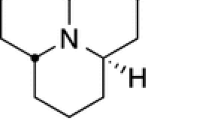
Abstract
Seventy skins of three mantellid frog species from Madagascan swamp-forest habitats, Mantella aurantiaca, M. crocea, and M. milotympanum, were individually examined for skin alkaloids using GC/MS. These poison frogs were found to differ significantly in their alkaloid composition from species of Mantella originating from non-flooded rainforest in eastern Madagascar, which were examined in earlier work. Only 16 of the previously detected 106 alkaloids were represented among the 60 alkaloids from the swamp-forest frogs of the present study. We hypothesize this difference is related mainly to habitat but cannot exclude a phylogenetic component as the three swamp-forest species are a closely related monophyletic group. The paucity of alkaloids with unbranched-carbon skeletons (ant-derived) and the commonness of alkaloids with branched-carbon skeletons (mite-derived) indicate that oribatid mites are a major source of alkaloids in these species of mantellids. Furthermore, most of the alkaloids have an oxygen atom in their formulae. Differences in alkaloids were observed among species, populations of the same species, and habitats. In M. aurantiaca, small geographic distances among populations were associated with differences in alkaloid profiles, with a remote third site illustrating even greater differences. The present study and an earlier study of three other mantellid species suggest that oribatid mites, and not ants, are the major source of alkaloids in the species of mantellids examined thus far.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkaloid-based chemical defenses have been identified in the skins of five evolutionarily distinct lineages of frogs, commonly referred to as poison frogs, found in the families Bufonidae, Dendrobatidae, Eleutherodactylidae, Mantellidae, and Myobatrachidae (Daly et al. 2005; Rodríguez et al. 2010; Saporito et al. 2012). More than 850 alkaloids have been detected in poison frogs, with the majority of the compounds characterized by low molecular weights (MW < 500 amu) and “izidine” carbon skeletons (Daly et al. 2005; Garraffo et al. 2012; Saporito et al. 2012). Nearly all of these alkaloids are sequestered from a diet of alkaloid-containing small arthropods, which largely includes brachypyline mites and myrmicine ants (Daly et al. 1994; Hantak et al. 2013; Saporito et al. 2009, 2012). The only known exceptions to sequestration are provided by myobatrachid frogs of the genus Pseudophryne (Smith et al. 2002) and by dendrobatid frogs of the genera Adelphobates and Dendrobates (Daly et al. 2003). Since nearly all alkaloids found in poison frogs arise from sequestration of arthropod alkaloids, the composition of alkaloids in poison frogs is dependent largely on the availability of alkaloid-containing arthropods.
The genus Mantella (family Mantellidae, subfamily Mantellinae) from Madagascar is currently composed of 16 described and at least one undescribed species (Glaw and Vences 2007; Vences et al. 1999). Alkaloid composition has been reported for 11 species of this genus, and more than 400 alkaloids have been detected (see Andriamaharavo et al. 2010; Clark et al. 2005, 2006; Daly et al. 1996, 2008; Garraffo et al. 1993a; Saporito et al. 2012). Alkaloid composition varies among species as well as among populations and among individuals of the same species, a finding that is consistent with studies of alkaloid defenses in bufonid and dendrobatid poison frogs (see Saporito et al. 2012 for a review).
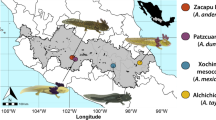
The present study continues our analysis of individual frog skins of Mantella, consisting of three closely related species: (1) three populations of Mantella aurantiaca, two of them from very close collection sites, and the other from a geographically distant location; (2) one population of M. crocea; and (3) one population of M. milotympanum (Fig. 1). All three species in the present study are found almost exclusively in swamp-forest habitats. Other studies of mantellid alkaloids have focused on species found in “drier” habitats (i.e., non-flooded rainforest) (Andriamaharavo et al. 2010).
Map of collection localities of specimens used in this study. Mantella. aurantiaca (circles); M. milotympanum (triangle); and M. crocea (square). Localities are numbered as follows: (1) Torotorofotsy (comprising two populations), (2) Andranomena (south of Moramanga), (3) Fierenana, and (4) Ampangandimbolana. The light grey shade represents the approximate extension of rainforest; major lakes and swamps are marked in dark grey; roads and major towns/villages are indicated on map
Swamp-forest habitats are located mainly at mid-altitudes (900–1000 m) in east-central Madagascar, and can be defined as open swamp valleys with surrounding hillside forests (Bora et al. 2008; Zimmermann and Andrianarivo 2000). Our observations indicate that these swamp habitats can measure from less than 20 m to more than 1 km in diameter. All three species (M. aurantiaca, M. crocea, M. milotympanum) are most typically found in periodically flooded rainforest characterized by the presence of screw pines (Pandanus spp.), and often bordering treeless swamp plains (Glaw and Vences 2007; Zimmermann and Andrianarivo 2000). During the rainy season (November–April), the forest edges contain small and shallow swampy regions composed of ponds, puddles, and slow flowing streams that are used by M. aurantiaca, M. crocea, and M. milotympanum for reproduction. During the dry season (May–October), these species are found mainly in the surrounding hillside forests.
Several other species of Mantella are found regularly in swampy areas of rainforest or rainforest edges, especially the lowland species M. bernhardi, as well as M. baroni, M. ebenaui, M. madagascariensis, and M. pulchra. Therefore, it should be emphasized that the dichotomy between non-flooded rainforest species and swamp-forest species is not rigid, and some forest species (e.g., M. bernhardi) are found frequently in swampy regions of their habitat. On the basis of mitochondrial RNA studies, M. madagascariensis and M. pulchra, together with the three species of Mantella examined in the present study, form a clade of closely related species called the M. madagascariensis group (Chiari et al. 2004; Vences et al. 2004; Vieites et al. 2006). While M. madagascariensis and M. pulchra appear to be found mainly in small swampy areas within their rainforest habitat, often next to streams, M. aurantiaca, M. crocea, and M. milotympanum are most commonly found in swamp-forest habitats as defined above.
Although the three species examined in the present study are collected for the pet trade, and until 2001, M. aurantiaca was the most heavily exported frog species from Madagascar (Rabemananjara et al. 2008a, b), there is little information on their natural history. Two swamp-forest species, M. aurantiaca and M. milotympanum, are considered to be in the Red List category “critically endangered” and one species, M. crocea, in the “endangered” category (Andreone et al. 2005). Only recently has an up to date geographical distribution of these three species been reported, with M. aurantiaca being found at 16 sites mostly in the Moramanga area, M. crocea at six sites slightly north of there, and M. milotympanum at four sites even further north (see map in Bora et al. 2008).
The diet of the swamp frog M. aurantiaca from one site (Torotorofotsy) has been published (Woodhead et al. 2007), and on the basis of that study, we anticipated that the alkaloid composition of all swamp-forest mantellid frogs in the present study (M. aurantiaca, M. crocea, and M. milotympanum) would be derived largely from mites. This finding was confirmed by the alkaloid analysis reported here, in which the chemical structures of the alkaloids suggests they are derived from oribatid mites.
Methods and Materials
Mantellid Frog Collections
A total of 70 mantellid frogs of three species were collected and examined in this study, including M. aurantiaca (N = 42), M. crocea (N = 18), and M. milotympanum (N = 10). Mantella aurantiaca was collected from three different localities, Torotorofotsy-1 (Tor-1; N = 20), Torotorofotsy-2 (Tor-2; N = 10), and Andranomena (south of Moramanga; S. Mora; N = 12), whereas M. crocea was collected from Ampangadimbolana and M. milotympanum was collected from Fierenana (Fig. 1; Online Resource 1). Individual frogs were collected by hand, measured for snout-to-vent length (SVL) to the nearest 0.1 mm, and sex was determined. Voucher specimens were deposited at the Zoological Museum Amsterdam, Netherlands; the Université d’ Antananarivo, Département de Biologie Animale, Madagascar; and the Zoologische Staatssammlung München, Germany.
Individual Frog Alkaloid Extractions
Individual mantellid skin samples were stored in small plastic vials sealed with a silicone rubber O-ring with 1 ml of methanol (hereafter, referred to as the methanolic frog skin extract). Because of the large number of samples examined, a rapid extraction (slightly modified from Garraffo et al. 1993a) of the samples for GC/MS and GC–FTIR analyses was used to free the samples of the silicone impurities and other non-alkaloid components (e.g., fatty acids, fatty acid methyl esters, etc.), and provide an alkaloid extract. To 1 ml of the methanolic frog skin extract, 0.05 ml of 1 M HCl were added, and the mixture was stirred with a Vortex mixer. The extract was carefully concentrated with a stream of nitrogen to a final volume of approximately 0.2 ml. An equal volume of H2O was added followed by extraction with hexane (3 × 0.4 ml). The hexane layers were discarded. The remaining acidic aqueous layer was adjusted to pH > 8 with saturated NaHCO3 and extracted with ethyl acetate (3 × 0.3 ml). The combined ethyl acetate layers were dried over anhydrous Na2SO4 and the solvent was carefully evaporated with nitrogen to a final volume of 0.1 ml. We estimated that each individual skin weighed about 0.1 g, so 1 μl of this alkaloid extract is equivalent to 1 mg of the original wet weight of skin.
Spectral Analyses
Mass spectral data [Electron Impact Mass Spectrometry, Chemical Ionization Mass Spectrometry (NH3), and Chemical Ionization Mass Spectrometry (ND3)] were obtained with a Thermo Electron Polaris Q mass spectrometer coupled to a Thermo Electron Focus GC. The GC was fitted with a Restek RTX-5MS capillary column (30 m, 0.25 mm i.d, 0.25 μm film thickness), and the analysis used a temperature program from 100 °C (1 min) to 280 °C at a rate of 10 °C/min. Molecular weights up to about 500 amu were detected. An approximate quantitation of alkaloids was performed using total ion current intensities; however, Online Resource 2 points out deficiencies in these analyses and suggests an improved method for future work.
Vapor phase Fourier-transform infrared spectral data (GC-FTIR) were obtained using a Hewlett-Packard model 5890 gas chromatograph, with a Phenomenex Zebron ZB-5 capillary column (30 m, 0.32 mm i.d., 0.25 μm phase-thickness), using the same temperature program as above and interfaced with a Hewlett-Packard model 5971 mass selective detector (MSD) and a model 5965B (IRD) with a narrow range (4000–750 cm−1) infrared detector. A Hewlett–Packard ChemStation was used to generate EIMS and FTIR spectra.
High resolution GC/MS data were generated with a Waters GCT instrument coupled to a Hewlett-Packard model 6890 gas chromatograph equipped with a Restek RTX-5MS column (30 m, 0.25 mm i.d, 0.25 μm) using the same temperature program as above.
Statistical Analyses
Nonmetric multidimensional scaling (nMDS) was used to graphically visualize alkaloid composition among species, among populations of the same species, and between habitats. A one-way analysis of similarity (ANOSIM) was used to determine statistical differences in alkaloid composition. All nMDS plots and ANOSIM results are based on Bray-Curtis dissimilarity matrices. See Daly et al. (2008) and Saporito et al. (2006, 2007a, 2010) for additional examples and discussion on the use of nMDS and ANOSIM in the study of poison frog alkaloids. All multivariate statistical analyses were performed using the software program PRIMER (version 5; Clark and Warwick 2001).
Results
A total of 60 alkaloids (including isomers) from 14 structural classes were detected in individual skin extracts of M. aurantiaca (N = 42; Online Resource 3, Tables S2–S4), M. crocea (N = 18; Online Resource 3, Table S5), and M. milotympanum (N = 10; Online Resource 3, Table S6). Structures of the most common alkaloids of each structural class are depicted in Fig. 2. A summary of the alkaloids detected in each species and population is presented in Table 1. The majority of alkaloids detected were allopumiliotoxins (aPTX), pumiliotoxins (PTX), dehydrodesmethylpumiliotoxins (dehydrodesmethyl-PTX), 5,8-disubstituted- and 5,6,8-trisubsituted-indolizidines (5,8-I and 5,6,8-I), dehydro-5,8-disubstitued indolizidines (dehydro-5,8-I), 3,5-disubstituted pyrrolizidines (3,5-P), and unclassified as to structure (unclass). Nearly all of these alkaloid classes (74 %) have branched-carbon skeletons and are presumed to originate from oribatid mites (Takada et al. 2005; Saporito et al. 2007b, 2011); however, 3,5-disubsituted pyrrolizidines, which account for 13 % of these alkaloids, have unbranched-carbon skeletons and likely are derived from myrmicine ants (Jones et al. 1999; Saporito et al. 2012; see Online Resource 4 for a discussion on the potential link between dietary preferences and alkaloid oxidation state, double bond equivalents, and isomers). The most abundant alkaloids were aPTX 323B, PTXs 307A and 323A, 5,6,8-I 265L, and unclass 392. Four previously unreported alkaloids were detected, namely dehydro-5,8-I 263X, 3,5-I (3,5-disubstituted indolizidine) 281R, unclass 281S, and 5,6,8-I 281T. The GC retention times and spectral data for these alkaloids are presented in Online Resource 5.
Alkaloid composition varied among species and among populations of the same species at different geographic locations. Alkaloid composition was significantly different among M. aurantiaca, M. crocea, and M. milotympanum (Fig. 3; Global R = 0.58; P < 0.001). A large difference in alkaloid profiles was observed between two species (M. crocea and M. milotympanum). The alkaloid compositions of M. madagascariensis of Daly et al. (2008) and M. aurantiaca, M. crocea, and M. milotympanum of the present study (all species of the M. madagascariensis group) varied significantly between each species (Fig. 4a; Global R = 0.67; P < 0.001). Data from two different populations of M. madagascariensis from Daly et al. (2008) were included in the analysis of Fig. 4a, one from the relatively disturbed location of Besariaka and one from the undisturbed location of Ranomafana. These two populations from Daly et al. (2008) are distinguished in Fig. 4b, along with M. milotympanum. Alkaloid composition varied significantly among each population (Fig. 4b; Global R = 0.78; P < 0.001). Alkaloid composition varied significantly among the three populations (Torotorofotsy-1, Torotorofotsy-2, and Andranomena (south of Moramanga) of M. aurantiaca (Fig. 5; Global R = 0.62; P < 0.001). A comparison of the shared and unique alkaloids of M. aurantiaca in Torotorofotsy-1, Torotorofotsy-2, and Andranomena are presented in the Venn diagram shown in Fig. 6. Alkaloid composition significantly differed between mantellids from “non-flooded” rainforest habitats (M. baroni, M. bernhardi, and M. madagascariensis, previously studied in Daly et al. 2008) and mantellids from swamp-forest habitats of the present study (M. aurantiaca, M. crocea, and M. milotympanum) (Fig. 7; Global R = 0.88; P < 0.001). In the present study, there was no statistical relationship between alkaloid composition and frog size or frog sex; however, see further discussion in Online Resource 6.
a. Multidimensional scaling plot of alkaloid composition among M. aurantiaca, M. crocea, M. milotympanum, and M. madagascariensis. Each point represents an individual frog, and the distance between any two points is directly proportional to the difference in alkaloid composition between these points. b. Multidimensional scaling plot of alkaloid composition among one population of M. milotympanum, and two of M. madagascariensis. Each point represents an individual frog, and the distance between any two points is directly proportional to the difference in alkaloid composition between these points. Note: one point for M. madagascariensis from Besariaka represents two frogs
Venn diagram illustrating the alkaloids identified in three populations of M. aurantiaca from Andranomena (A), Torotorofotsy-1 (B), and Torotorofotsy-2 (C). Letter combinations within circles (AB, AC, BC, ABC) indicate shared alkaloids among populations. The shared alkaloids are shown next to the respective combination of letters: Sites A & B share 1 alkaloid; Sites A & C share 3 alkaloids; Sites B & C share 8 alkaloids; Sites A, B, & C share 9 alkaloids. Alkaloids with linear carbon skeletons (likely derived from myrmicine ants) are underlined, and pumiliotoxin (PTX) and allopumiliotoxin (aPTX) alkaloids are in bold font
Multidimensional scaling plot of alkaloid composition between mantellids from swamp-forest (M. aurantiaca, M. crocea, and M. milotympanum) and non-flooded rainforest (M. baroni, M. bernhardi, and M. madagascariensis) habitats (data for the latter species from Daly et al. 2008a). Each point represents an individual frog, and the distance between any two points is directly proportional to the difference in alkaloid composition between these points
There are some specific differences between alkaloids detected in swamp-forest frogs and those from non-flooded rainforest (“dry-land”). For example, although both groups of frogs contained aPTXs 305A, 305C, 321C, and 323B, each population also had unique aPTXs. Three unique aPTXs were found in “dry-land” frogs (253A, 323 J, and 325A) and two were unique to swamp-forest frogs (337D and 339A). Many of the alkaloids common to “dry-land” Mantella, such as the deoxy- and dihydro-PTX classes were absent from the swamp-forest frogs, but desmethyl PTXs were present in swamp-forest frogs. The 1,4-disubstituted quinolizidines and tricyclic alkaloids were absent from swamp-forest frogs. Of the fifteen 5,8-disubstituted indolizines detected in “dry-land” frogs, only one (253B) has been detected in the swamp-forest frogs. Furthermore, only one unclassified alkaloid (323G) was common to both swamp-forest and “dry-land” frogs, and only one of the eleven 5,6,8-Is (265L) detected in “dry-land” frogs was detected in swamp-forest frogs, whereas swamp-forest frogs had four different 5,6,8-Is (251S, 251T, 267T, and 281T). Lastly, the dehydro-5,8-I class was represented by four alkaloids (206L, 207W, 245F, and 247O) in the “dry-land” frogs, whereas four different dehydro-5,8-Is (249K, 251P, 263X, and 265F) have been detected in swamp-forest frogs. Only one alkaloid of this class (265F) was common to both “dry-land” and swamp-forest frogs.
Discussion
Differences in alkaloid composition were observed within and among the species of Mantella examined (see nMDS plots of Figs. 3, 4, 5 and 7). Alkaloid defenses in these mantellids are entirely dependent on a diet of alkaloid-containing arthropods, and it is hypothesized that differences in their chemical defenses are due largely to the availability of arthropods among geographic locations (Saporito et al. 2009). Variation in alkaloid composition among geographic locations has been described in other species of Mantella (see Andriamaharavo et al. 2010; Clark et al. 2006; Daly et al. 1996, 2008; Garraffo et al. 1993a), and appears to be a general characteristic common to all poison frogs (see Daly et al. 2007; Garraffo et al. 1993b; Saporito et al. 2006, 2007a, 2009, 2012; Smith et al. 2002 and references therein). Additional research on the availability and distribution of arthropods among different habitats is necessary to understand this complex relationship in mantellid frogs as well as other poison frogs.
Interestingly, some dietary studies of mantellid frogs have reported that ants represent a major food source for certain species (Clark et al. 2005; Vences and Kniel 1998; Vences et al. 1998). Vences and Kniel (1998) studied the stomachs of seven M. haraldmeieri, three M. nigricans, four M. laevigata, and one M. ebenaui (as M. betsileo), and reported ants as prevalent prey in all of these species, with an overall numeric proportion of 74 %. Additionally, Clark et al. (2005) reported that ants accounted for the majority of arthropods identified in stomach contents of populations of M. baroni, M. bernhardi, and M. madagascariensis (ranging from 51 % to 80 % of the total arthropods present, depending on the species/population); however, most of these ants belonged to the genus Pheidole, which to date, has not been shown to contain alkaloids in the New World tropics or Madagascar. Currently, the only detailed study of diet in M. aurantiaca (collected earlier at the present Tor-1 site) reported a low proportion of ants present in stomach contents (14 % of total arthropods present; Woodhead et al. 2007). The ants consumed by M. aurantiaca consisted largely of Pheidole and Monomorium species. It should be noted that alkaloids have only been detected in members of the genus Monomorium from the New World and mainland Africa (Jones et al. 1999, 2003). Although it is likely that Madagascan Monomorium will contain unbranched alkaloids common to this genus, to the best of our knowledge, Monomorium have not been examined for alkaloids in Madagascar. Interestingly, an examination of the alkaloid structures present in these species of mantellids suggests a minor contribution of alkaloids from ants, as compared to oribatid mites (see Supplementary Information of Clark et al. 2005 and Supplementary Information of Daly et al. 2008). Collectively, these findings suggest that although ants are consumed by mantellids, they do not appear to be a major source of alkaloids – in particular, species that consume a large proportion of ants do not necessarily contain a large amount or high diversity of ant-derived alkaloids (Clark et al. 2005; Daly et al. 2008; present study).
Ant-derived alkaloids appear to be a minor contribution to the sequestered alkaloids of M. baroni, M. bernhardi, and M. madagascariensis (Daly et al. 2008), and contribute even less to the swamp-forest species M. aurantiaca, M. crocea, and M. milotympanum in the present study. Although the majority of alkaloids in members of the genus Mantella (including those examined here) appear to arise from oribatid mites, this does not appear to translate into mites being the most abundant arthropods in the stomach contents of mantellid species examined thus far. Vences and Kniel (1998) found that mites constituted only 2 % of all prey. Clark et al. (2005) reported that Acari represented 12 % of the total arthropods present in stomach contents of populations of M. baroni, M. bernhardi, and M. madagascariensis (ranging from 6 % to 29 % of the total arthropods present, depending on the species/population), and Woodhead et al. (2007) reported that Acari represented 23 % of the arthropods present in stomach contents of M. aurantiaca but only 5 % of the volume. We note that Acari is a large taxonomic group that also includes non-oribatid mites, and currently, alkaloids have been detected only in oribatid mites (Saporito et al. 2007b, 2009, 2011). Future studies on the stomach contents of mantellids and other poison frogs should aim to improve the taxonomic resolution of Acari. Although Acari (presumably including oribatid mites) may not always represent the largest number of prey items in mantellids and may make an even smaller contribution to prey volume, they may be a dietary source rich in alkaloids, whereas ants may be a small source of alkaloids but a major source of nutrition.
Our findings of few alkaloids with unbranched-carbon skeletons in the swamp-forest frogs suggest that ant prey is not an important source of alkaloids in these mantellids. In earlier studies of riparian or forest-inhabiting Madagascan frogs (e.g., Daly et al. 1996, 2008; Garraffo et al. 1993a), only a small number of alkaloids suspected to originate from ants were present in frog skins. The limited number of ant alkaloids is even more pronounced in the swamp-forest frogs examined here. Of the frogs examined, only one 3,5-I (211E) and six 3,5-Ps [233H, cis/trans 239K (3 isomers), 239R, and 267H] were detected among the 60 total alkaloids from all three species, representing the only suspected ant alkaloids. Only two of these pyrrolizidines, 239K (one stereoisomer) and 267H, have been detected in previous studies of Mantella from non-flooded rainforests (Daly et al. 2008). The 3,5-Is detected in previous studies of “dry-land” mantellids, 249A and 275C, are absent in swamp-forest frogs. However, substantial amounts of the 3,5-I 211E and 3,5-P 267H were detected in the swamp-forest frog M. milotympanum in the present study, suggesting that ant alkaloids can (in some cases) contribute significantly to the alkaloid composition of certain mantellid populations. In general, however, putative ant-derived alkaloids are poorly represented in the skin alkaloids of swamp-forest frogs, and the majority of alkaloids are most likely derived from oribatid mites.
A significant difference in alkaloid composition was observed among the populations of M. aurantiaca, M. crocea, and M. milotympanum examined (Table 1; Online Resource 3, Tables S2–S6; Fig. 3). A study based on mitochondrial and nuclear DNA sequences revealed that M. crocea and M. milotympanum are closely related species, and possibly even conspecifics (Chiari et al. 2004). Although closely related, these two species have only four alkaloids in common [aPTX 323B, 5,8-I 239C (2 isomers), and unclassified 392; see Table 1]. The nMDS plot in Fig. 3 reflects the detection of up to twice the number of alkaloids (24) in M. milotympanum compared to that of M. crocea. A more recent study based on mitochondrial DNA sequences has confirmed that M. aurantiaca and M. madagascariensis also are closely related within the M. madagascariensis group (Vieites et al. 2009); however, these two species have no alkaloids in common (data from Table 1 and Daly et al. 2008). A comparison of alkaloids among all members of the M. madagascariensis group (with the exception of M. pulchra, for which no alkaloid data are available) illustrates that these species differ significantly from one another in alkaloid composition (Fig. 4a, b). These findings indicate that alkaloid composition varies greatly among mantellid species, including closely related species, further confirming that alkaloids are not reliable taxonomic markers for mantellids.
A significant difference in alkaloid composition was observed even among the populations of M. aurantiaca at Torotorofotsy-1 (“Tor-1”), Torotorofotsy-2 (“Tor-2”), and Andranomena (“S. Mora”, south of Moramanga; Online Resource 3, Tables S2–S4; Figs. 5 and 6). The nMDS plot in Fig. 5 graphically illustrates that individuals from Tor-1 and Tor-2 share more alkaloids with each other than they do with individuals from S. Mora. Alkaloid composition in M. aurantiaca skins, however, did differ significantly between Tor-1 and Tor-2, two populations that are virtually sympatric, being separated by only a small distance. These data suggest that the availability of alkaloid-containing arthropods appears to be important on both small and large spatial scales (also see Daly et al. 2008; Saporito et al. 2006, 2007a). The similarities and differences in alkaloids among the three populations of M. aurantiaca can be visualized in the Venn diagram in Fig. 6. Most of the alkaloids detected in these three populations have branched-carbon skeletons and likely are derived from oribatid mites (Saporito et al. 2007b, 2011).
In summary, in an attempt to further understand the importance of differences in habitat in explaining the variation in alkaloid composition common to all poison frogs (including mantellids), we compared alkaloid composition between the swamp-forest inhabiting species of the present study (M. aurantiaca, M. crocea, M. milotympanum) and the rainforest species examined previously (Daly et al. 2008; M. baroni, M. bernhardi, M. madagascariensis). The alkaloid composition of species found in swamp-forest habitat was significantly different than that of species found in non-flooded rainforest habitats (see nMDS plot in Fig. 7), and there were differences in alkaloid composition among species (and populations; see nMDS plots of Figs. 3, 4 and 5 and Daly et al. 2008). These results suggest that even subtle differences in habitat (and the associated changes in arthropods) may be an important factor in explaining the observed differences in alkaloid composition among species and populations of mantellid frogs, and likely other poison frogs as well (for further discussion, see Online Resource 6). The abundance of arthropods is known to vary with habitat due to a variety of factors (e.g., differences in forest and vegetation type, forest succession, frequency and type of disturbance, and season), and therefore the proportion of alkaloid-containing arthropods is expected to differ among habitats (see Daly et al. 2008 and Saporito et al. 2007a, 2009 for further discussion). These differences in arthropod availability presumably translate into the variation in alkaloid composition observed in mantellids and other poison frogs. Still, in the particular case of Mantella, we cannot fully exclude that a phylogenetic component (a sequestering mechanism or preference for certain prey) might contribute to the differences observed, given that the three species of swamp-forest frogs studied herein all are close relatives (Chiari et al. 2004); however, the differences between the three swamp-forest frog species in the present study and their equally close relatives, M. madagascariensis and M. pulchra from non-flooded rainforest supports habitat-related differences in arthropod communities as the primary driver of these differences. Future studies should be aimed at understanding the differences in the availability of alkaloid-containing arthropods among habitats, particularly the less commonly studied oribatid mites.
References
Andreone F, Cadle JE, Cox N, Glaw F, Nussbaum RA, Raxworthy CJ, Stuart SN, Vallan D, Vences M (2005) Species review of amphibian extinction risks in Madagascar: conclusions from the global amphibian assessment. Conserv Biol 19:1790–1802
Andriamaharavo NR, Garraffo HM, Saporito RA, Daly JW, Razafindrabe C, Andriantsiferana M, Spande TF (2010) Roughing it: a mantellid poison frog shows greater alkaloid diversity in some disturbed habitats. J Nat Prod 73:322–330
Bora P, Dolch R, Jenkins R, Jovanovic O, Rabemananjara CEF, Randrianirina J, Rafanomezantsoa J, Raharivololoniaina L, Ramilijaona O, Raminosoa N, Randrianavelona R, Raselimanana A, Razafimahatratra B, Razafindraibe T, Vences M (2008) Geographical distribution of three species of Malagasy poison frogs of high conservation priority: Mantella aurantiaca, M. crocea and M. milotympanum. Herp Notes 1:39–48
Chiari Y, Vences M, Vieites DR, Rabemananjara F, Bora P, Ramilijaona O, Meyer A (2004) New evidence for parallel evolution of colour patterns in Malagasy poison frogs (Mantella). Mol Ecol 13:3763–3774
Clark VC, Raxworthy CJ, Rakotomalala V, Sierwald P, Fisher BL (2005) Convergent evolution of chemical defense in poison frogs and arthropod prey between Madagascar and the Neotropics. Proc Natl Acad Sci USA 102:11617–11622
Clark VC, Rakotomalala V, Ramilijaona O, Abrell L, Fisher BL (2006) Individual variation in alkaloid content of poison frogs of Madagascar (Mantella; Mantellidae). J Chem Ecol 32:2219–2233
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Daly JW, Secunda S, Garraffo HM, Spande TF, Wisnieski A, Cover JF Jr (1994) An uptake system for dietary alkaloids in poison frogs (Dendrobatidae). Toxicon 32:657–663
Daly JW, Andriamaharavo NR, Andriantsiferana M, Myers CW (1996) Madagascan poison frogs (Mantella) and their skin alkaloids. Amer Mus Novitates 3177:1–34
Daly JW, Garraffo HM, Spande TF, Clark VC, Ma J, Ziffer H, Cover JF Jr (2003) Evidence for an enantioselective pumiliotoxin 7-hydroxylase in dendrobatid poison frogs of the genus Dendrobates. Proc Natl Acad Sci USA 100:11092–11097
Daly JW, Spande TF, Garraffo HM (2005) Alkaloids from amphibian skin: a tabulation of over eight-hundred alkaloids. J Nat Prod 68:1556–1575
Daly JW, Wilham JM, Spande TF, Garraffo HM, Gil RR, Silva GL, Vaira M (2007) Alkaloids in bufonid toads (Melanophryniscus): temporal and geographic determinants for two Argentinian species. J Chem Ecol 31:871–887
Daly JW, Garraffo HM, Spande TF, Giddings L-A, Saporito RA, Vieites DR, Vences M (2008) Individual and geographic variation of skin alkaloids in three species of Madagascan poison frogs (Mantella). J Chem Ecol 32:252-279 [This paper contains a significant error in Table 7 (pp 266–267) in that the data columns for M. madagascariensis from the Ranomafana and Besariaka sites were interchanged. Tables 3 and 4 where the original data are presented are correct. Other errors were detected in the transcription of data in that paper into the summaries of Tables 7 and 11 as listed in the Erratum for that paper.]
Garraffo HM, Caceres J, Daly JW, Spande TF (1993a) Alkaloids in Madagascan frogs (Mantella): pumiliotoxins, indolizidines, quinolizidines, and pyrrolizidines. J Nat Prod 56:1016–1038
Garraffo HM, Spande TF, Daly JW, Baldessari A, Gros EG (1993b) Alkaloids from bufonid toads (Melanophyrniscus): decahydroquinolines, pumiliotoxins, and homopumiliotoxins, indolizidines, pyrrolizidines and quinolizidines. J Nat Prod 56:357–373
Garraffo HM, Andriamaharavo NR, Vaira M, Quiroga MF, Heit C, Spande TF (2012) Alkaloids from single skins of the Argentinian toad Melanophryniscus rubriventris (ANURA, BUFONIDAE): an unexpected variability in alkaloid profiles and a profusion of new structures. Springer Plus 1:51
Glaw F, Vences M (2007) A field guide to the amphibians and reptiles of Madagascar, 3rd Edition. Cologne, Vences & Glaw Verlag, Germany
Hantak MM, Grant T, Reinsch S, McGinnity D, Loring M, Toyooka N, Saporito RA (2013) Dietary alkaloid sequestration in a poison frog: An experimental test of alkaloid uptake in Melanophryniscus stelzneri (Bufonidae). J Chem Ecol 39:1400–1406
Jones TH, Gorman JST, Snelling RR, Delabie JHQ, Blum MS, Garraffo HM, Jain P, Daly JW, Spande TF (1999) Further alkaloids common to ants and frogs: decahydroquinolines and a quinolizidine. J Chem Ecol 25:1179–1193
Jones TH, Zottig VH, Robertson HG, Snelling RR (2003) The venom alkaloids from some african Monomorium species. J Chem Ecol 29:2721–2727
Rabemananjara FCE, Rasoamampionona Raminosoa N, Ravoahangimalala Ramilijaona O, Rakotondravony D, Andreone F, Bora P, Carpenter AI, Glaw F, Razafindrabe T, Vallan D, Vieites DR, Vences M (2008a) Malagasy poison frogs in the pet trade: a survey of levels of exploitation of species in the genus Mantella. In: Andreone F (editor) A conservation strategy for the amphibians of Madagascar. Monografie del Museo Regionale di Scienze Naturali di Torino 45:277–300
Rabemananjara FCE, Bora P, Razafindrabe TJ, Randiamitso E, Ravoahangimalala Ramilijaona O, RaSamampionona Raminosoa N, Rakotondravony D, Vieites DR, Vences M (2008b) Rapid Assessment of population sizes in ten species of Malagasy poison frogs, genus Mantella. In: Andreone, F. (ed) A conservation strategy for the amphibians of Madagascar. Monografie del Museo Regionale di Scienze Naturali di Torino 45:253–264
Rodríguez A, Poth D, Schulz S, Vences M (2010) Discovery of skin alkaloids in a miniaturized eleutherodactylid frog from Cuba. Biology Lett B 414–418
Saporito RA, Donnelly MA, Garraffo HM, Spande TF, Daly JW (2006) Geographic and seasonal variation in alkaloid-based chemical defenses of Dendrobates pumilio from Bocas del Toro, Panama. J Chem Ecol 32:795–814
Saporito RA, Donnelly MA, Jain P, Garraffo HM, Spande TF, Daly JW (2007a) Spatial and temporal patterns of alkaloid variation in the poison frog Oophaga pumilio in Costa Rica and Panama over 30 years. Toxicon 50:757–778
Saporito RA, Donnelly MA, Norton RA, Garraffo HM, Spande TF, Daly JW (2007b) Oribatid mites as a major dietary source for alkaloids in poison frogs. Proc Natl Acad Sci USA 104:8885–8890
Saporito RA, Spande TF, Garraffo HM, Donnelly MA (2009) Arthropod alkaloids in poison frogs: a review of the ‘dietary hypothesis’. Heterocycles 79:277–297
Saporito RA, Donnelly MA, Madden AA, Garraffo HM, Spande TF (2010) Sex-related differences in alkaloid chemical defenses of the dendrobatid frog Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama. J Nat Prod 73:317–321
Saporito RA, Norton RA, Andriamaharavo NR, Garraffo HM, Spande TF (2011) Alkaloids in the mite Scheloribates laevigatus: Further alkaloids common to oribatid mites and poison frogs. J Chem Ecol 37:213–218
Saporito RA, Donnelly MA, Spande TF, Garraffo HM (2012) A review of chemical ecology in poison frogs. Chemoecology 22:159–168
Smith BP, Tyler MJ, Kaneko T, Garraffo HM, Spande TF, Daly JW (2002) Evidence of biosynthesis of pseudophrynamine alkaloids by an Australian myobatrachid frog (Pseudophryne) and for sequestration of dietary pumiliotoxins. J Nat Prod 65:439–447
Takada W, Sakata T, Shimano S, Enami Y, Mori N, Nishida R, Kuwahara Y (2005) Scheloribatid mites as the source of pumiliotoxins in dendrobatid frogs. J Chem Ecol 31:2403–2415
Vences M, Kniel C (1998) Mikrophage und myrmecophage Ernährungsspezialisierung bei madagassischen Giftfröschen der Gattung Mantella. Salamandra 34:245–254
Vences M, Glaw F, Böhme W (1998) Evolutionary correlates of microphagy in alkaloid-containing frogs (Amphibia: Anura). Zool Anz 236:217–230
Vences M, Glaw F, Böhme W (1999) A review of the genus Mantella (Anura, Ranidae, Mantellinae): taxonomy, distribution and conservation of Malagasy poison frogs. Alytes 17:3–72
Vences M, Chiari Y, Raharivololoniaina L, Meyer A (2004) High mitochondrial diversity within and among populations of Malagasy poison frogs. Mol Phyl Evol 30:295–307
Vieites DR, Chiari Y, Vences M, Andreone F, Rabemananjara F, Bora P, Nieto-Román S, Meyer A (2006) Mitochondrial evidence for distinct phylogeographic units in the endangered Malagasy poison frog Mantella bernhardi. Mol Ecol 15:1617–1625
Vieites DR, Wollenberg KC, Andreone F, Kohler J, Glaw F, Vences M (2009) Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proc Natl Acad Sci USA 106:8267–8272
Woodhead C, Vences M, VIEites DR, Gamboni I, Fisher B, Griffiths RA (2007) Specialist or generalist? Feeding ecology of the Malagasy poison frog Mantella aurantiaca. Herpetological J 17:225–236
Zimmerman H, Andrianarivo C (2000) La protection de la biodiversité des forêts et des marais de Torotorofotsky à Andasibe, Madagascar-Est. In: Lourenco WR, Goodman SM (eds) Diversity and endemism in Madagascar. Mémoires de la Société de Biogeographie, Paris, pp 261–272
Acknowledgments
Research in Madagascar was made possible by collaboration with the Département de Biologie Animale, Université d’Antananarivo, and research and export permits (CITES export permits # 1100-EAL/MG01/CWO from 19 December 2001 and 070C to 074C-EA02/MG05 from 18 Feb 2005) kindly issued by the Malagasy authorities. The Volkswagen Foundation, Biopat, and the Deutsche Forschungsgemeinschaft supported fieldwork of M.V. and D.R.V. The work at NIH was supported by intramural funds of NIDDK.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 105 kb)
Online Resource 2
(DOC 106 kb)
Online Resource 3
(PDF 273 kb)
Online Resource 4
(PDF 504 kb)
Online Resource 5
(PDF 78.4 kb)
Online Resource 6
(PDF 448 kb)
ESM 1
(PDF 83.7 kb)
Rights and permissions
About this article
Cite this article
Andriamaharavo, N.R., Garraffo, H.M., Spande, T.F. et al. Individual and Geographic Variation of Skin Alkaloids in Three Swamp-Forest Species of Madagascan Poison Frogs (Mantella). J Chem Ecol 41, 837–847 (2015). https://doi.org/10.1007/s10886-015-0616-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0616-4