Abstract
During field screening trials in Brazil, adults of both sexes of the cerambycid beetle Achryson surinamum (L.) (Cerambycinae: Achrysonini) were significantly attracted to racemic anti-2,3-octanediol, previously identified as a sex and aggregation-sex pheromone of various cerambycid species across different continents. Analyses of beetle-produced volatiles revealed that males of A. surinamum sex-specifically produce (2S,3R)-2,3-octanediol, as well as lesser amounts of (S)-2-methylbutan-1-ol. In field trials, both sexes of beetles were attracted by reconstructions of the species’ pheromone blend with synthesized components, confirming males produce an aggregation-sex pheromone. During the trials, the cerambycine Sphaerion inerme White (Elaphidiini) was attracted to some of the test lures, providing leads to its attractant pheromone. Subsequent analysis of extracts of headspace volatiles from live adults of S. inerme revealed that males produce a blend of (R)-2-methylbutan-1-ol and (R)-2-methylpentan-1-ol. In field tests, blends of racemic 2-methylbutan-1-ol+2-methylpentan-1-ol attracted significant numbers of beetles of both sexes. This study provides further examples of how identification of attractant pheromones of cerambycid species can be expedited by leveraging prior knowledge of the pheromone chemistry of related species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past two decades, research has consistently revealed a noteworthy level of conservation of pheromone structures within the large beetle family Cerambycidae (Millar and Hanks 2017). This conservation phenomenon crosses taxonomic levels, ranging from genera to tribes and occasionally even subfamilies, with numerous demonstrated instances of species sharing the same or closely related pheromone components (reviewed in Millar and Hanks 2017). This parsimony in biosynthesis and utilization of shared structural motifs also extends across multiple continents, even though species have been geographically isolated for millions of years. An example is the compound 3-hydroxyhexan-2-one, which is a pheromone component for numerous cerambycid species from all habitable continents (Millar and Hanks 2017). A number of other compounds also are widely shared among cerambycid species (Hanks and Millar 2016).
From a practical perspective, this conservation of pheromone structures suggests traps baited with single components or blends of related pheromone compounds might attract multiple cerambycid species, and this hypothesis has been borne out in several studies (Bobadoye et al. 2019; Flaherty et al. 2019; Hayes et al. 2016; Millar et al. 2018; Rassati et al. 2019; Sweeney et al. 2014). As a result, traps baited with pheromones or pheromone blends are becoming pivotal tools in mapping geographic ranges of native cerambycids (Santos-Silva et al. 2020) and strengthening surveillance programs aimed at detecting incursions of invasive species (Fan et al. 2019; Roques et al. 2023). This is particularly critical due to the significant threat posed by invasive wood-boring cerambycids to orchards and natural and managed forests, because the long-lived larvae are easily transported between continents in wooden products and packing materials via global commerce (Meurisse et al. 2019). The widespread sharing of pheromone structures also holds promise for expediting identification of pheromones or their analogs for related target species, leveraging the identification and testing of pheromone components for one species into identification of pheromones of related species (e.g., Millar et al. 2018, 2019; Silva et al. 2017, 2018, 2020, 2021, 2024).
During field screening trials of known cerambycid pheromones in Brazil, adult males and females of Achryson surinamum (L.) (Cerambycinae: Achrysonini) were significantly attracted to traps baited with racemic anti-2,3-octanediol (Silva et al. 2024). Whereas previous studies have established 2,3-octanediols as sex and aggregation-sex pheromones, or candidates for pheromones of at least 10 cerambycid species endemic to Asia, Europe, and North America (Millar and Hanks 2017), to our knowledge this was the first time a South American species had been attracted to an isomer of 2,3-octanediol. The significant and specific attraction led us to predict that one of the enantiomers of anti-2,3-octanediol is a pheromone component for A. surinamum. It had been previously noted that males of this species have sex-specific pores on the tergum of the prothorax which in other cerambycine species are associated with the emission of volatile pheromones (Ray et al. 2006).
The range of A. surinamum encompasses southern South America, primarily Brazil, and extends northward to the southern United States (Monné 2024). The beetle has a wide host range, the larvae being associated with > 80 species of native and introduced woody plants in 21 families, especially Fabaceae (Monné 2024). This polyphagy underscores the potential invasiveness of A. surinamum, as evidenced by prior studies (Duffy 1953, 1960; Girard 1968; Maier 2017). Given the potential risk posed by this species, proactively developing pheromone-based attractants for incorporation into quarantine surveillance and monitoring programs could prove useful should it invade new regions.
Here, we present our findings on the identification and field testing of the attractant pheromone of A. surinamum. Serendipitously, our experiments also found significant attraction of the cerambycine Sphaerion inerme White (tribe Elaphidiini) to certain test compounds, providing an opportunity to identify pheromone components for this species as well. This species is restricted to South America, primarily Brazil, and little is known of its host preferences (Monné 2024).
Materials and methods
Source of chemicals
Racemic 2-methylbutan-1-ol (MeBuOH), (S)-2-methylbutan-1-ol (S-MeBuOH), and racemic 2-methylpentan-1-ol (MePeOH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). (R)-2-Methylbutan-1-ol (R-MeBuOH) was prepared by lipase-based kinetic resolution of racemic MeBuOH (Bello et al. 2015), and (S)-2-methylpentan-1-ol (S-MePeOH) was synthesized as described in Cossé et al. (2020). Racemic anti-2,3-octanediol (anti-C8-diol) was synthesized as described in Lacey et al. (2004), and (2S,3R)-2,3-octanediol as in Wickham et al. (2016).
Study sites
Field experiments to test synthetic attractant pheromones, and to collect live adults of target cerambycid species for collection of headspace volatiles, were conducted at two widely separated sites in the Brazilian state of Sao Paulo: Piracicaba (University of Sao Paulo campus, – 22.711 lat., – 47.625 long.) and Valentim Gentil (private agricultural property, – 20.372 lat., – 50.080 long.). The Piracicaba site spans a 28-ha regenerated fragment of Atlantic Forest, comprising native and exotic species of mature hard- and softwood trees. It primarily features tree species in genera such as Inga, Leucaena, Libidibia, Piptadenia, Schizolobium, Tipuana (all Fabaceae), Cariniana, Lecythis (Lecythidaceae), Mangifera (Anacardiaceae), and Eucalyptus (Myrtaceae). The understory is a mix of young trees, lianas, shrubs, and herbaceous vegetation. The Valentim Gentil site encompasses a 4.8-ha remnant of Cerrado (Brazilian savanna), bordered by pasture lands and rubber tree plantations. It is predominantly composed of mature mixed hardwood trees, featuring native species such as Albizia, Anadenanthera, Hymenaea, Senegalia (Fabaceae), Handroanthus (Bignoniaceae), and Sterculia (Malvaceae). The understory consists of young trees, lianas, shrubs, herbaceous vegetation, and scattered fallen decaying trees.
General field trapping methods
Synthetic attractant pheromones were tested using cross-vane panel traps custom-built from black corrugated plastic (1.2 m tall × 0.3 m wide). Internal surfaces of traps were coated with a 50% aqueous suspension of the lubricant Fluon® (Insect-a-Slip, BioQuip Products Inc., Rancho Dominguez CA, USA). Traps were suspended from PVC pipe frames (2.1 × 0.3 m long; 2 cm i.d.) mounted on 1-m steel reinforcing bar posts driven into the ground. Collection jars, suspended below the traps, were filled with 300 ml of saturated aqueous NaCl solution and a few drops of dish detergent to preserve captured beetles. Pheromone lures were prepared from clear polyethylene press-seal bags (5 × 7 cm, 50 μm wall thickness, BCIEEL S.A., Brazil), containing a dental cotton roll loaded with test compound solutions (50 mg for racemic compounds and 25 mg for pure enantiomers) in 1 ml of isopropanol. Release rates of compounds from these lures generally range from 0.02 to 1.5 mg/d (Millar et al. 2018). Control lures comprised similar bags with cotton wicks loaded with 1 ml of isopropanol. Lures were hung in the central open slot of traps and replaced every 15 d. Lures were randomly assigned to traps within a block. Each block included one trap for each treatment and a control trap, spaced ~ 15 m apart, and blocks were ~ 30 m apart. The number of blocks varied with experiment (see below). To control for positional bias, treatments were rotated within blocks each time traps were serviced.
Sources of cerambycid species for collection of insect-produced volatiles
Live adults of A. surinamum and S. inerme were captured for collection of headspace volatiles using the aforementioned traps, but with minor modifications. Holes approximately 2 mm in diameter were drilled in the bottom of each trap’s collection jar to facilitate rainwater drainage. One trap baited with anti-C8-diol was deployed in Piracicaba (1 October–6 November 2015), targeting A. surinamum. Adults of S. inerme were caught in Valentim Gentil (20–30 October 2016) with a trap baited with a six-component blend of cerambycid pheromones that has been shown to attract multiple cerambycid species across Brazil (for details, see Silva et al. 2024). Traps were checked daily for captured beetles. Adults of A. surinamum and S. inerme were sexed based on antennal length, the antennae being almost twice the body length in males and ~ equal to body length in females. Subsequently, beetles were held individually in plastic containers, each containing a glass vial filled with 10% sucrose solution to provide moisture and nourishment. Beetles captured in Valentim Gentil were transported to Piracicaba for aeration within the same collection week. All beetles underwent a 24 h acclimation period under laboratory conditions (25 ± 2 ℃, 60 ± 10% RH, 12:12 h L:D, and 5000 lx LED lights) before initiating volatile collections.
Collection of headspace volatiles from beetles
Beetles, either individually or in pairs of the same species and sex, were placed in 500 ml cylindrical glass chambers positioned horizontally on a laboratory bench. To offer perching places, half of the chamber's internal surface was lined with paper towels. Each chamber contained two glass vials filled with 10% sucrose solution for nourishment. Volatiles were trapped with collectors fabricated from glass pipettes (8.5 cm long × 0.5 cm i.d.) packed with 150 mg of 80/100 mesh HayeSep® Q adsorbent (Supelco, Bellefonte, PA, USA) held in place with glass wool plugs. Collectors were connected to chamber outlets using screw caps fitted with PTFE ferrules. A constant airflow of ~ 150 ml/min of activated charcoal-filtered air was passed through the chambers and pipettes, regulated by flowmeters. Beetles underwent continuous aeration for 1–4 periods, each lasting 24–72 h. Simultaneously, control chambers containing only paper towels and feeder vials were aerated to monitor for potential system contaminants. Three successive aliquots of 500 μl of dichloromethane were used to extract volatiles from collectors into 2 ml silanized amber glass vials. Vials were stored at − 30 ℃ until analysis. In total, we aerated eight males and three females of A. surinamum from 10 October to 6 November 2015 and eight males and four females of S. inerme from 24 to 31 October 2016.
Identification of candidate pheromone components of A. surinamum and S. inerme
Volatiles collected from A. surinamum (12 extracts from males and three from females) and S. inerme (six from males and three from females) were initially analyzed in Brazil by gas chromatography with flame ionization detection (GC–FID) or gas chromatography–mass spectrometry (GC–MS) to confirm they contained potential compounds of interest, i.e., sex-specific compounds. Two-microliter aliquots were injected into a GC–2010 gas chromatograph (Shimadzu Corp., Kyoto, Japan) fitted with an Rtx–1 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film; Restek, Bellefonte, PA, USA). Injections were made splitless (purge valve off for 1 min) with an injector temperature of 250 ℃ and helium carrier gas at a linear velocity of 30 cm/s. The GC oven was programmed at 35 ℃ (hold 1 min), increased to 40 ℃ at 2 ℃/min (hold 1 min), and then increased to 250 ℃ at 10 ℃/min (hold 15 min). Similarly, 1 µl of the sample was injected splitless into a Shimadzu QP2010 Ultra GCMS fitted with an Rtx–1MS nonpolar column (30 m × 0.25 mm × 25 µm film; Restek). Injector and GC oven temperatures were set as described above, with helium carrier gas at 44 cm/s and 80.8 kPa inlet pressure. Ion source and quadrupole temperatures were 250 ℃. Mass spectra were recorded in electron impact mode (70 eV) from m/z 35–260 amu, with a 4-min solvent delay.
Samples that contained detectable quantities of sex-specific compounds were shipped to the University of California, Riverside. These samples were reanalyzed using an Agilent 7820A GC interfaced to a 5977E mass selective detector (Agilent Technologies, Santa Clara CA, USA) with helium as the carrier gas. The GC was equipped with an HP–5 column (30 m × 0.25 mm i.d. × 0.25 μm film; Agilent Technologies), and 1 µl aliquots of samples were injected in splitless mode. The GC oven was programmed from 40 ℃/5 min, with a ramp of 10 ℃/min to 280 ℃ (hold for 10 min). Quadrupole, ion source, and injector temperatures were set at 150, 230, and 250 ℃, respectively. Mass spectra were obtained using electron impact ionization (70 eV) with a mass range from 40–400 amu. Absolute configurations of chiral compounds were determined by analysis of extracts on a Cyclodex-B chiral stationary phase GC column (30 m × 0.25 mm, 0.25 μm film, J&W Scientific, Folsom CA, USA), with a flame ionization detector, comparing retention times of insect-produced compounds with those of authentic standards. Identities of compounds were confirmed by coinjection of aliquots of insect extract, spiked with the appropriate standard. The oven was programmed from 50 ℃/1 min, 5 ℃/min to 200 ℃.
Field bioassays of identified candidate attractant pheromones of A. surinamum and S. inerme
Candidate synthetic pheromones of A. surinamum were bioassayed at the Piracicaba site. Three sequential bioassays were performed with treatments randomly assigned to traps across five blocks (Bioassay 1), six blocks (Bioassay 2), and seven blocks (Bioassay 3), with traps checked every 2–4 d for captured beetles.
Bioassay 1 was conducted from 23 October to 8 November 2021 (total of seven collection dates) and assessed attraction to individual compounds or 1:1 blends, including: 1) SR-C8-diol; 2) anti-C8-diol; 3) MeBuOH; 4) SR-C8-diol + MeBuOH; 5) anti-C8-diol + MeBuOH; and 6) Control.
Because bioassay 1 showed A. surinamum was most strongly attracted by anti-C8-diol + MeBuOH (see Results), bioassay 2 tested this blend, the analogous blend of anti-C8-diol with the insect-produced (S)-MeBuOH. as well as anti-C8-diol alone, (S)-MeBuOH alone, and the control. This bioassay ran from 9 to 24 November 2021 (seven collection dates).
Bioassay 3 tested ratios of anti-C8-diol:S-MeBuOH in a semilogarithmic dosage scale, as follows: (1) 100:0; (2) 100:3.3; (3) 100:10; (4) 100:33; (5) 100:100; and (6) control. This bioassay ran from 18 October to 1 November 2022 (four collection dates).
Candidate pheromone compounds for S. inerme were tested at the Valentim Gentil site, testing MeBuOH and MePeOH as individual compounds, the 1:1 blend, and the control. These treatments were allocated to traps across four blocks, with traps checked every 2–4 d from 8 October to 24 December 2017 (26 collection dates).
Cerambycid beetles were identified to species by Antonio Santos-Silva of the Museum of Zoology of the University of São Paulo (MZUSP). Voucher specimens of the study species have been deposited in the museum collection in the Department of Entomology and Acarology (USP/ESALQ), Piracicaba, SP, Brazil.
Statistical analyses
We evaluated treatment differences for species represented by at least eight specimens per bioassay using the nonparametric Friedman’s test (PROC FREQ, option CMH; SAS Institute Inc. 2011) due to field data not meeting ANOVA assumptions due to heteroscedasticity (Sokal and Rohlf 1995). Replicates were defined by both spatial (block) and temporal (collection date) data. Spatial blocks with the greatest numbers of beetles (i.e., that represented the independent responses of multiple beetles to bioassay treatments) were lent greater weight by dropping from analyses those spatial blocks having fewer than a threshold number of the target species. These threshold numbers were selected, separately for A. surinamum (threshold 4, 2, and 31 for bioassays 1, 2 and 3, respectively), S. inerme (1), and non-target cerambycid species (1), so as to maximize the number of beetles captured per replicate while maintaining sufficient replication for a robust statistical test (at least eight replicates).
To address the multiple statistical tests of treatment effects, we adjusted significance levels in bioassays 1 (α = 0.017; N = 3 analyses), 2 (α = 0.025; N = 2), and 3 (α = 0.017; N = 3) for A. surinamum using the Bonferroni procedure (Quinn and Keough 2002). When the Friedman’s test showed overall significance, we conducted pairwise comparisons of treatment means using the Ryan-Einot-Gabriel-Welsch multiple range test (REGWQ) which controls the Type I experiment-wise error rate (SAS Institute Inc. 2011).
Results
Identification of candidate pheromone components of A. surinamum and S. inerme
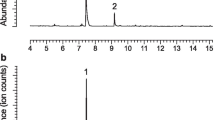
GC-FID analysis of aeration extracts from A. surinamum detected two volatile compounds emitted by males which were absent in extracts from conspecific females or control aerations (Fig. 1a). Among the 12 aeration samples from males, five contained both major and minor sex-specific compounds, five contained only the major compound, and two samples lacked these compounds altogether. The major compound was tentatively identified as a 2,3-octanediol from its mass spectrum, and was confirmed to be anti-C8-diol by comparing its retention time and mass spectrum with those of authentic standards of syn- and anti-diastereomers, which are readily separable and distinguishable on the nonpolar DB-5 GC column used for analyses. Further analyses on the chiral stationary phase Cyclodex B GC column determined the beetles produced exclusively the (2S,3R)-enantiomer.
Representative gas chromatograms of headspace volatiles collected from males of Achryson surinamum and Sphaerion inerme. Peaks are numbered to denote male-specific compounds: 1 = (S)-2-methylbutan-1-ol, 2 = (2S,3R)-2,3-octanediol, 3 = (R)-2-methylbutan-1-ol, and 4 = (R)-2-methylpentan-1-ol. Asterisks (*) indicate analytical artifacts or contaminants from the aeration system
The minor component, which eluted considerably earlier than anti-C8-diol, was tentatively identified as the known cerambycid pheromone component MeBuOH, and identification was confirmed by matches with an authentic standard. Analyses on the Cyclodex B column then determined that the beetles produced the (S)-enantiomer.
GC-FID chromatograms of extracts of volatiles emitted by male S. inerme revealed two peaks which were not observed in equivalent chromatograms from females or in aeration controls (Fig. 1b). The first peak was readily identified as MeBuOH from its retention time and mass spectrum, as described above for A. surinamum. The second peak was tentatively identified as the homologous MePeOH from matching its mass spectrum to the NIST mass spectral database, and identification was confirmed by matching its mass spectrum and retention time with those of an authentic standard. Analysis of an extract on the Cyclodex B column, in conjunction with appropriate standards, determined that males of this species produce R-MeBuOH and R-MePeOH.
Field bioassays of identified candidate pheromones of A. surinamum and S. inerme
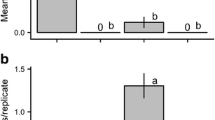
In bioassay 1, 152 adults of A. surinamum (86 females and 66 males) were captured. Among the treatments, SR-C8-diol alone or combined with MeBuOH, as well as the racemic anti-C8-diol + MeBuOH attracted significantly more beetles than MeBuOH alone or the control (Fig. 2a). Catches with anti-C8-diol alone were intermediate (Fig. 2a). During this bioassay, 27 adults of the non-target cerambycid S. inerme were also captured, most being in traps baited with SR-C8-diol + MeBuOH (Fig. 2b). Moreover, 20 adults of the cerambycid Andraegoidus rufipes (F.) (Cerambycinae: Trachyderini) were captured, but catches of this species appeared to be randomly distributed across treatments (means not significantly different; Friedman’s Q5,78 = 10.5, p > 0.017).
Mean number (± 1 SE) of beetles of both sexes combined, of (a) Achryson surinamum and (b) Sphaerion inerme captured during field bioassay 1 in Piracicaba. Compound abbreviations: SR-C8-diol = (2S,3R)-2,3-octanediol, anti-C8-diol = racemic anti-(2,3)-octanediol, MeBuOH = racemic 2-methylbutan-1-ol, and Control = solvent (neat isopropanol). The effect of treatment type on trap catches was statistically significant for both A. surinamum (Friedman’s Q5,114 = 40.4, p < 0.001, N = 19 replicates) and S. inerme (Q5,84 = 19.4, p < 0.001, N = 14). Differing letters within a species denote statistically significant differences determined by the REGWQ multiple range test (p < 0.05)
Seventy-seven adults of A. surinamum (50 females and 27 males) were captured during bioassay 2, with beetles showing a significant preference for the anti-C8-diol + S-MeBuOH blend (Fig. 3). Although nine adults of A. rufipes were also captured, catches again appeared random with respect to treatments (means not significantly different; Q4,40 = 2.0, p > 0.025).
Mean number (± 1 SE) of adult Achryson surinamum of both sexes combined, captured during field bioassay 2 in Piracicaba. Compound abbreviations: anti-C8-diol = racemic anti-(2,3)-octanediol, MeBuOH = racemic 2-methylbutan-1-ol, S-MeBuOH = (S)-2-methylbutan-1-ol, and Control = solvent (neat isopropanol). The effect of treatment type on trap catches of A. surinamum was statistically significant (Friedman’s Q4,90 = 48.2, p < 0.001, N = 18 replicates). Differing letters denote statistically significant differences determined by the REGWQ multiple range test (p < 0.05)
In bioassay 3, which tested different ratios of anti-C8-diol with S-MeBuOH, 894 adults of A. surinamum (433 females and 461 males) were captured. Attraction steadily increased with increasing amounts of S-MeBuOH in blends (Fig. 4). All blends attracted significantly more beetles than the control, and the 100:100 ratio attracted significantly more beetles than the 100:0, 3.3, and 10 ratios. Catches with the 100:100 blend did not significantly differ from those with the 100:33 blend (Fig. 4). Twenty-four adults of the cerambycine Ambonus interrogationis (Blanchard) (tribe Elaphidiini) and 11 of A. rufipes were also captured. As in the other bioassays, these captures seemed incidental rather than a response to the treatments because mean captures of both species were not significantly different across all treatments (Q5,84 = 18.2, p > 0.017; Q5,66 = 4.4, p > 0.017, respectively).
Mean number (± 1 SE) of adult Achryson surinamum of both sexes combined, captured during field bioassay 3 in Piracicaba. The numbers in treatments denote different ratios of racemic anti-(2,3)-octanediol:(S)-2-methylbutan-1-ol. Control = solvent (neat isopropanol). The effect of treatment type on trap catches of A. surinamum was statistically significant (Friedman’s Q5,96 = 54.2, p < 0.001, N = 16 replicates). Differing letters denote statistically significant differences determined by the REGWQ multiple range test (p < 0.05)
During the bioassay that targeted S. inerme, 273 adults (149 females and 124 males) were captured. Beetles were more strongly attracted to the MeBuOH + MePeOH blend than to either compound alone, and all treatments were significantly more attractive than the control (Fig. 5). No other cerambycid species was caught in sufficient numbers for statistical analysis.
Mean number (± 1 SE) of adult Sphaerion inerme, both sexes combined, captured during a field bioassay in Valentim Gentil. Compound abbreviations: MeBuOH = racemic 2-methylbutan-1-ol, MePeOH = racemic 2-methylpentan-1-ol, and Control = solvent (neat isopropanol). The effect of treatment type on trap catches of S. inerme was statistically significant (Friedman’s Q3,64 = 46, p < 0.001, N = 16 replicates). Differing letters denote statistically significant differences determined by the REGWQ multiple range test (p < 0.05)
Discussion
This study showed a sex-specific emission pattern in adult males of A. surinamum, characterized by the production of (2S,3R)-2,3-octanediol and lower amounts of (S)-2-methylbutan-1-ol. Through field trapping bioassays employing synthetic compounds, our findings indicate that blending SR- or racemic anti-C8-diol with MeBuOH led to significantly increased catch rates compared to individual compounds or controls. The non-natural enantiomer in anti-C8-diol did not disrupt attraction of A. surinamum. From a practical point of view, this information is useful because the racemate is much cheaper to synthesize than the pure stereoisomer. However, subsequent field tests comparing the effects of racemic MeBuOH and S-MeBuOH showed that attraction of A. surinamum to anti-C8-diol was significantly increased when combined with S-MeBuOH, indicating that the non-natural R-enantiomer present in racemic MeBuOH was partially disrupting attraction. However, in this case, because S-MeBuOH is relatively inexpensive, using the pure enantiomer should not be a hindrance to developing a practical lure for this species. In a final bioassay, testing ratios of the two compounds, the results suggested that ratios containing approximately equal amounts of the two components were most attractive.
To our knowledge, our study is the first confirmation of a 2,3-alkanediol being produced by a South American cerambycid species, although there has been a previous report of attraction of cerambycids to 2,3-hexanediols in field trials in Chile (Curkovic et al. 2022). 2,3-Alkanediols were among the first attractant pheromones identified within the family Cerambycidae (Sakai et al. 1984), and they have been reported from a number of Asian, North American, and European species in the subfamilies Cerambycinae and Prioninae (Millar and Hanks 2017). Thus, this appears to be another structural motif that is widely shared within the family.
Serendipitously, live specimens of both sexes of the sympatric S. inerme were captured during the first bioassay targeting A. surinamum, with traps baited with MeBuOH combined with SR-C8-diol yielding highest captures. Subsequent analysis of extracts of volatiles collected from live beetles confirmed sex-specific production of R-MeBuOH and R-MePeOH by males. Subsequent field tests employing racemic versions of these compounds demonstrated that both sexes were significantly attracted to both compounds, but more strongly to MeBuOH, and even more strongly to the blend of MeBuOH+MePeOH. The fact that beetles were attracted to the blend of racemic compounds is fortuitous, because the racemic versions of both compounds are cheap and readily available commercially should monitoring lures be required for this species. To our knowledge, this is also the first time MePeOH has been identified as a cerambycid pheromone component, expanding the known “chemical space” occupied by pheromones of this family.
Data availability
Not applicable.
Code availability
Not applicable.
References
Bello JE, McElfresh JS, Millar JG (2015) Isolation and determination of absolute configurations of insect-produced methyl-branched hydrocarbons. Proc Nat Acad Sci USA 112:1077–1082
Bobadoye B, Torto B, Fombong A, Zou Y, Adlbauer K, Hanks LM, Millar JG (2019) Evidence of aggregation-sex pheromone use by longhorned beetles (Coleoptera: Cerambycidae) species native to Africa. Environ Entomol 48:189–192
Cossé AA, Zilkowski BW, Zou Y, Millar JG, Bauer L, Poland T (2020) Female-produced sex pheromone of Tetrastichus planipennisi, a parasitoid introduced for biological control of the invasive emerald ash borer, Agrilus planipennis. J Chem Ecol 46:508–519
Curkovic T, Arraztio D, Huerta A, Rebolledo R, Cheuquel A, Contreras A, Millar JG (2022) Generic pheromones identified from northern hemisphere Cerambycidae (Coleoptera) are attractive to native longhorn beetles from Central-Southern Chile. Insects 13:1067
Duffy EAJ (1953) A monograph of the immature stages of British and imported timber beetles (Cerambycidae). Order of the Trustees of the British Museum, London
Duffy EAJ (1960) A monograph of the immature stages of Neotropical timber beetles (Cerambycidae). Order of the Trustees of the British Museum, London
Fan J, Denux O, Courtin C, Bernard A, Javal M, Millar JG, Hanks LM, Roques A (2019) Multi-component blends for trapping native and exotic longhorn beetles at potential points-of-entry and in forests. J Pest Sci 92:281–297
Flaherty L, Gutowski JMG, Hughes C, Mayo P, Mokrzycki T, Pohl G, Silk P, Van Rooyen K, Sweeney J (2019) Pheromone-enhanced lure blends and multiple trap heights improve detection of bark and wood-boring beetles potentially moved in solid wood packaging. J Pest Sci 92:309–325
Girard DH (1968) List of intercepted plant pests, 1967: pests recorded from July 1, 1966, through June 30, 1967. USDA-ARS, Hyattsville
Hanks LM, Millar JG (2016) Sex and aggregation-sex pheromones of cerambycid beetles: basic science and practical applications. J Chem Ecol 42:631–654
Hayes RA, Griffiths MW, Nahrung HF, Arnold PA, Hanks LM, Millar JG (2016) Optimizing generic cerambycid pheromone lures for Australian biosecurity and biodiversity monitoring. J Econ Entomol 109:1741–1749
Lacey ES, Ginzel MD, Millar JG, Hanks LM (2004) Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. J Chem Ecol 30:1493–1507
Maier CT (2017) Cerambycidae (Coleoptera) accidentally introduced into connecticut from China or from other areas in the United States. Proc Entomol Soc Wash 119:423
Meurisse N, Rassati D, Hurley BP, Brockerhoff EG, Haack RA (2019) Common pathways by which non-native forest insects move internationally and domestically. J Pest Sci 92:13–27
Millar JG, Hanks LM (2017) Chemical ecology of cerambycids. In: Wang Q (ed) Cerambycidae of the World: biology and pest management. CRC Press, Boca Raton, pp 167–202
Millar JG, Mitchell RF, Mongold-Diers JA, Zou Y, Bográn CE, Fierke MK, Ginzel MD, Johnson CW, Meeker JR, Poland TM, Ragenovich IR, Hanks LM (2018) Identifying possible pheromones of cerambycid beetles by field testing known pheromone components in four widely separated regions of the United States. J Econ Entomol 111:252–259
Millar JG, Richards AB, Halloran S, Zou Y, Boyd EA, Quigley KN, Hanks LM (2019) Pheromone identification by proxy: identification of aggregation-sex pheromones of North American cerambycid beetles as a strategy to identify pheromones of invasive Asian congeners. J Pest Sci 92:213–220
Monné MA (2024) Catalogue of the Cerambycidae (Coleoptera) of the neotropical region. Part I. subfamily Cerambycinae. https://cerambycids.com/catalog/. Accessed 4 Apr 2024
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rassati D, Marini L, Marchioro M, Rapuzzi P, Magnani G, Poloni R, Di Giovanni F, Mayo P, Sweeney J (2019) Developing trapping protocols for wood-boring beetles associated with broadleaf trees. J Pest Sci 92:267–279
Ray AM, Lacey ES, Hanks LM (2006) Predicted taxonomic patterns in pheromone production by longhorned beetles. Naturwissenschaften 93:543–550
Roques A, Ren L, Rassati D, Shi J, Akulov E, Audsley N, Auger-Rozenberg M, Avtzis D, Battisti A, Bellanger R, Bernard A, Bernadinelli I, Branco M, Cavaletto G, Cocquempot C, Contarini M, Courtial B, Courtin C, Denux O, Dvořák M, Fan J, Feddern N, Francese J, Franzen EKL, Garcia A, Georgiev G, Georgieva M, Giarruzzo F, Gossner M, Gross L, Guarneri D, Hoch G, Hölling D, Jonsell M, Kirichenko N, Loomans A, Luo Y, McCullough D, Maddox C, Magnoux E, Marchioro M, Martinek P, Mas H, Mériguet B, Pan Y, Phélut R, Pineau P, Ray AM, Roques O, Ruiz M, Sarto i Monteys V, Speranza S, Sun J, Sweeney JD, Touroult J, Valladares L, Veillat L, Yuan Y, Zalucki MP, Zou Y, Žunič-Kosi A, Hanks LM, Millar JG (2023) Worldwide tests of generic attractants, a promising tool for early detection of non-native cerambycid species. NeoBiota 84:169–209
Sakai T, Nakagawa Y, Takahashi J, Iwabuchi K, Ishii K (1984) Isolation and identification of the male sex pheromone of the grape borer Xylotrechus pyrrhoderus Bates (Coleoptera: Cerambycidae). Chem Lett 13:263–264
Santos-Silva A, Botero JP, de Nascimento FEL, Silva WD (2020) A new synonym and seventeen new distributional records in South American Cerambycidae (Coleoptera), with notes on Chlorethe scabrosa Zajciw, 1963. Pap Avulsos Zool 60:e20206010
SAS Institute (2011) SAS/STAT v 93 User’s Guide. SAS Institute Inc, Cary
Silva WD, Zou Y, Bento JMS, Hanks LM, Millar JG (2017) Aggregation-sex pheromones and likely pheromones of 11 South American cerambycid beetles, and partitioning of pheromone channels. Front Ecol Evol 5:101
Silva WD, Millar JG, Hanks LM, Costa CM, Leite MOG, Tonelli M, Bento JMS (2018) Interspecific cross-attraction between the South American cerambycid beetles Cotyclytus curvatus and Megacyllene acuta is averted by minor pheromone components. J Chem Ecol 44:268–275
Silva WD, Hanks LM, Alvarez JCS, Madalon FZ, Bento JMS, Bello JE, Millar JG (2020) Variations on a theme: two structural motifs create species-specific pheromone channels for multiple species of South American cerambycid beetles. Insects 11:222
Silva WD, Hanks LM, Bento JMS, Millar JG (2021) 3-Hydroxyhexan-2-one and 3-methylthiopropan-1-ol as pheromone candidates for the South American cerambycid beetles Stizocera phtisica and Chydarteres dimidiatus dimidiatus, and six related species. J Chem Ecol 47:941–949
Silva WD, Hanks LM, Bento JMS, Zou Y, Millar JG (2024) Evidence for 3-hydroxyhexan-2-one as a shared pheromone component for 12 South American species of cerambycid beetles. J Econ Entomol. https://doi.org/10.1093/jee/toae075
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W. H. Freeman, New York
Sweeney JD, Silk PJ, Grebennikov V (2014) Efficacy of semiochemical-baited traps for detection of longhorn beetles (Coleoptera: Cerambycidae) in the Russian Far East. Eur J Entomol 111:397–406
Wickham JD, Millar JG, Hanks LM, Zou Y, Wong JCH, Harrison RD, Chen Y (2016) 2R,3S)-2,3-Octanediol, a female-produced sex pheromone of Megopis costipennis (Coleoptera: Cerambycidae: Prioninae. Environ Entomol 45:223–228
Acknowledgements
We thank Araci R. Silva, Cassio D. Silva (Valentim Gentil), Fernando Z. Madalon (USP/ESALQ, Piracicaba), and the staff at the Department of Forest Sciences of USP/ESALQ (Experimental Station of Forest Science at Itatinga) for their invaluable support during the field experiments. Special recognition goes to Antonio Santos-Silva of the Museum of Zoology of the University of São Paulo, MZUSP, whose expertise was pivotal in identifying the cerambycid species. Field collections of the study species in Brazil were conducted under SISBIO permit #46395 from the Brazilian Ministry of the Environment. Our work adheres to ethical guidelines, registered with the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen, Brazil) under #AE3897B.
Funding
This work was supported by INCT-Semioquímicos na Agricultura (FAPESP grant # 2014/50871–0 and CNPQ grant # 465511/2014–7 to J.M.B.), Sao Paulo Research Foundation (grant # 2013/26936–2 to W.D.S.) and the United States Department of Agriculture—Animal and Plant Health Inspection Service (grant #s 15, 16, 17, 18, 19, 20, and 21–8130-1422-CA to J.G.M. and L.M.H., and grant #s 21, 22, and 23–8130-0909-CA to W.D.S.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. W.D.S. collected insects, prepared extracts, performed initial chemical analyses, and conducted field bioassays. J.G.M. identified compounds, and Y.Z. synthesized several of the compounds used in this study. W.D.S. and L.M.H. carried out the statistical analyses. W.D.S. wrote the first draft of the manuscript, J.G.M. added sections related to chemistry, and J.G.M. and L.M.H. reviewed and edited the manuscript. Funding was acquired via grants submitted by L.M.H., J.M.B., and J.G.M. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Günther Raspotnig.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, W.D., Zou, Y., Hanks, L.M. et al. Pheromone chemistry of the Neotropical cerambycid beetles Achryson surinamum and Sphaerion inerme. Chemoecology 34, 61–69 (2024). https://doi.org/10.1007/s00049-024-00401-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-024-00401-w