phys Chem A 86 (2012) 1068–1072.
Reichardt C, Welton T (2011) Solvents and Solvent Effects in Organic Chemistry. Fourth Edition, Wiley-VCH Verlag GmbH & Co KGaA
Catalan J, Lopez V, Perez P (1996) Use of the SPP Scale for the Analysis of Molecular Systems with Dual Emissions Resulting From The Solvent Polarity. J Fluoresc 6:15–22
Marfin YS, Merkushev DA, Usoltsev SD, Shipalova MV, Rumyantsev EV (2015) Fluorescent properties of 8-substituted BODIPY dyes: influence of solvent effects. J Fluoresc. doi:10.1007/s10895-015-1643-9
Caltagirone C, Arca M, Falchi AM, Lippolis V, Meli V, Monduzzi M, Nylander T, Rosa A, Schmidt J, Talmond Y, Murgia S (2015) Solvatochromic Fluorescent BODIPY Derivative as imaging agent in Camptothecin loaded Hexosomes for possible Theranostic applications. RSC Adv 5:23443–23449
Marfin YS, Merkushev DA, Levshanov GA, Rumyantsev EV (2014) Fluorescent properties of 8-phenylBODIPY in ethanol – ethylene glycol mixed solutions. J Fluoresc. doi:10.1007/s10895-014-1447-3
Sunahara H, Urano Y, Kojima H, Nagano T (2007) Design and synthesis of a library of BODIPY-based environmental polarity sensors utilizing Photoinduced electron-transfer-controlled fluorescence ON/OFF switching. J Am Chem Soc 129:5597–5604
Banuelos PJ, Lopez Arbeloa F, Martinez Martinez V, Lopez Arbeloa T, Lopez Arbeloa I (2004) Photophysical properties of the Pyrromethene 597 dye: solvent effect. J Phys Chem A 108:5503–5508
Marfin YS, Rumyantsev EV (2014) Analysis of solvation and structural contributions in spectral characteristics of dipyrrin Zn(II) complexes. Spectrochim Acta A Mol Biomol Spectrosc 130:423–428
Catalan J (2009) Toward a generalized treatment of the solvent effect based on four empirical scales: dipolarity (SdP, a new scale), polarizability (SP), acidity (SA), and basicity (SB) of the medium. J Phys Chem B 113:5951–5960
Acknowledgments
The work was supported by the grants of the Russian Foundation for Basic Research (No 16-03-01028, No 15-33-20002) and the grant of the President of the Russian Federation for young scientists (Grant No. МК-8835.2016.3).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Marfin, Y.S., Vodyanova, O.S., Merkushev, D.A. et al. Effect of π-Extended Substituents on Photophysical Properties of BODIPY Dyes in Solutions. J Fluoresc 26, 1975–1985 (2016). https://doi.org/10.1007/s10895-016-1891-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1891-3