Abstract
Evidence for the the ability of birds to detect olfactory signals is now well documented, yet it remains unclear whether birds secrete chemicals that can be used as social cues. A potential source of chemical cues in birds is the secretion from the uropygial gland, or preen gland, which is thought to waterproof, maintain, and protect feathers from ectoparasites. However, it is possible that preen oil also may be used for individual recognition, mate choice, and signalling social/sexual status. If preen oil secretions can be used as socio-olfactory signals, we should be able to identify the volatile components that could make the secretions more detectable, determine the seasonality of these secretions, and determine whether olfactory signals differ among relevant social groups. We examined the seasonal differences in volatile compounds of the preen oil of captive white-throated sparrows, Zonotrichia albicollis. This species is polymorphic and has genetically determined morphs that occur in both sexes. Mating is almost exclusively disassortative with respect to morph, suggesting strong mate choice. By sampling the preen oil from captive birds in breeding and non-breeding conditions, we identified candidate chemical signals that varied according to season, sex, morph, and species. Linear alcohols with a 10–18 carbon chains, as well as methyl ketones and carboxylic acids, were the most abundant volatile compounds. Both the variety and abundances of some of these compounds were different between the sexes and morphs, with one morph secreting more volatile compounds in the non-breeding season than the other. In addition, 12 compounds were seasonally elevated in amount, and were secreted in high amounts in males. Finally, we found that preen oil signatures tended to be species-specific, with white-throated sparrows differing from the closely related Junco in the abundances and/or prevalence of at least three compounds. Our data suggest roles for preen oil secretions and avian olfaction in both non-social as well as social interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical signals mediate more animal interactions than any other signal type (Wyatt 2003), but historical assumptions about the lack of olfaction in birds have served to downplay the potential role of avian olfaction and hamper advances in the area of avian chemical signaling. However, recent studies have found that birds possess more functional olfactory receptor (OR) genes than previously thought, suggesting that olfaction could be important in this group (Steiger et al. 2008). In addition, data have emerged showing that birds do employ olfaction for foraging (e.g., Hagelin 2004; Nevitt et al. 2008), navigation and orientation (e.g., Bonadonna et al. 2003; Nevitt and Bonadonna 2005; Wallraff 2004), nest construction (e.g., Petit et al. 2002), and recognition (Caspers and Krause 2013; Krause and Caspers 2012), and even assessing predation risk (e.g., Amo et al. 2008; Whittaker et al. 2009).
There also is evidence suggesting that birds use chemical signals as social cues. Ducks and chickens subjected to bilateral olfactory nerve section engaged in significantly fewer mating behaviors compared to sham-operated males (Hirao et al. 2009). Antarctic prions, Pachiptila desolata, preferred the body scent of their partner to that of other conspecifics and avoided their own odor (Bonadonna and Nevitt 2004) in a manner consistent with avoidance of inbreeding (Mateo and Johnston 2000). Crested auklets (Aethia cristatella) preferred the odor of conspecific plumage assessed during “ruffsniff” social displays (Hagelin 2007b; Hagelin et al. 2003). However, despite these advances, our knowledge of the social role of avian odor is still rudimentary and studies of other avian taxonomic groups are warranted.
There are many potential sources of avian chemical signals (Campagna et al. 2012; Hagelin 2007a; Hagelin and Jones 2007), but preen gland secretions arising from the uropygial gland at the base of the tail could prove to be one of the most important. Preen oil has a role in waterproofing (Elder 1954) and feather maintenance (Jacob and ZisweIler 1982). It also provides protection against bacteria, fungi, mites, or other feather parasites (Jacob and ZisweIler 1982; Moyer et al. 2003; Shawkey et al. 2003), and accordingly, access to preen oil secretions is important for maintaining self-health and high reproductive investment (Giraudeau et al. 2010). However, there also is reason to believe that preen oil is an important social chemosignal (Hirao et al. 2009; Johansson and Jones 2007), as it contains volatile compounds that may act as strong signals detectable via olfaction. Preen oil compounds exhibit sexual (Amo et al. 2012; Leclaire et al. 2011, 2012; Mardon et al. 2010; Soini et al. 2007; Zhang et al. 2010;) and age variation (Shaw et al. 2011), as well as being repeatable signatures of an individual (Mardon et al. 2010; Whittaker et al. 2010). Finally, preen oil compounds are also species-specific (Haribal et al. 2005; Soini et al. 2013; Zhang et al. 2013).
One of the strongest pieces of evidence supporting a sociochemical role for uropygial secretions is that the components of preen oil often differ between wintering and breeding seasons (Bohnet et al. 1991; Kolattukudy et al. 1987; Piersma et al. 1999; Reneerkens et al. 2002, 2007; Soini et al. 2007; Whittaker et al. 2011). For example, as the season progresses from non-breeding to breeding, there is a shift from monoester to diester waxy components in the preen oil secretions of ducks (Bohnet et al. 1991) and sandpipers (Reneerkens et al. 2002). Since diesters are less volatile than monoesters, this switch is hypothesized to reduce odor and depredation in incubating females (Reneerkens et al. 2002). However, some researchers have suggested that a seasonal change in compounds also could be used to convey information relevant to intraspecific communication, such as an individual’s identity (Coffin et al. 2011; Mardon et al. 2010; Soini et al. 2007; Whittaker et al. 2010) and/or sexual attractiveness (Bohnet et al. 1991; Mardon et al. 2010; Whittaker et al. 2010, 2011). There is also some evidence that uropygial secretions are used to differentiate the sex of conspecifics (Zhang et al. 2010), and mate quality (Leclaire et al. 2011; Whittaker et al. 2013).
The first studies suggesting preen oil as a potential source of chemosignals focused on non-passerine species (e.g., Bohnet et al. 1991; Hirao et al. 2009; Mardon et al. 2010). However, Passeriformes is the most specious order of birds, and, more recently, several songbirds have been found to produce volatile compounds (Amo et al. 2012; Haribal et al. 2005; Krause and Caspers 2012; Soini et al. 2007; Whittaker et al. 2010; Shaw et al. 2011). One passerine, the dark-eyed junco, Junco hyemalis, has been shown to exhibit seasonal (Soini et al. 2007), individual, and sexual variation (Whittaker et al. 2010) in preen oil chemistry. In addition, the junco discriminates between conspecific- and heterospecific-based preen oil odor (Whittaker et al. 2009, 2013), as well as between population of origin (Whittaker et al. 2010). The Junco and Zonotrichia genera are closely related, with occassional hybridization (Jung et al. 1994), and so we expect similar patterns in the white-throated sparrow, Zonotrichia albicollis. However, the white-throated sparrow also has a unique polymorphism, where both sexes occur as either tan or white plumage morphs (Lowther 1961). Morph is genetically determined - white birds are heterozygous for a complex chromosomal rearrangement (2m/2), and tan birds are homozygous (2/2) for the non-rearranged chromosome (Romanov et al. 2009; Thomas et al. 2008; Thorneycroft 1975).
The morphs display distinct behavioral differences. White males sing more than tan males (Tuttle 1993) and engage in extra-pair copulations (Tuttle 2003). The morphs exhibit alternative reproductive strategies in which white males trade off reduced within-pair paternity for increased extra-pair paternity (Tuttle 2003). By contrast, tan males invest in monogamy (Tuttle 2003) and high parental care (Knapton and Falls 1983). Over 97 % of the pair types are disassortative, which maintains all morph-sex classes within the population (Lowther 1961; Thorneycroft 1975; Tuttle 1993, 2003), and this suggests that strong, and, perhaps, multiple cues for mate discrimination are likely. The alternative reproductive strategies and the unique disassortative mating system make the white-throated sparrow an ideal model to test not only for the effect of season on preen oil chemistry, but also for any intraspecific variation between genotypes, i.e., morph-sex classes.
The aims of this study were to examine whether the composition of volatile compounds in preen oil differs between both season and morph-sex class of the white-throated sparrow, and to identify candidate social chemosignals. We hypothesized that since mate selection is such an important process for maintaining the disassortative mating system (Houtman and Falls 1994; Tuttle 1993), the signatures of the volatile compounds in preen oil may differ between morph-sex classes. In addition, we hypothesized that if preen oil provides an important social chemical signal, the signatures will be more complex during the breeding season than during the non-breeding season. Finally, although we reasoned that Zonotrichia might share some preen oil compounds with Junco, we suspected that the signature (i.e., the presence and amount) of compounds would be sufficiently different to allow for the differentiation of species.
Methods and Materials
Sample Collection
White-throated sparrows were captured at Indiana State University, Terre Haute, IN, USA using passive mist netting (Master Banding Permit 22296 to E.M. Tuttle) between October 2009 and February 2010. Eleven white males, 7 tan males, 2 white females, and 2 tan females were captured for a total of 22 birds. All birds were housed in indoor aviaries at Indiana State University and fed an ad libitum diet of water, sunflower hearts, white millet, thistle, probiotics (Benebac, PetAg, Hampshire, IL, USA), meal worms, and wax worms. Diet did not vary over the course of the experiment. Daily bird care averaged 30 min per day over approximately 150 day. Initially, lighting conditions were set to a natural Indiana winter day length and preen oil was first sampled after the birds were in captivity for at least 2 weeks. The aviary was kept on a natural winter photoperiod until late February 2010, and then it was slowly brought to a photoperiod of L:D 16:8 h to bring the birds into breeding condition. Preen oil then was again sampled at least 2 weeks after reaching this breeding photoperiod. At this point, all birds had molted, and the males had developed cloacal protuberances and were singing. Preen oil was collected in 80 μl glass capillary tubes (Fisher Scientific, Pittsburgh, PA, USA) by gently pressing the tube against the preen gland and collecting its contents via capillary action (Kolattukudy et al. 1987). For consistency in sampling technique, the same person (Tuttle) sampled all birds in both seasons. The volume of each preen oil sample was calculated from the length of the sample measured with digital calipers and using the average internal radius (r) of the glass capillary tube (0.55 mm). Samples were kept frozen at −20 °C until they were shipped on dry ice to Indiana University, Department of Chemistry, Institute for Pheromone Research, Bloomington, IN, USA.
Reagents and Materials
Standard chemicals, the internal standard 7-tridecanone and ammonium sulfate (99.999 %) were purchased from Sigma-Aldrich Chemical Company (St Louis, MO, USA). High-purity OmniSolv™ water was obtained from EMD Chemicals, Inc., Gibbstown, NJ, USA. Methanol was from Baker Analyzed, Mallinckrodt Baker, Inc., Phillipsburg, NJ, USA.
Sample Preparation
Sample preparation followed the methods of Soini et al. (2007) and Whittaker et al. (2010) utilizing the stir bar sorptive extraction technique (Soini et al. 2005). Thawed preen oil samples were pushed out of the microcapillary glass pipettes into 20 ml glass vials. Then 2.0 ml of high-purity water, 100 mg of ammonium sulfate, and an internal standard of 8 ng of 7-tridecanone dissolved in 5 μl methanol were added to each vial. A Twister™ stir bar (Gerstel GmbH, Mülheim an der Ruhr, Germany, 10 × 0.5 mm polydimethylsiloxane) was added and stirred at 800+rpm for 60 min on the 15-place stirplate (Variomag Multipoint HP15, H+P Labortechnic, Oberschleissheim, Germany). After 60 min, the stir bar was removed and rinsed with high-purity water, dried gently, and placed in the thermal desorption autosampler tube for gas chromatography-mass spectrometry (GC/MS) analysis.
Analytical Instruments
GC/MS analyses were conducted using an Agilent 6890N gas chromatograph connected to a 5973iMSD mass spectrometer (Agilent Technologies, Inc., Wilmington, DE, USA) and equipped with the thermal desorption autosampler and cooled injection system (TDSA-CIS 4 from Gerstel) (Soini et al. 2007; Whittaker et al. 2010). All analytical instruments were set to the same specifications as described by Whittaker et al. (2010).
Samples were thermally desorbed in a TDSA automated system and then injected into the column with the CIS-4 cooled injection assembly. Splitless mode was used for desorption with a temperature program of 20 °C for 0.5 min, then 60 °C/min up to 250 °C for 3 min. Transfer line temperature was set at 280 °C, and CIS was cooled using liquid nitrogen to −80 °C. After desorption and cryotrapping were complete, the CIS was heated at 12 °C/sec to 270 °C and held for 12 min. Solvent vent mode was used for the CIS inlet, with a vent pressure of 9.1 psi, a vent flow of 50 ml/min, and a purge flow of 50 ml/min. The GC column was a DB-5MS capillary (30 m × 0.25 mm, i.d., 0.25 μm film thickness; Agilent). Carrier gas was helium at constant flow (1.1 ml/min), and the oven temperature program started at 50 °C held for 2 min, then increased to 200 °C at the rate of 3 °C min, and held for 12 min. For the MS detection, positive electron ionization (EI) mode at 70eV was used with a scanning rate of 2.47 scans/sec, and mass range of 40–350 amu. The transfer line temperature was 280 °C, the ion source was kept at 230 °C, and the quadrupole temperature was 150 °C.
Quantitative Comparisons
Major volatile compounds in the preen oil samples were identified by comparison of mass spectra and retention times with those of authentic standards. The peak areas for each compound were used for quantitative comparisons and were integrated either from the total ion current (TIC) profiles or from post-run, single ion current (SIC) profiles at m/z 55 for n-alcohols, m/z 58 for methyl ketones, or m/z 60 for n-carboxylic acids, which are the base peaks in the mass spectra. The analyte peak areas in SIC profiles at m/z 55, 58, or 60 were normalized by dividing by the peak area of the internal standard in the SIC profile at m/z 113 in the same run. These normalized values were used in pairwise comparisons of compound amounts during the breeding and the non-breeding seasons.
Statistics
Peak areas, or abundances, of compounds were non-normally distributed, so that nonparametric, Wilcoxon-signed rank tests were used to compare the relative concentrations of each compound in winter samples to the samples collected under conditions that simulated spring breeding. Since captive bird populations were heavily male-biased, Wilcoxon-signed rank tests were run separately for all males (N = 16), and for white (N = 10) and tan morph males (N = 6). Next, Mann-Whitney U Tests were run to determine if there were any within-season variations in compound abundance between sexes and male morphs, but we did not include a Kruskal-Wallis ANOVA comparing morph-sex classes since only two winter samples were available for white females and tan females, and no breeding condition samples were available for tan females. The Wilcoxon-signed rank tests and Mann-Whitney U tests run dependent variables in a univariate fashion in SPSS 19.0, with only one independent variable comparison, and so no corrections for Bonferroni or sequential Bonferroni adjustments were needed. Statistically significant values less than P = 0.05 were accepted. These statistical analyses were completed using SPSS 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY, USA: IBM Corp.).
To reduce the variables in analyses, we also conducted principal component analyses (PCA) on the abundances and proportions of the 22 identified volatile compounds. Since we did not sample enough females during the breeding season, we restricted these analyses to males only. We retained only those PCs with eigenvalues greater than one. We then tested for differences between morphs and season using multivariate analysis of variance (MANOVA). PCA and MANOVA were completed in JMP®, Version 9. (SAS Institute Inc., Cary, NC, USA 1989–2007).
Results
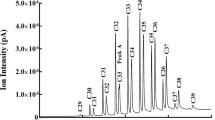
In the white-throated sparrow preen oil samples, we identified 42 compounds based on comparison with the spectra and retention times of authentic standards (Fig. 1, Table 1). Additionally, we tentatively identified another 13 compounds (Table 1). Using the post-run selected ion current (SIC) profiles, 27 compounds were used for quantitative comparisons. A selected ion current profile of m/z 55 revealed the major components to be 9 linear 1-alkanols with a carbon chain-length ranging from 10 to 18. The secondary compounds that were also consistently found included 9 methyl ketones, from 2-nonanone to 2-heptadecanone, and 9 carboxylic acids, including pentanoic acid and nonanoic acid, through to stearic acid (octadecanoic acid). Of the 27 major compounds identified, some compounds were detected in the majority of samples, while others were found in only few individuals (Tables 2, 3, 4, 5, and 6). Pentanoic acid (1 sample), 2-nonanone (4 samples), and 2-decanone (5 samples) were conspicuous compounds that were observed only in some breeding condition samples, but not in any wintering condition specimens (Table 2).
Preen oil extract profiles from the white-throated sparrow: a total ion chromatogram (TIC) profile of the volatile compounds extracted from the preen oil of a male white-throated sparrow; b post-run selected ion m/z 55 profile from the TIC shown in (a). Numbers refer to 1 = 1-nonanol, 2 = 1-decanol, 3 = 1-undecanol, 4 = 1-dodecanol, 5 = 1-tridecanol, 6 = 1-tetradecanol, 7 = 1-pentadecanol, 8 = 1-hexadecanol, 9 = 1-heptadecanol, 10 = 1-octadecanol
Breeding season samples contained higher abundances of 12 different compounds that were seasonally elevated in spring breeding condition as compared to winter non-breeding condition. Seasonally elevated compounds included linear alcohols from 1-decanol to 1-pentadecanol, dodecanoic acid, octadecanoic acid, 2-decanone, 2-tridecanone, 2-tetradecanone, and 2-pentadecanone (Table 2, Figs. 2, 3, and 4). Since males made up almost the entire sample, additional Wilcoxon-signed rank tests were run to determine if males (N = 16), white males (N = 10), and tan males (N = 6) also exhibited higher compound abundance in breeding condition. All 12 of the seasonally elevated compounds also were seasonally elevated in males. In addition, 1-hexadecanol was seasonally elevated in males (Table 3). Ten of the compounds that were seasonally elevated in males also were seasonally elevated in white males (N = 10, Table 4), whereas none of these compounds were seasonally elevated in tan males (N = 6).
Normalized peak areas of C10-C18 alcohols: averages (±SE) of the normalized peak areas of C10-C14 (A) and C15-C18 (B) linear alcohols in the four morph-sex classes during the non-breeding and breeding seasons. Designation: tan males = TM (N = 7 non-breeding, 6 breeding), white males = WM (N = 11 non-breeding, 10 breeding), tan females = TF (N = 2 non-breeding, 0 breeding), and white females = WF (N = 2 non-breeding, 2 breeding)
Normalized peak areas of methyl ketones: averages (±SE) of the normalized peak areas of methyl ketones in the four morph-sex classes during the non-breeding and breeding seasons. Designation: tan males = TM (N = 7 non-breeding, 6 breeding), white males = WM (N = 11 non-breeding, 10 breeding), tan females = TF (N = 2 non-breeding, 0 breeding), and white females = WF (N = 2 non-breeding, 2 breeding)
Normalized peak areas of linear carboxylic acids acids (C9-C16): averages (±SE) of the normalized peak areas of linear acids (C9-C16) in the four morph-sex classes during the non-breeding and breeding seasons. Designation: tan males = TM (N = 7 non-breeding, 6 breeding), white males = WM (N = 11 non-breeding, 10 breeding), tan females = TF (N = 2 non-breeding, 0 breeding), and white females = WF (N = 2 non-breeding, 2 breeding)
Mann-Whitney U tests showed that 11 different compounds found in winter samples were more abundant in females (N = 4) than in males (N = 18, Table 5). Additionally, 3 linear alcohols were more abundant in tan males (N = 7) than white males (N = 11, Table 6, Fig. 2). During the winter season, 2-tridecanone and 2-tetradecanone appeared to be more abundant in tan males than in white males, but the results were marginally non-significant (2-tridecanone: U = 17.5, Z = −1.908, P = 0.056; 2-tetradecanone: U = 17, Z = −1.954, P = 0.056).
For the absolute abundance of volatile profiles in males, the first six principal components had eigenvalues greater than one, and they explained 90.0 % of the variance in the absolute concentration of volatile compounds (Table 7). The principal components of abundance differed only significantly by season (MANOVA, F = 9.024, P = 0.005). However, for relative proportions of volatile compounds in males, the first seven principal components had eigenvalues greater than one, and they explained 83.2 % of the variance in the proportional concentration of volatile compounds (Table 7). The principal components of relative proportion differed significantly by season (MANOVA, F = 6.573, P = 0.015) and by the interaction of morph and season (MANOVA, F = 7.275, P = 0.011).
Discussion
Preen oil samples from white-throated sparrows contained primarily linear alcohols, methyl ketones, and linear carboxylic acids that ranged from 9 to 18 carbons in length (Table 2). Twenty-seven of these volatile compounds were present in the uropygial oil of captive white-throated sparrows in breeding condition, while only 23 of these were present in samples taken from winter-condition, non-breeding birds. Twelve compounds were found in both non-breeding and breeding-condition birds but were seasonally elevated for all individuals, including 7 n-alcohols, 4 methyl ketones, and dodecanoic acid (Table 3); thirteen compounds were seasonally elevated only in males (Table 4). Four more volatile compounds (1-decanol, 2-decanone, 2-nonanone, and pentanoic acid) were found exclusively only in breeding season samples. Together, these findings suggest that the composition of preen oil during the breeding season is more complex and more volatile than preen oil in the non-breeding season. Our results also show that this seasonal variation in preen oil composition is independent of diet, and suggest that preen oil has additional roles and increased importance during the breeding season. Explanations for a seasonal shift in odor composition include nest crypsis (Reneerkens et al. 2002, 2007; Soini et al. 2007), increased protection from ectoparasites (Moyer et al. 2003; Shawkey et al. 2003; Soini et al. 2007) and use as social chemosignals (Campagna et al. 2012; Hagelin 2007a, b; Hagelin and Jones 2007; Mardon et al. 2010; Whittaker et al. 2010). All the above factors could be relevant in the white-throated sparrow, but since our samples were composed primarily of males, who, in this species, do not incubate at the nest, we suspect that preen oils function as social signals and, possibly, as an aid in parasite resistance.
Some of the compounds found in white-throated sparrow preen oil could confer health benefits. The production of 2-tridecanone, a naturally occurring insecticide (Williams et al. 1980), increased significantly as birds progressed from non-breeding to breeding. A similar pattern has been observed in a related species, the dark-eyed junco, Junco hyemalis (Soini et al. 2007, 2013). In addition, dodecanoic acid (lauric acid), which was found to increase in breeding males, has been reported to have antimicrobial properties (Huang et al. 2011). Finally, linear n-alcohols, which are a primary component of white-throated sparrow preen oil, are natural antimicrobial and antifungal substances (Martin-Vivaldi et al. 2010; Ruiz-Rodriguez et al. 2009; Soini et al. 2007; Vivaldi et al. 2010). These and other chemicals could serve to deter the numerous biting flies, midges, mosquitos, as well as bacterial and fungal infections the birds can encounter while on the warmer breeding grounds. Since darker pigmented feathers are more resistant to bacterial degradation, we expected tan morphs to show greater increases in preen oil compounds than white morphs. However, no significant difference between the male morphs in breeding condition was observed for these particular chemical compounds.
We compared the volatile chemical composition of white-throated sparrow preen oil with putative chemical signaling compounds from other taxa (Table 8) and found some overlapping compounds. For example, 2-pentadecanone increased significantly in white males and white females, but decreased in tan males, during the breeding season. There were too few tan females to determine whether or not they too exhibited a similar pattern. Several invertebrate species, including 3 species of butterflies (Schultz et al. 1993) and leaf-cutter ants (Ortius-Lechner et al. 2000), as well as many species of lizards (Louw et al. 2007) and mammals such as Sika deer (Wood 2003), white-lipped pecari (Waterhouse et al. 2001), African wild dog (Apps et al. 2012), and lion, leopard, Bengal tiger, and cougar (Soini et al. 2012) likely use 2-pentadecanone for intraspecific communication, and it is possible that this compound also serves the same function in the white-throated sparrow (Table 8). Similarly, octadecanoic acid (stearic acid), which increases in white and tan males during the breeding season, has been assigned with the chemosignaling function in several other taxa (Table 8).
Many of the compounds found in white-throated sparrow preen oil also were found in preen oil samples from a closely related species, the dark-eyed junco (Junco hyemalis), and several of the same compounds have been found to be seasonally elevated in both avian species (Soini et al. 2007, 2013). When in breeding-condition, both white-throated sparrows and juncos had higher concentrations of linear alcohols with 10–15 carbons in at least one sex. Both also had higher levels of 2-tridecanone, 2-tetradecanone, 2-pentadecanone, and dodecanoic acid during the breeding condition, suggesting that they are phylogenetically conserved. However, white-throated sparrow uropygial secretions from birds in breeding condition contained 2-decanone and octadecanoic acid, which were not present in junco secretions (Soini et al. 2007). Since the breeding distributions of these two species overlap, and occassional hybrids have been found (Jung et al. 1994), it is intriguing to speculate that volatile preen compounds could be used in species recognition (Haribal et al. 2005; Soini et al. 2007, 2013; Whittaker et al. 2010).
Our analyses suggest that within white-throated sparrows, both sexes vary in the abundance of multiple preen oil compounds. Although, the number of females we studied was low, we did find that non-breeding females tended to have higher amounts of 8 different linear alcohols, 2-tetradecanone, 2-pentadecanone, 2-hexadecanone, and 2-heptadecanone (Table 5) than non-breeding males. It is unclear why females have higher levels of n-alcohols and methyl ketones, while males have lower levels of these compounds during the non-breeding season, especially if we assume that these compounds are costly for birds to produce and that they could attract predators. Winter preen oil volatiles might be a benefit if higher levels help females, who are normally more subordinant to males in winter flocks (Piper and Wiley 1989a), to establish a higher dominance status and gain better access to food. Alternatively, the lower levels of preen oil compounds found in males may help maintain social flock cohesion much like dull plumage during the winter might help maintain stable social relations within wintering flocks (Atkinson and Ralph 1980). Regardless of the role of preen oil secretions during the winter, when males have lower amounts of these compounds during the non-breeding season, there is a greater shift in the abundances of these compounds as they increase in the spring. This may act as a potent sexually-selected cue that females or other males can easily detect.
Interestingly, some preen oil compounds as well as the overall relative abundance of those compounds also differed between morphs of the white-throated sparrow (Fig. 5; Table 7). Tan males in non-breeding condition exhibited significantly higher levels of three n-alcohols (1-tetradecanol, 1-pentadecanol, and 1-hexadecanol) than did white males in non-breeding condition (Table 6), suggesting that the two morphs utilize preen oil compounds differently during the winter months. These three linear alcohols often are used in human skin care products as emolients, and so they could serve a similar function in tan males by softening and protecting their plumage from moisture loss during the cold, dry winters. In general, tan plumage contains more melanins than white plumage does, and since melanins are known to enhance feather stiffness (Bonser 1995), the added emolients may maintain better feather condition in these morphs. The preen oil volatiles in tan males were uniformly higher across the non-breeding and breeding season, whereas white males had low levels of volatiles in the non-breeding season and significant increases in 8 of these compounds, as they progressed into the breeding season (Table 4; Fig. 5). Sexual selection is thought to be more intense in white males as they seek extra-pair copulations with as many neighboring females as possible (Formica and Tuttle 2009; Tuttle 2003). Accordingly, white males molt in brighter, more contrasting plumage in the spring. If preen oil volatiles also are a sexually selected signal, then it is possible that preen oil composition in white males mirrors these drastic changes.
Mean proportional composition of volatiles in preen oil by morph/sex class and season: average proportion of alcohols, acids, and ketones in preen oil sampled from white males (WM), tan males (TM), white females (WF), and tan females (TF) in non-breeding and breeding condition. No data are shown for tan females in breeding condition because samples sizes were low for that morph/sex class
Preen oil composition should have both genetic and environmental components. In white-throated sparrows, different dietary supplements have been shown to affect the nonvolatile compounds in preen oil (Thomas et al. 2010), and so diet might also affect volatile compounds. Although both morphs are part of the same breeding population, white and tan males tend to settle in slightly different microhabitats (Formica et al. 2004; Formica and Tuttle 2009). Any differences in diet could exaggerate the differences in preen oil signature we report here. However, since white-throated sparrows mate disassortatively (i.e., white males X tan females, tan males X white females; Lowther 1961), we would expect males and their opposite morph mates to be similar in preen oil composition if it is strongly under the influence of diet. In this study, all individuals were fed the same diet for an extended period of time and so the differences we report are likely to be genetically derived. Genetic background has been shown to influence preen oil compounds in other species (Leclaire et al. 2012). Similarly, the differential production of preen oil compounds in white and tan morphs might be under genetic control since morph itself is determined by the presence or absence of large complex rearrangement of the \ chromosome (Romanov et al. 2009; Thomas et al. 2008; Thorneycroft 1975) that encompasses ~86 % of the chromosome and limits recombination in heterozygotes (Thorneycroft 1975; Thomas et al. 2008).
In summary, the preen oil of the white-throated sparrow contains volatile compounds that are elevated when birds are in breeding condition. Some seasonally elevated compounds differ between the sexes, some differ between male morphs. Future work should include a greater sample of females so that complete morph-sex class comparisons can be made. In addition, these analyses should be complemented with analyses of preen oil sampled from wild birds - such studies could elucidate the relative influences of environment and genetics on the chemical composition of avian preen oil. Together, the results suggest that, in addition to feather conditioning, waterproofing, antimicrobial, antifungal, and antiparasitic agents, preen oil secretions also may function as breeding season sociochemicals in this species.
References
Amo L, Galvan I, Tomas G, Sanz JJ (2008) Predator odour recognition and avoidance in a songbird. Funct Ecol 22:289–293
Amo L, Avilés JM, Parejo D, Pepa A, Rodriguez J, Tomás G (2012) Sex recognition by odour and variation in the uropygial gland secretions in starlings. J Anim Ecol 81:605–613
Apps P, Mmualefe L, McNutt JW (2012) Identification of volatiles from the secretions and excretions of African Wild Dogs (Lycaon pictus). J Chem Ecol 38:1450–1461
Atkinson CT, Ralph CJ (1980) Acquisition of plumage polymorphism in white-throated sparrows. Auk 97:245–252
Bohnet S, Rogers L, Sasaki G, KolattuKudy PE (1991) Estradiol influences proliferation of 3-hydroxy fatty acid diesters, the female pheromones, in the uropygial glands of male and female mallards. J Biol Chem 266:9795–9804
Bonadonna F, Nevitt GA (2004) Partner-specific odor recognition in an Antarctic seabird. Science 306:835
Bonadonna F, Cunningham GB, Jouventin P, Hesters F, NeVitt GA (2003) Evidence for nest-odour recognition in two species of diving petrel. J Exp Biol 206:3719–3722
Bonser RHC (1995) Melanin and the abrasion resistance of feathers. Condor 97:590–591. doi:10.2307/1369048
Campagna S, Mardon J, Celerier A, Bonadonna F (2012) Potential semiochemical molecules from birds: A practical and comprehensive compilation of the last 20 years studies. Chem Senses 37:3–25
Caspers BA, Krause ET (2013) Intraspecific olfactory communication in zebra finches (Taeniopygia guttata): Potential information apart from visual and acoustic cues. In: Chemical signals in vertebrates 12, Springer Science+Business Media, New York
Coffin HR, Watters JV, Mateo JM (2011) Odor-based recognition of familiar and related conspecifics: a first test conducted on captive Humboldt penguins (Spheniscuc humboldti). PLoS One 6:e25002
Elder WH (1954) The oil gland of birds. Wilson Bull 66:6–31
El-Sayed AM (2012) The pherobase: Database of pheromones and semiochemicals <http://www.pherobase.com>
Formica VA, Tuttle EM (2009) Examining the social landscapes of alternative reproductive strategies. J Evol Biol 22:2395–2408
Formica VA, Gonser RA, Ramsay SM, Tuttle EM (2004) Spatial dynamics of alternative reproductive strategies: the role of neighbors. Ecology 85:1125–1136
Giraudeau M, Czirjak GA, Duval C, Bretagnolle V, Eraud C, McGraw KJ, Heeb P (2010) Effect of restricted preen-gland access on maternal self maintenance and reproductive investment in mallards. PLoS ONE 5:e13555
Hagelin JC (2004) Observations on the olfactory ability of an endangered nocturnal parrot: the New Zealand Kakapo. Ibis 146:161–164
Hagelin JC (2007a) Odors and chemical signaling. In: Jamieson BGM (ed) Reproductive biology and phylogeny of birds. Science Publishers, Enfield, pp 75–119
Hagelin JC (2007b) The citrus-like scent of crested auklets: reviewing the evidence for an avian olfactory ornament. J Ornithol 148(Suppl 2):S195–S201
Hagelin JC, Jones IL (2007) Bird odors and other chemical substances: a defense mechanism or overlooked mode of intraspecific communication? Auk 124:741–761
Hagelin JC, Jones IL, Rasmussen LE (2003) A tangerine-scented social odour in a monogamous seabird. Proc R Soc Lond Biol 270:1323–1329
Haribal M, Dhondt AA, Rosane D, Rodriguez E (2005) Chemistry of preen gland secretions of passerines: different pathways to the same goal? Why? Chemoecology 15:251–260
Hirao A, Aoyama M, Sugita S (2009) The role of uropygial gland on sexual behavior in domestic chicken Gallus gallus domesticus. Behav Process 80:115–120
Houtman A, Falls JB (1994) Negative assortative mating in the white-throated sparrow, Zonotrichia albicollis: the role of mate choice and intra-sexual competition. Anim Behav 48:377–383
Huang CB, Alimova Y, Myers TM, Ebersole JL (2011) Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol 56:650–654. doi:10.1016/j.archoralbio.2011.01.011
IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp
Jacob J, ZisweIler V (1982) The uropygial gland. In: Farner DS, King JR, Parkes KC (eds) Avian biology. Academic, New York, pp 199–314
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289
Jung RE, Morton ES, Fleischer RC (1994) Behavior and parentage of a white-throated sparrow × dark-eyed junco hybrid. Wilson Bull 106:189–202
Knapton RW, Falls JB (1983) Differences in parental contribution among pair types in the polymorphic White-throated sparrow. Can J Zool 61:1288–1292
Kolattukudy PE, Bohnet S, Rogers L (1987) Diesters of 3-hydroxy fatty acids produced by the uropygial glands of female mallards uniquely during the mating season. J Lipid Res 28:582–588
Krause ET, Caspers BA (2012) Are olfactory cues involved in nest recognition in two social species of estrildid finches? PLoS One 7:e36615
Leclaire S, Merkling T, Raynaud C, Giacinti G, Bessière J-M, Hatch SA, Danchin E (2011) An individual and sex odor signature in kittiwakes? Study of the semiochemical composition of preen secretion and preen down feathers. Naturwissenschaften 98:615–624
Leclaire S, Merkling T, Raynaud C, Mulard H, Bessiere J-H, Lhuillier E, Hatch SA, Danchin E (2012) Semiochemical compounds of preen secretion reflect genetic make-up in a seabird species. Proc R Soc B 279:185–193
Louw S, Burger BV, Le Roux M, Van Wyk JH (2007) Lizard epidermal gland secretions I: Chemical characterization of the femoral gland secretion of the sungazer, Cordylus giganteus. J Chem Ecol 33:1806–1818
Lowther JK (1961) Polymorphism in the white-throated sparrow, Zonotrichia albicollis (Gmelin). Can J Zool 39:281–292
Mardon J, Saunders SM, Anderson MJ, Couchoux C, Bonadonna F (2010) Species, gender, and identity: cracking petrels’ sociochemical code. Chem Senses 35:209–321
Martin-Vivaldi M, Pena A, Peralta-Sánchez JM, Sánchez L, Ananou S, Ruiz-Rodríguez M, Soler JJ (2010) Antimicrobial chemical in hoopoe preen secretions are prodiced by symbiotic bacteria. Proc R Soc Lond B 7:123–130
Mateo JM, Johnston RE (2000) Kin recognition and the ‘armpit effect’: evidence for self-referent phenotype matching. Proc R Soc Lond B 267:695–700
Moyer BR, Rock AN, Clayton DH (2003) Experimental test of the importance of preen oil in Rock Doves (Columba livia). Auk 120:432–435
Nevitt GA, Bonadonna F (2005) Sensitivity to dimethyl sulphide suggests a mechanism for olfactory navigation by seabirds. Biol Lett 1:303–305
Nevitt GA, Losekoot M, Weimerskirch H (2008) Evidence for olfactory search in wandering albatross, Diomedea exulans. Proc Natl Acad Sci U S A 105:4576–4581
Ortius-Lechner D, Maile R, Morgan ED, Boomsma JJ (2000) Metapleural gland secretion of the leaf-cutter ant Acromyrmex octospinosus: new compounds and their functional significance. J Chem Ecol 26:1667–1683
Petit C, Hossaert-Mckey M, Perret P, Blondel J, Lambrechts MM (2002) Blue tits use selected plants and olfaction to maintain an aromatic environment for nestlings. Ecol Lett 5:585–589
Piersma T, Dekker M, Damste JSS (1999) An avian equivalent of make up? Ecol Lett 2:201–203
Piper WH, Wiley RH (1989) Correlates of dominance in wintering white-throated sparrows: age, sex and location. Anim Behav 37:298–310
Reneerkens J, Piersma T, Damste JSS (2002) Sandpipers (Scopopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proc R Soc Lond B 269:2135–2139
Reneerkens J, Almeida JB, Lank DB, Jukema J, Lanctot RB, Morrison RIG, Rijpstra WIC, Schamel D, Schekkerman H, Damste JSS, Tomkovich PS, Tracy DM, Tulp I, Piersma T (2007) Parental role division predicts avian preen wax cycles. Ibis 149:721–729
Romanov MN, Tuttle EM, Houck ML, Modi WS, Chemnick LG, Korody ML, Stremmel E, Mork M, Otten CA, Renner T, Jones KC, Dandekar S, Papp JC, Da Y, Comparative Sequencing Program NISC, Green ED, Magrini V, Hickenbotham MT, Glasscock J, Mcgrath S, Mardis ER, Ryder OA (2009) The value of avian genomics to the conservation of wildlife. BMC Genomics 10:S10
Ruiz-Rodriguez M, Valdivia E, Soler JJ, Martin-Vivaldi M, Martin-Platero AM, Martinez-Bueno M (2009) Symbiotic bacteria living in the hoopoe’s uropygial gland prevent feather degradation. J Exp Biol 212:3621–3626
Schultz S, Boppre M, Vane-Wright RI (1993) Specific mixtures of secretions from male scent organs of African milkweed butterflies (Danainae). Proc R Soc Lond B 342(1300):161–181. doi:10.1098/rstb.1993.0144
Shaw CL, Rutter JE, Austin AL, Garvin MC, Whelan RJ (2011) Volatile and semivolatile compounds in gray catbird uropygial secretions vary with age and between breeding and wintering grounds. J Chem Ecol 37:329–339
Shawkey MD, Pillai SR, Hill GE (2003) Chemical Warfare? Effects of uropygial oil on feather-degrading bacteria. J Avian Biol 34:345–349
Soini HA, Bruce KE, Wiesler D, David F, Sandra P, Novotny MV (2005) Stir bar sorptive extraction: a new quantitative and comprehensive sampling technique for determination of chemical signal profiles from biological media. J Chem Ecol 31:377–392
Soini HA, Schrock SE, Bruce KE, Wiesler D, Ketterson ED, Novotny MV (2007) Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J Chem Ecol 33:183–198
Soini HA, Linville SU, Wiesler D, Posto AL, Williams DR, Novotny MV (2012) Investigation of scents on cheeks and foreheads of large felines in connection to the facial marking behavior. J Chem Ecol 38:145–156
Soini HA, Whittaker DJ, Wiesler D, Ketterson ED, Novotny MV (2013) Chemosignaling diversity in songbirds: chromatographic profiling of preen oil volatiles in difference species. J Chromatogr A 1317:186–192
Steiger SS, Fidler AE, Valcu M, Kempanaers B (2008) Avian olfactory receptor gene repertoires: evidence for a well-developed sense of smell in birds? Proc R Soc Lond B 275:2309–2317
Thomas JW, Caceres M, Lowman JJ, Morehouse CB, Short ME, Baldwin EL, Maney DL, Martin CL (2008) The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement and suppressor of recombination. Genetics 179:1455–1468
Thomas RH, Price ER, Seewagen CL, Mackenzie SA, Bernards MA, Guglielmo CG (2010) Use of TLC-FID and GC-MS/FID to examine the effects of migratory state, diet and captivity on preen wax composition in white-throated sparrows Zonotrichia albicollis. Ibis 152:782–792
Thorneycroft HD (1975) A cytogenetic study of the white-throated sparrow, Zonotrichia albicollis (Gmelin). Evolution 29:611–621
Tuttle EM (1993) Mate choice and the maintenance of stable polymorphisms in the White-throated sparrow. PhD Dissertation. State University of New York at Albany, Albany, N.Y.
Tuttle EM (2003) Alternative reproductive strategies in the White-throated sparrow: behavioral and genetic evidence. Behav Ecol 14:425–432
Wallraff HG (2004) Avian olfactory navigation: its empirical foundation and conceptual state. Anim Behav 67:189–204
Waterhouse JS, Hudson M, Pickett JA, Weldon PJ (2001) Volatile components in dorsal gland secretions of the white-lipped peccary, Tayassu pecari, from Bolivia. J Chem Ecol 27:2459–2469
Whittaker DJ, Reichaard DG, Dapper AL, Ketterson ED (2009) Behavioral responses of nesting female dark-eyed juncos Junco hyemalis to hetero- and conspecific passerine preen oils. J Avian Biol 40:579–583
Whittaker DJ, Soini HA, Atwell JW, Hollars C, Novotny M, Ketterson ED (2010) Songbird chemosignals: volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav Ecol 21:608–614
Whittaker DJ, Soini HA, Gerlach NM, Posto AL, Novotny MV, Ketterson ED (2011) Role of testosterone in stimulating seasonal changes in a potential avian chemosignal. J Chem Ecol 37:1349–1357
Whittaker DJ, Gerlach NM, Soini HA, Novotny MV, Ketterson ED (2013) Bird odor predicts reproductive success. Anim Behav 86:697–703
Williams WG, Kennedy GG, Yamamoto RT, Thacker JD, Bordner J (1980) 2-Tridecanone: a naturally occurring insecticide from wild tomato Lycopersicon hirsutum f.glabratum. Science 207:888–889
Wood WF (2003) Volatile components in metatarsal glands of sika deer, Cervus nippon. J Chem Ecol 29:2729–2733
Wyatt TD (2003) Pheromones and animal behaviour: communication by smell and taste. Cambridge University Press, Cambridge
Zhang J-X, Wei W, Zhang J-H, Yang W-H (2010) Uropygial gland-secreted alkanols contribute to olfactory sex signals. Chem Senses 35:375–382
Zhang Y-H, Du Y-F, Zhang J-X (2013) Uropygial gland volatiles facilitate species recognition between two sympatric sibling bird species. Behav Ecol 24:1271–1278
Acknowledgments
This work was sponsored by the National Institute of Health NIGMS R01GM084229 grant awarded to Elaina M. Tuttle and Rusty Gonser, and by the College of Graduate and Professional Studies at Indiana State University. The Lilly Chemistry Alumni Chair Funds to Milos V. Novotny supported the chemical analyses at the Institute for Pheromone Research, Indiana University. We thank Julie Hagelin for advice and two anonymous reviewers for input. Additional thanks goes to C.A.T. Gonser and D. Sebastian.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tuttle, E.M., Sebastian, P.J., Posto, A.L. et al. Variation in Preen Oil Composition Pertaining to Season, Sex, and Genotype in the Polymorphic White-Throated Sparrow. J Chem Ecol 40, 1025–1038 (2014). https://doi.org/10.1007/s10886-014-0493-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-014-0493-2