Abstract
Adoxophyes honmai, a serious pest of tea plants, prefers to lay eggs on mature tea leaves rather than young leaves. Here, we examined a hypothesis that Ascogaster reticulata, an egg-larval parasitoid of A. honmai, increases the likelihood of encountering host egg masses by searching mature tea leaves when host-derived cues are not available. In a dual-choice bioassay using a four-arm olfactometer, A. reticulata preferred odor from intact, mature leaves versus young leaves. Based on volatile analysis with gas chromatography-mass spectrometry (GC-MS), we identified 5 and 10 compounds from mature and young leaf volatiles, respectively. The 5 components in the extract from intact mature leaves included (Z)-3-hexenyl acetate, (E)-β-ocimene, linalool, (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), and methyl salicylate. When each individual compound, or quaternary and quintenary blends of them, ratios of which were adjusted to match those of mature leaf volatiles, were provided, parasitoids preferred the full mixture and the quaternary blend devoid of DMNT to the solvent control. Methyl salicylate, one of the components of preferred blends, was not detected among young leaf volatiles. We concluded that the volatile composition of tea leaves changes, depending on their maturity, and that this composition affects foraging behavior of the parasitoid, which is closely related to the host herbivore’s oviposition preference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To utilize parasitoids efficiently in an agroecosystem, it is important to understand their behavioral traits in detail. It is postulated that parasitoids when searching for hosts or host habitat, utilize both short- and long-range host-finding cues, depending on the distance to the target host or host plant (Fatouros et al. 2008; Hilker and Fatouros 2015; Turlings and Erb 2018; Vet and Dicke 1992; Webster and Cardé 2017). Parasitoid wasps exploit olfactory cues released by host plants as long-range cues to search for host habitats (Steidle and van Loon 2002; Vinson 1991). After landing on a host plant, parasitoids rely on olfactory or gustatory chemical cues (e.g., kairomones) that directly indicate the presence of the host (Colazza et al. 1999; Fatouros et al. 2008). However, direct cues are often difficult to detect due to the small size of emitters and the minute quantities of such cues (Webster and Cardé 2017). In short-range host searching, parasitoids use not only direct cues, but also indirect cues, such as volatiles released from host plants, indicating where host feeding or oviposition sites are most likely to be found (Bell 1990; Meiners 2015). Short-range plant volatiles comprise two kinds of cues: uninduced host-habitat cues, based on phenological changes in host plants themselves, and induced host-habitat cues, based on changes in host plants induced by host infestation (Fatouros et al. 2008). While responses of parasitoids to induced cues have been well studied (Hilker and Fatouros 2015; Turlings and Erb 2018), few studies have been examined parasitoid exploitation of host-habitat cues that change during plant development (i.e., phenological or plant maturity changes; Hou et al. 2005; Jönsson et al. 2005). In order to utilize parasitoids more efficiently in agricultural fields, more information is needed on different responses of parasitoids to host-habitat cues that vary due to plant maturity, because parasitoids seem to search for host-habitat cues and host-induced cues in a sequential manner, and make choices to find more reliable direct cues under natural conditions.
Only a few studies have shown that plant maturity and/or phenological changes can provide information on appropriate host habitat for parasitoids (Hou et al. 2005; Jönsson et al. 2005). Jönsson et al. (2005) found that odors from oilseed rape flower buds were preferred to odors from opened flowers by Phradis interstitialis, a parasitoid of pollen beetles (Meligethes spp.). This strategy seems adaptive for the wasp to find suitable stages (eggs and 1st instar larvae) of the prey. Hou et al. (2005) showed that the larval parasitoid, Cotesia cariyai, prefers volatiles from infested young leaves to those from infested old leaves, indicating that induced host cues differed in successive stages of plant maturity. However, responses of wasps to intact leaves differing in maturity were not tested. Here, we focused on responses of wasps to intact leaves, young and mature, to understand behavioral strategies of parasitoid in host-habitat location, which have not been reported previously.
In the tea plantation ecosystem, the smaller tea tortrix, Adoxophyes honmai Yasuda (Lepidoptera: Tortricidae), is a serious pest of tea plants, and larvae of this moth cause great damage to tea plants (Tamaki 1991). In this species, rapid development of resistance to diamide insecticides was observed in 2010–2011 in Shizuoka Prefecture, the largest tea-producing region in Japan. Globally, this was the second case of high resistance to diamides (Uchiyama and Ozawa 2014), illustrating the difficulty of controlling this moth by chemical means alone, and the need for other approaches, including biological control. Ascogaster reticulata Watanabe (Hymenoptera: Braconidae) is an egg-larval specialist parasitoid wasp of 7 tortricid species, including A. honmai (Kamijo 1973; Takagi 1974; Watanabe 1967). A. reticulata is one of the dominant parasitoid species in Japanese tea fields (Takagi 1974). Female parasitoids oviposit in host eggs. Parasitized eggs hatch normally and develop to fourth instar larvae. Parasitoid larvae egress from fourth instars of the host, feed on them externally, and then pupate (Kawakami 1985). Several studies have investigated responses of A. reticulata to host plants infested by A. honmai in terms of associative learning and contact cues (Deshpande and Kainoh 2012; Seino and Kainoh 2008; Seino et al. 2010). However, little is known about innate responses of these wasps to intact host plants, and about the relationships between those responses and host plant chemistry. As for A. honmai, Piyasaengthong et al. (2016) showed that the host moth prefers to lay egg masses on mature tea leaves rather than on young leaves. Based on this finding, we hypothesized that if A. reticulata effectively searches for A. honmai egg masses without host-derived cues, it is reasonable for these wasps to search near mature tea leaves for short-range host-habitat location. Deshpande and Kainoh (2012) reported that A. reticulata may not respond to volatiles from intact tea leaves, but their results were marginally insignificant. These situations prompted us to re-examine responses of A. reticulata to intact tea leaf volatiles.
In this study, we observed the effect of foliar aging on behavior of A. reticulata females, and their responses to volatile chemicals from tea leaves. This study reveals how volatile compounds released by the same plant structures at different stages of maturity enable parasitoids to locate host habitats effectively.
Methods and Materials
Plants
Potted tea plants, Camellia sinensis cv. Yabukita, which were approximately 10 years old and grown from seedlings were kept in a greenhouse at L14 (25 ± 1 °C):D10 (20 ± 1 °C) and 60 ± 10% RH. Plants were watered every other day. Leaf maturity was determined as described by Piyasaengthong et al. (2016). Both leaf color and leaf position on a branch were used as factors to determine foliar maturity. See Supplementary Fig. 1 in Piyasaengthong et al. (2016) for details about color criteria for young leaves (YL) and mature leaves (ML). Leaf color of YL was lighter than that of ML. Leaves with colors that were difficult to classify as YL or ML were not used in this study. In addition, YL were located at tips of branches, whereas ML were closer to bases of branches.
Insects
The parasitoid, A. reticulata, was reared as described by Kainoh (1988), and its host A. honmai was reared as described by Tamaki (1966). Both parasitoids and hosts were obtained from colonies reared for more than 10 years in the laboratory (25 ± 1 °C, 60 ± 10% RH, and a L16:D8 photoperiod). During this time, both laboratory populations were occasionally bolstered with wild specimens to avoid inbreeding. Parasitized and unparasitized A. honmai larvae were reared in plastic boxes (25 × 18 × 8 cm) on an artificial diet containing dried tea leaf powder and crumpled pieces of wax paper (9 × 4.5 cm). A. reticulata cocoons and A. honmai pupae were taken out of rearing boxes, and A. reticulata cocoons were placed in individual test tubes (1 cm in diameter, 7.5 cm in length, 5 mL) with cotton plugs until they emerged. Emerged A. reticulata adults were sexed under a stereomicroscope, and 10 to 20 of each were kept in a plastic container (15 cm in diameter, 9 cm in height) with wet cotton on the bottom and honey streaked on the inner wall. Two- to four-day-old naive females (without any contact with males or plants after eclosion) were used in all experiments. Behavior of mated and unmated female wasps does not differ (Deshpande and Kainoh 2012). A. honmai pupae were placed in rearing boxes (25 × 18 × 8 cm) with wet cotton on the bottom. The inner side of the box lid was covered with a sheet of wax paper (37 × 25 cm) for oviposition. Collected egg masses were used to maintain colonies of parasitoids and hosts.
Volatile Collection
Volatiles from intact tea leaves were collected as described by Kobayashi et al. (2012). All leaves were plucked 30 to 60 min before starting volatile collection, washed carefully with tap water to remove impurities, and rinsed with distilled water. Preliminary volatile collection using 6 YL or 6 ML for 4 h showed that ML released much smaller quantities of volatiles than YL. Levels of ML volatiles were close to the detection limit of our instrumentation. Therefore, 20 YL and 60 ML were used for each YL and ML volatile collection. Washed leaves were placed in 500-mL gas washing conical flasks. The inlet of each flask was connected to an activated charcoal filter, and the outlet was attached to an adsorbent cartridge and a vacuum pump (KNF Neuberger, Freiburg, Germany). The adsorbent cartridge was composed of a glass tube (i.d. 6.0 mm, o.d. 7.0 mm) containing the adsorbent, HayeSep Q (ca. 100 mg, 60–80 mesh; Restek Corporation, USA), sandwiched between quartz wool (fine grade; Daico Mfg, Kyoto, Japan). For conditioning, adsorbent cartridges were washed with hexane and heated at 150 °C for 2 h under nitrogen flow (30 mL/min) before use. Replicates were performed 3 times for each ML and YL. Volatiles were collected for 4 h during the daytime at 25 ± 1 °C and 60 ± 10% RH under fluorescent lights (ca. 2950 lx). Airflow was adjusted to 1000 mL per min. After collection, volatiles were extracted in 1.0 mL hexane, and the extract was stored at −20 °C until analysis. For controls, after conditioning of adsorbent cartridges, volatiles were collected from empty flasks and were extracted and analyzed to check background volatiles from the collection system.
Chemicals
(Z)-3-Hexenyl acetate (H) and indole were purchased from Tokyo Chemical Industry Co., Ltd., Tokyo, Japan. Linalool (L) and methyl salicylate (M) were obtained (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan). (E)-β-Ocimene (O) and (E)-4,8-dimethyl-1,3,7-nonatriene [(E)-DMNT; D] were from our stock samples, synthesized previously (Liu et al. 2019). (Z)-3-Hexenyl butyrate, (Z)-3-hexenyl 2-methylbutyrate, (Z)-3-hexenyl 3-methylbutyrate, and (Z)-3-hexenyl hexanoate were prepared by Steglich esterification (Neises and Steglich 1978) of (Z)-3-hexen-1-ol (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) and corresponding acids, butyric acid, hexanoic acid, and 3-methylbutyric acid were purchased from FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan. 2-Methylbutyric acid was obtained from Kanto Chemical Co., Inc., Tokyo, Japan. (E,E)-α-Farnesene was obtained, as described previously (Liu et al. 2019).
Chemical Analysis
Compounds in volatile extracts were analyzed by gas chromatography (GC) and by gas chromatography-mass spectrometry (GC-MS). GC analysis was performed on an HP6890 gas chromatograph (Hewlett-Packard, Palo Alto, CA, USA) equipped with a DB-5MS column (25 m × 0.25 mm, 0.25 μm film thickness; J&W Scientific Folsom, CA, USA). Each sample (1 μL) was injected at 280 °C in splitless mode (sampling time: 0.75 min) using helium as a carrier gas (1 mL/min) in constant flow mode. The oven temperature was held at 35 °C for 1 min, increased at a rate of 10 °C per min to a final temperature of 280 °C, and then held for 0.5 min. Components in samples were detected with a flame-ionization detector at 280 °C and were analyzed quantitatively using ChemStation software (Agilent Technologies, ver. A 10.00). For GC-MS, an HP 6890 N gas chromatograph (Hewlett-Packard, Palo Alto, CA, USA) was coupled with an MS600H mass spectrometer (JEOL Ltd., Tokyo, Japan) to obtain electron ionization mass spectra at 70 eV. The ion source temperature was set at 210 °C. GC conditions were the same as above, except that the oven temperature was programmed to start with an initial temperature of 45 °C for 1 min, raised to 280 °C at a rate of 10 °C per min, then held for 0.5 min. Mass spectra were analyzed with the software package TSS 2000 (v. 2.00.0062, Shrader Analytical and Consulting Laboratories, MI, USA). Compounds were provisionally identified by searching the NIST Mass Spectral Program (v. 1.6) and then confirmed by comparison of mass spectral data and retention times with those of standards. Amounts of individual volatile compounds were calculated by comparing their peak areas relative to those of standards.
Bioassay
In all bioassays, behavioral responses of A. reticulata females to odors from natural tea leaves, headspace volatile extracts, and standard chemicals were observed in a four-arm olfactometer (24 × 24 × 2 cm; Fig. 1) (Fujinuma et al. 2010; Vet et al. 1983) in two-choice tests. Odor sources were prepared as follows. When tea leaves were provided as odor sources, 6 ML or 6 YL were placed in a 500-mL flask as described above in the section about volatile collection. Volatile extracts from ML or standard chemicals were applied to filter paper strips (Advantec No. 2, 4 × 40 mm). These strips were put into glass tubes (i.d. 5 mm, o.d. 7.5 mm, length 100 mm). For ML volatile extracts, an aliquot (4.2 μL) of extracts containing 6 LE (Leaf Equivalents; 1 LE represents the amount of volatiles collected from 1 ML in 10 min) was applied to a filter paper strip, since ML volatiles were collected from 60 leaves for 4 h, extracted in 1.0 mL hexane, and used for 10 min bioassays. Based on the chemical analysis of ML volatiles, three chemical mixtures were prepared: (a) a blend containing all 5 authentic chemical components of ML volatiles in the natural ratio at four different concentrations, corresponding to 3, 6, 30, and 300 LE, (b) a blend containing 4 of the 5 components in an amount equal to 30 LE, (c) 5 individual compounds equivalent to 30 LE. From results of bioassays using these chemicals, we found a combination preferred by the parasitoid, and then we prepared one more blend. In this blend, 1 μg of each component of the preferred combination was mixed, creating an even-ratioed mixture, to test whether A. reticulata respond to a mixture of ML volatile components in an unnatural ratio. All mixtures of authentic chemicals were analyzed by GC to check the ratios of components before using them in bioassays.
For bioassays, charcoal-filtered air was introduced into the olfactometer arena from each odor source at a flow rate of 1000 mL per min. In a two-choice system using a four-arm olfactometer, the same odor was fed into two opposing arms. A female wasp was released at the center of the arena, and her behavior was recorded for 10 min with a camera (SUV-Cam II, ELMO, Aichi, Japan) installed 37 cm above the arena. Only the time spent actively moving (walking, antennating; hereinafter referred to as “residence time”) in each treated area of the arena was summarized using The Observer XT v. 9.0 software (Noldus Information Technology, Wageningen, The Netherlands). For all bioassays, a test using an individual wasp was counted as one replicate. Replicates were performed >12 times for each two-choice test. Natural leaves were changed every two to three replicates, whereas filter paper strips were replaced after each replicate. Before and after a replicate, the arena was cleaned with 70% ethyl alcohol. After finishing observations of two wasps, positions of the odor sources were changed to eliminate directional bias. All bioassays were performed between 11:00 and 15:00.
Statistical Analysis
All statistical analyses were performed with R, v. 3.5.3 (R Core Team 2019). In consideration of pseudoreplications caused by reuse of sets of natural leaves for 2–3 wasps, generalized linear mixed models (GLMMs, in the package lme4; Bates et al. 2015) with a Gaussian distribution and the log link function were used for data analyses. In this model, the explanatory variable was the maturity of leaves (ML or YL), the dependent variable was the residence time in either ML or YL treated areas of the four-arm arena, and the random factor was the sets of leaves. Also, to account for differences in activity among individual wasps and the relationship between residence times in control and treatment areas of the arena, we used an offset with the log-transformed total residence time in the model [known as a rate model; for details see p. 518 in Crawley 2007 and p. 63 in Faraway 2006]. On the other hand, there was no pseudoreplication in bioassays using volatile extracts or compound solutions. Thus, paired t-tests were used to compare residence times at either treatment or control areas in the arena. For all bioassays, wasps that remained inactive for more than 3 min after release into the arena, were regarded as non-responding individuals, and their data were excluded from statistical analyses. Amounts of volatiles from YL and ML were corrected for ng per 1 g fresh leaf weight per 4 h and analyzed using Wilcoxon rank sum test.
Results
Behavioral Responses of Female A. reticulata to ML and YL Volatiles
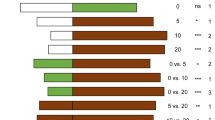
When headspace odor from YL and charcoal-filtered air (control) were provided in two-choice tests, residence times were not significantly different (t = 1.23, df = 14, P > 0.05). In contrast, a highly significant preference was observed for ML odor vs control (t = 4.217, df = 14, P < 0.001). Moreover, ML odor was preferred significantly over YL odor (t = −2.06, df = 16, P = 0.039; Fig. 2).
Preferences of A. reticulata in two-choice bioassays between volatiles from young versus mature tea leaves in a four-arm olfactometer. For each two-choice test, responses of A. reticulata to 6 young leaves versus a blank (charcoal filtered air; n = 15), 6 mature leaves versus a blank (n = 15), and 6 young versus 6 mature leaves (n = 17) were recorded using a four-arm olfactometer. Bars represent mean residence times (± SE) of A. reticulata in each treatment area. NR indicates the number of individuals that did not respond (i.e., the number that remained inactive for more than 3 min). Data for each group were tested separately using GLMMs. *p < 0.05, ***p < 0.001, ns: not significant
Volatile Compositions of ML and YL
Volatiles were collected from 60 ML and 20 YL, respectively. GC and GC-MS results revealed that volatile components were qualitatively different between ML and YL (Table 1). Four components were detected in both ML and YL: (Z)-3-hexenyl acetate (H), (E)-β-ocimene (O), linalool (L), and (E)-DMNT (D). Methyl salicylate (M) was detected only in ML volatiles. Six other compounds were detected only among YL volatiles: (Z)-3-hexenyl butyrate, (Z)-3-hexenyl 2-methylbutyrate, (Z)-3-hexenyl 3-methylbutyrate, indole, (Z)-3-hexenyl hexanoate, and (E,E)-α-farnesene. Although total the amount of volatiles collected from YL was 15.1 times higher than from ML, the difference was statistically nonsignificant owing to the small sample size (W = 9, df = 4, P = 0.10). For the same reason, even though mean amounts of individual volatiles collected from YL were 2.4 (D) to 13.5 times (H) higher than from ML, these differences were also nonsignificant (P = 0.10): H (W = 9, df = 4), O (W = 9, df = 4), L (W = 9, df = 4), and D (W = 7, df = 4).
Behavioral Responses of Female A. reticulata to ML Volatile Extract and Chemical Standards
Wasp responses to the volatile extract from ML were tested in an olfactometer experiment. Females spent more time in treatment areas than in control areas (t = −4.36, df = 14, P < 0.001). This indicated that ML volatile extracts contained components that attracted the wasps; therefore, we focused on the 5 main components (H, O, L, D, and M) identified from ML extracts as candidates for wasp attractants.
In order to investigate behavioral responses of wasps to the above 5 components in detail, blends and single compounds were offered as odor sources. The 5-component blend (HOLDM) was used in different amounts [3, 6, 30, and 300 leaf equivalents (LE)], to test dose-responses. The lowest concentration of the blend (3 LE) was no more attractive to the wasps than the control (t = −0.62, df = 12, P > 0.05; Fig. 3); however, all 3 higher concentrations attracted them significantly [6 LE (t = 2.32, df = 12, P = 0.039), 30 LE (t = 2.64, df = 12, P = 0.022), and 300 LE (t = 3.12, df = 12, P = 0.009)]. Moreover, residence times in treated areas increased in proportion to increasing amounts of LE. On the other hand, females showed no significant preference for individual compounds (P > 0.05; Table 2): H (t = 0.21, df = 11), O (t = −0.24, df = 11), L (t = −0.35, df = 11), D (t = −0.24, df = 11), and M (t = −0.95, df = 11) at 30 LE, a dose that wasps preferred in the 5-compound blend test.
Behavioral responses of A. reticulata to blends of 5 volatile compounds derived from mature tea leaves at different doses in a four-arm olfactometer. Mean residence times (± SE) of A. reticulata in each treatment (hexane solution of volatile blends) and control (hexane) areas were measured in two-choice tests (n = 13). Ratios of the blends were adjusted to match those of mature tea leaf volatiles. Doses were quantified as leaf equivalents, the number of tea leaves emitting same amounts of volatiles as provided. NR indicated the number of individuals that did not respond for more than 3 min. Data for each dose were compared separately using paired t-tests. *p < 0.05, **p < 0.01, ns: not significant
In order to determine which component among the 5 is responsible for eliciting positive responses from the wasps, 4-component blends (HOLD, HOLM, HODM, HLDM, and OLDM) were tested at 30 LE in bioassays against the solvent blank. Females spent significantly more time in HOLM-treated areas than controls (t = −3.46, df = 14, P = 0.004; Fig. 4a). In contrast, female wasps showed no significant responses to the other four blends (P > 0.05): HOLD (t = 0.81, df = 14), HODM (t = −0.92, df = 14), HLDM (t = −0.24, df = 14), and OLDM (t = −0.32, df = 14). Additionally, when even-ratioed HOLM was provided, it was significantly preferred by females over control (t = −3.50, df = 13, P = 0.004; Fig. 4b).
Behavioral responses of A. reticulata to mixtures of 4 of the 5 compounds; (Z)-3-hexenyl acetate (H), (E)-β-ocimene (O), linalool (L), (E)-4,8-dimethyl-1,3,7-nonatriene (D) and methyl salicylate (M), at 30 LE in a four-arm olfactometer. Mean residence times (± SE) of A. reticulata were measured in two-choice tests between a control and (a) each of five 4-compound mixtures, component ratios of which were adjusted to match those of mature tea leaf volatiles (n = 15), or (b) a blend comprising equal amounts of H, O, L, and M (n = 14). For both a and b, since the solvent was hexane, hexane alone was used as the control. NR indicates the number of individuals that did not respond for more than 3 min. Data for each category of chemical and the control were tested separately with paired t-tests. **p < 0.01, ns: not significant
Discussion
In this study, we focused on the leaf maturity preference of the egg-larval parasitoid, Ascogaster reticulata. Responses of female wasps to odors from intact tea leaves showed that wasps preferred ML to YL. This result indicates that the preference for mature leaves in A. reticulata is similar to the oviposition preference of Adoxophyes honmai reported by Piyasaengthong et al. (2016). Searching around ML can be considered as a reasonable strategy for host-habitat location for the parasitoid, since the female moth prefers ML to YL for laying eggs; therefore, host eggs are more likely to be found on ML. Host localization conventionally consists of four steps (Fatouros et al. 2008; Iacovone et al. 2016; Webster and Cardé 2017): 1. host-habitat location using odors from host plants over long-range; 2. host-habitat location using odors from intact host plants and/or from plants infested by the host herbivore at short-range; 3. host location using cues produced by the host herbivore at short range; 4. host recognition. Many studies have reported that chemical cues produced by plants in response to host infestation are more specific and reliable information for foraging parasitoids (reviewed by Hilker and Fatouros 2015; Turlings and Erb 2018; Vet and Dicke 1992). On the other hand, some studies have shown that odor cues emitted by intact host plants attract parasitoids over short distances at the same level as those from infested host plants (Mohammed et al. 2019). In addition, it is obvious that habitat recognition by parasitoids can occur continuously or almost simultaneously with host infested-plant odor recognition at short range. In such cases, other factors available from a host plant, like plant maturity, can provide specific information about the host habitat on the host plant. This study and others about parasitoid responses to plant maturity (Hou et al. 2005; Jönsson et al. 2005) showed that coincidence of plant maturity preference between natural enemies and herbivores seems to be adaptive for the predators to locate direct host cues.
Although YL emit higher amounts of volatiles than ML, wasps showed no significant responses to YL volatiles. Also, the HOLDM and HOLM blends were preferred by A. reticulata, but other blends and single chemicals did not elicit any responses. These results show that a combination of compounds H, O, L, and M is recognized by female wasps. The absence of M in YL volatiles may be a key to understanding wasp preferences, but this needs further study (e.g., a dual-choice bioassay between HOLDM and HOLD). In addition to these responses, when female wasps were exposed to a mixture of HOLM in equal ratios, they responded significantly to the mixture compared to the solvent control. This implies that wasps are able to respond to a broad range of ratios under varying natural circumstances. Volatile compounds in the atmosphere gradually degrade (Blande et al. 2014). Degradation of compounds can alter the chemical composition of the blend by changing the ratio of compounds within the blend (Šimpraga et al. 2016). Therefore, broad tuning to ratios of components in the blend seems to be advantageous for the wasps.
With regard to searching strategies by parasitoids, three possible modes of odor discrimination have been proposed (McCormick et al. 2012): 1. presence or absence of compounds specific to a certain plant species are used for plant recognition, so-called “species-specific odor recognition”; 2. predators discriminate changes in ratios of volatile compounds and alter their behavior, so-called “ratio-specific odor recognition”; 3. odor discrimination is based on the entire blend or many of its components detected as a whole, so-called “whole-blend odor recognition.” A study involving a herbivore predator reported a phenomenon consistent with whole-blend odor recognition (van Wijk et al. 2011). The predatory mite, Phytoseiulus persimilis, showed a positive response to methyl salicylate, a negative response to (Z)-3-hexenyl acetate, and no response to other major volatiles from herbivore-infested lima beans, when compounds were provided individually. In contrast, a mixture of all compounds from infested lima beans was more attractive than individual compounds or partial mixtures, when offered with background odor from non-infested lima beans (van Wijk et al. 2011). This is similar to our results showing that the response of A. reticulata females to individual compounds differed from that to a blend of compounds. Female parasitoids do not rely on a single component of the preferred blend or a specific ratio of ML volatiles, suggesting that odor recognition is receptive to volatiles as a whole-blend. In the current study, A. reticulata seemed to distinguish differences in volatiles reflecting foliar aging using whole-blend odor recognition. However, tests were limited to individual compounds, and quaternary or quintenary blends of volatile components. If there are synergistic effects between components, wasps might respond differently to mixtures of two or three compounds, as shown in a study by Liu et al. (2019). In order to test synergistic effects, especially in binary and tertiary blends containing M, further experiments with other combinations and different ratios of components are required.
In this study, a parasitoid wasp, A. reticulata showed preference for odors from mature tea leaves over those from young tea leaves, and subsequent chemical analyses of the odors and bioassays using chemical standards suggest that the preference is for specific mixtures of tea leaf volatiles. The preference for mature tea leaves by the parasitoid wasp parallels the previously reported oviposition preference of the host moth, A. honmai. These results support our hypothesis that it is reasonable for wasps to search near mature tea leaves for short-range host-habitat location to increase the probability of finding direct host cues. To understand parallel preferences for mature tea leaves between parasitoid wasps and host moths, detailed experiments should be performed on both insect species. Identification of chemical components that are responsible for mature leaf preference of both A. reticulata and A. honmai may enable us to develop alternative ‘push-pull’ strategies (Cook et al. 2007) involving a combination of parasitoid attraction and pest moth avoidance for tea plantation management in the future.
Data Availability
All data and materials from this study are available from the corresponding author upon request.
References
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bell WJ (1990) Locating patches and distant resources. In: Bell WJ (ed) Searching behaviour: the behavioural ecology of finding resources. Chapman and Hall, London, pp 69–82
Blande JD, Holopainen JK, Niinemets Ü (2014) Plant volatiles in polluted atmospheres: stress responses and signal degradation. Plant Cell Environ 37:1892–1904. https://doi.org/10.1111/pce.12352
Colazza S, Salerno G, Wajnberg E (1999) Volatile and contact chemicals released by Nezara viridula (Heteroptera: Pentatomidae) have a kairomonal effect on the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae). Biol Control 16:310–317
Cook SM, Khan ZR, Pickett JA (2007) The use of push-pull strategies in integrated pest management. Annu Rev Entomol 52:375–400. https://doi.org/10.1146/annurev.ento.52.110405.091407
Crawley MJ (2007) The R book. Wiley, Chichester
Deshpande SA, Kainoh Y (2012) Herbivore egg deposition induces tea leaves to arrest the egg-larval parasitoid Ascogaster reticulata Watanabe (Hymenoptera: Braconidae). Entomol Exp Appl 144:172–180. https://doi.org/10.1111/j.1570-7458.2012.01275.x
Faraway JJ (2006) Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. Chapman & Hall/CRC, Boca Raton
Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M (2008) Foraging behavior of egg parasitoids exploiting chemical information. Behav Ecol 19:677–689. https://doi.org/10.1093/beheco/arn011
Fujinuma M, Kainoh Y, Nemoto H (2010) Borago officinalis attracts the aphid parasitoid Aphidius colemani (Hymenoptera: Braconidae). Appl Entomol Zool 45:615–620. https://doi.org/10.1303/aez.2010.615
Hilker M, Fatouros NE (2015) Plant responses to insect egg deposition. Annu Rev Entomol 60:493–515. https://doi.org/10.1146/annurev-ento-010814-020620
Hou M, Takabayashi J, Kainoh Y (2005) Effect of leaf age on flight response of a parasitic wasp Cotesia kariyai (Hymenoptera: Braconidae) to a plant-herbivore complex. Appl Entomol Zool 40:113–117. https://doi.org/10.1303/aez.2005.113
Iacovone A, French AS, Tellier F, Cusumano A, Clement G, Gaetner C, Conti E, Salerno G, Marion-Poll F (2016) The role of contact chemoreception in the host location process of an egg parasitoid. J Insect Physiol 91–92:63–75. https://doi.org/10.1016/j.jinsphys.2016.07.001
Jönsson M, Lindkvist A, Anderson P (2005) Behavioural responses in three ichneumonid pollen beetle parasitoids to volatiles emitted from different phenological stages of oilseed rape. Entomol Exp Appl 115:363–369. https://doi.org/10.1111/j.1570-7458.2005.00271.x
Kainoh Y (1988) Some factors influencing sex ratio in Ascogaster reticulatus Watanabe (Hymenoptera: Braconidae). Appl Entomol Zool 23:35–40. https://doi.org/10.1303/aez.23.35
Kamijo K (1973) The parasite complex of Choristoneura diversana Hübner injurious to todo-fir, Abies sachalinensis masters. Jpn J Appl Entomol Zool 17:77–83 (in Japanese with English summary)
Kawakami T (1985) Development of immature stages of Ascogaster reticulatus Watanabe (Hymenoptera: Braconidae), an egg-larval parasitoid of the smaller tea tortrix moth, Adoxophyes sp. (Lepidoptera: Tortricidae). Appl Entomol Zool 20:380–386. https://doi.org/10.1303/aez.20.380
Kobayashi K, Arai M, Tanaka A, Matsuyama S, Honda H, Ohsawa R (2012) Variation in floral scent compounds recognized by honeybees in Brassicaceae crop species. Breed Sci 62:293–302. https://doi.org/10.1270/jsbbs.62.293
Liu C, Matsuyama S, Kainoh Y (2019) Synergistic effects of volatiles from host-infested plants on host-searching behavior in the parasitoid wasp Lytopylus rufipes (Hymenoptera: Braconidae). J Chem Ecol 45:684–692. https://doi.org/10.1007/s10886-019-01088-y
McCormick AC, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310. https://doi.org/10.1016/j.tplants.2012.03.012
Meiners T (2015) Chemical ecology and evolution of plant-insect interactions: a multitrophic perspective. Curr Opin Insect Sci 8:22–28. https://doi.org/10.1016/j.cois.2015.02.003
Mohammed K, Agarwal M, Du XB, Newman J, Ren YL (2019) Behavioural responses of the parasitoid Aphytis melinus to volatiles organic compounds (VOCs) from Aonidiella aurantii on its host fruit Tahitian lime fruit Citrus latifolia. Biol Control 133:103–109. https://doi.org/10.1016/j.biocontrol.2019.03.015
Neises B, Steglich W (1978) Simple method for the esterification of carboxylic acids. Angew Chem Int Ed 17:522–524. https://doi.org/10.1002/anie.197805221
Piyasaengthong N, Sato Y, Kinoshita N, Kainoh Y (2016) Oviposition preference for leaf age in the smaller tea tortrix Adoxophyes honmai (Lepidoptera: Tortricidae) as related to performance of neonates. Appl Entomol Zool 51:363–371. https://doi.org/10.1007/s13355-016-0408-5
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org/
Seino H, Kainoh Y (2008) Associative learning and discrimination of 10 plant species by the egg-larval parasitoid, Ascogaster reticulata Watanabe (Hymenoptera: Braconidae). Appl Entomol Zool 43:83–90. https://doi.org/10.1303/aez.2008.83
Seino H, Shoji K, Kainoh Y (2010) Utilization of learned plant chemicals in host searching behavior by the egg-larval parasitoid Ascogaster reticulata Watanabe (Hymenoptera: Braconidae). Appl Entomol Zool 45:339–345. https://doi.org/10.1303/aez.2010.339
Šimpraga M, Takabayashi J, Holopainen JK (2016) Language of plants: where is the word? J Integr Plant Biol 58:343–349. https://doi.org/10.1111/jipb.12447
Steidle JLM, van Loon JJA (2002) Chemoecology of parasitoid and predator oviposition behaviour. In: Hilker M, Meiners T (eds) Chemoecology of insect eggs and egg deposition. Blackwell Publishing, Berlin, pp 291–317
Takagi K (1974) Monitoring of hymenopterous parasite in the field. Bull Tea Res Stn 10:91–131 (in Japanese with English summary)
Tamaki Y (1966) Mass rearing of the smaller tea tortrix, Adoxophyes orana Fischer von Röslerstamm, on a simplified artificial diet for successive generations (Lepidoptera: Torticidae). Appl Entomol Zool 1:120–124. https://doi.org/10.1303/aez.1.120
Tamaki Y (1991) Tortricid in tea. In: van der Geest LPS, Evenhuis HH (eds) Tortricids pests: their biology, natural enemies and control. Elsevier, Amsterdam, pp 541–551
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452. https://doi.org/10.1146/annurev-ento-020117-043507
Uchiyama T, Ozawa A (2014) Rapid development of resistance to diamide insecticides in the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae), in the tea fields of Shizuoka prefecture, Japan. Appl Entomol Zool 49:529–534. https://doi.org/10.1007/s13355-014-0283-x
van Wijk M, de Bruijn PJA, Sabelis MW (2011) Complex odor from plants under attack: herbivore’s enemies react to the whole, not its parts. PLoS One 6:e21742. https://doi.org/10.1371/journal.pone.0021742
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172. https://doi.org/10.1146/annurev.en.37.010192.001041
Vet LEM, van Lenteren JC, Heymans M, Meelis E (1983) An airflow olfactometer for measuring olfactory responses of hymenopterous parasitoids and other small insects. Physiol Entomol 8:97–106. https://doi.org/10.1111/j.1365-3032.1983.tb00338.x
Vinson SB (1991) Chemical signals used by parasitoids. Redia 74(3):15–42
Watanabe C (1967) Description of a new species of the genus Ascogaster Wesmael and notes on synonymy of Apanteles species (Hymenoptera: Braconidae). Insecta Mastumurana 29:41–44
Webster B, Cardé RT (2017) Use of habitat odour by host-seeking insects. Biol Rev 92:1241–1249. https://doi.org/10.1111/brv.12281
Acknowledgements
We appreciate the helpful comments of Prof. DeMar Taylor and Assoc. Prof. Seiichi Furukawa on this study. We are grateful to Dr. Yukie Sato for her advice on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
Not applicable.
Code Availability
Software packages and codes used for statistical analyses in this study are available from the corresponding author.
Supplementary Information
ESM 1
(PPTX 636 kb)
Rights and permissions
About this article
Cite this article
Komatsuzaki, S., Piyasaengthong, N., Matsuyama, S. et al. Effect of Leaf Maturity on Host Habitat Location by the Egg-Larval Parasitoid Ascogaster reticulata. J Chem Ecol 47, 294–302 (2021). https://doi.org/10.1007/s10886-021-01250-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01250-5