Abstract
Lung recruitment manoeuvres (RMs) during mechanical ventilation may reduce atelectasis, however, the optimal recruitment strategy for patients undergoing thoracic surgery remains unknown. Our study was designed to investigate whether ultrasound-guided lung RMs is superior to conventional RMs in reducing perioperative atelectasis during thoracic surgery with one-lung ventilation. We conducted a randomised controlled clinical trial from August 2022 to September 2022. Sixty patients scheduled for video-assisted thoracoscopic surgery (VATS) under general anaesthesia were enrolled. Subjects were randomly divided into the ultrasound-guided RMs group (manual inflation guided by lung ultrasound) or conventional RMs group (manual inflation with 30 cmH2O pressure). Lung ultrasound were performed at three predefined time points (1 min after anaesthetic induction; after RMs at the end of surgery; before discharge from postanesthesia care unit [PACU]). The primary outcome was lung ultrasound score before discharge from the PACU after extubation. In the early postoperative period, lung aeration deteriorated in both groups even after lung RMs. However, ultrasound-guided lung RMs had significantly lower lung ultrasound scores when compared with conventional RMs in bilateral lungs (2.0 [0.8–4.0] vs. 8.0 [3.8–10.3], P < 0.01) at the end of surgery, which remained before patients discharged from the PACU. Accordingly, the lower incidence of atelectasis was found in ultrasound-guided RMs group than in conventional RMs group (7% vs. 53%; P < 0.01) at the end of surgery. Ultrasound-guided RMs is superior to conventional RMs in improving lung aeration and reducing the incidence of lung atelectasis at early postoperative period in patients undergoing VATS. The study protocol was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center (No. 220,825,810; date of approval: August 5, 2022) and registered on Chinese Clinical Trial Registry (registration number: ChiCTR2200062761).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atelectasis commonly occurs in patients undergoing general anaesthesia (GA), with an incidence as high as 90% [1]. The development of atelectasis induces impairment of gas exchange, leading to refractory hypoxemia and other perioperative lung complications, especially in patients undergoing video-assisted thoracoscopic surgery (VATS) [2]. Lung resection can significantly affect patients’ oxygenation and forced vital capacity [3], and one-lung ventilation (OLV) could lead to ischemic–reperfusion lung injury, barotrauma, and atelectrauma, which may further impair postoperative lung function [4].
The protective lung ventilation strategy in OLV includes the use of low tidal volume, positive end-expiratory pressure (PEEP), as well as recruitment manoeuvres (RMs) in thoracic anaesthesia [5]. RMs have been reported to reduce or even reverse lung collapse during GA by increasing the transpulmonary pressure [6]. In previous studies, the alveolar RMs is usually performed to reduce atelectasis, and thus improve oxygenation, lung mechanics, and ventilation efficiency through the re-opening of atelectatic lung areas [7, 8]. However, due to the special physiological and clinical circumstances of OLV that complicate lung-protective ventilation techniques [9], data on the optimal recruitment strategy regarding timing, frequency, and inflation pressures for lung resection surgery is still limited.
Lung ultrasound (LUS) is a fast, radiation-free and portable tool for accurately assessing perioperative lung aeration and pulmonary complications, including pulmonary oedema and atelectasis [10, 11]. The dynamic alveolar response to lung RMs can be easily evaluated using LUS [12]. Thus, as a bedside tool, it can guide RMs in real-time manner to reduce atelectasis formation during GA [13, 14]. However, whether LUS-guided lung RMs can reduce perioperative atelectasis in patients during VATS has not yet been reported.
Therefore, we conducted a randomised controlled clinical trial to examine whether LUS-guided RMs could improve LUS scores after surgery, and thus reduce the postoperative incidence of atelectasis compared with conventional RMs in patients undergoing VATS under GA.
2 Materials and methods
2.1 Patients enrollment and grouping
The ethical approval for this study was provided by the Institutional Review Board of Fudan University Shanghai Cancer Center (No. 220,825,810; Chairperson Prof Zhen Chen) on August 5, 2022 and registered in the Chinese Clinical Trial Registry (number: ChiCTR2200062761, principal investigator: Jun Zhang, date of registration: August 18, 2022).
All adult patients with American Society of Anesthesiologists (ASA) physical status I–II scheduled for elective lung cancer resection under GA were screened for eligibility between August 2022 and September 2022. The written informed consent was obtained from all eligible patients before enrollment. The exclusion criteria were as follows: emergency surgery, history of thoracic and heart surgery, body mass index (BMI) > 40 kg·m–2, recent respiratory tract infection, anatomical abnormality of the airway and thoracic cage, genetic disease, and abnormal radiographic findings, including pneumothorax, pleural effusion, or pneumonia.
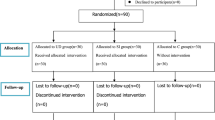
The patients were randomly divided into either ultrasound-guided RM group (Group UR) or conventional RM group (Group CR) using a computer-generated random number table. Allocation concealment was achieved by coded, sealed envelopes, which were opened by a nurse not involved in the study before anaesthetic induction. The outcome evaluator was blinded to group allocation.
2.2 Anesthetic managements and monitoring
Patients were pre-oxygenated with 100% oxygen prior to anaesthetics administration. Intravenous midazolam (1 mg), propofol targeted controlled infusion (TCI: effect site concentration 3–4 µg·ml–1, Marsh model), sufentanil (0.3 µg·kg–1), and rocuronium (0.6 mg·kg–1) were used for anaesthetic induction. Intraoperative anaesthesia was maintained with propofol TCI (effect site concentration 3–4 µg·ml–1), remifentanil TCI (effect site concentration 1–2 ng·ml–1, Minto model), and intermittent sufentanil or rocuronium was used if necessary. A standard monitoring protocol including electrocardiography (EEG), pulse oxygen saturation (SpO2), invasive arterial blood pressure, and end-tidal carbon dioxide partial pressure (PetCO2), was used during perioperative period.
All patients underwent VATS in the lateral position. OLV was achieved through the insertion of an appropriately-sized double-lumen tube, and correct placement was validated using a fibrobronchoscope. Patients were mechanically ventilated in a volume-controlled mode. The OLV parameters were set as following: tidal volume = 5–6 ml·kg–1 and ventilatory rate = 12–14 beats·min–1 to maintain intraoperative PetCO2 at 35–45 mmHg, fraction of oxygen (FiO2) = 0.8 and PEEP = 5 cmH2O. Before closing the chest, the collapsed lung was completely expanded using an alveolar RM under direct visualization by the surgeon. The peak airway pressure for recruitment was limited to 40 cmH2O. The OLV was then changed to two-lung ventilation (TLV) until extubation with a FiO2 = 0.5. A chest tube attached to a water-sealed bottle was used to drain subsequent air and fluid leakage.
The continuous patient-controlled intravenous analgesia was provided to control postoperative pain. In the postanesthesia care unit (PACU), mechanical ventilation was maintained for all patients, with settings same to those for pre-recruitment. Sugammadex was used to reverse the neuromuscular blockade before extubation. After extubation, oxygen therapy was administered through a facemask at 3 L·min–1 for at least 15 min.
2.3 Interventions
The RMs were performed twice with 50/50 oxygen/air, one after anesthetic induction and another at the end of surgery in both groups. In Group UR, real-time ultrasound-guided lung RMs were conducted when atelectasis was found by ultrasound examination. Based on a previous study [7], the peak airway pressure was limited to 30 cmH2O, and maintained for 10–15 s. Manual inflation was applied until no obvious areas of atelectasis could be detected on ultrasound image in bilateral lungs, Otherwise, the manual lung RMs are performed again. In the Group CR, the RMs were conducted manually at a pressure of 30 cmH2O and maintained for 10–15 s, after which the previous ventilator settings were reinstated.
When the SpO2 fell below 90% during OLV, rescue strategies were conducted in a stepwise manner in both groups [7]. The following sequence was used: (1) three rounds of RMs were performed in the dependent lung at a pressure of 30 cmH2O for 10 s, (2) three rounds of RMs were conducted in the dependent lung at 35 cmH2O pressure for 10 s, and (3) FiO2 was increased to 100%. If profound hypoxemia developed after these three strategies, other rescue strategies, such as continuous positive airway pressure to the surgical lung or switching to TLV, were performed based on the attending anaesthesiologist’s clinical judgement.
2.4 LUS examination
The patients were examined with LUS at three predefined time points (Fig. 1). The first examination was conducted 1 min after anaesthetic induction (T1), the second one was performed after RMs at the end of surgery (T2), and the third one was conducted before discharge from the PACU (T3). All LUS scans were conducted by one anaesthesiologist, who has 5 years LUS experience, using a Vivid-iq Ultrasound System (GE Healthcare, Wauwatosa, WI, USA) with a 2–5 MHz C5-2 convex probe.
The patients were examined in the supine position with both arms above the head. Six intercostal areas were imaged in each hemithorax for a total of 12 scans per subject (Fig. 2a), as described previously [1, 7]. Taking the nipple line as the dividing line, scans 1 and 2 were conducted in the clavicular midline area, scans 3 and 4 were performed in the midaxillary line region, and scans 5 and 6 were conducted at the intercostal lung areas of the posterior axillary line. Ultrasonography images were evaluated and recorded for signs including A lines, B lines, the lung ‘sliding’ sign, atelectasis, and air bronchograms.
Division of the chest into segments for lung ultrasound examination in patients and typical lung ultrasound examples with different scores of severity. The asterisks denote the six regions subjected to ultrasound examination. A total of 12 regions were examined in one patient a; lung ultrasound score 0, 0 to 2 B lines b; 1, at least three B lines, one or more small subpleural consolidations separated by a normal pleural line (c, d); 2, multiple coalescent B lines, or multiple small subpleural consolidations separated by a thickened or irregular pleural line (e, f); 3, consolidation or subpleural consolidation of more than 1 cm×2 cm g
Based on a previous study (1), four grades were defined to assess lung aeration (Fig. 2), with scores ranging from 0 to 3 as follows: (0) A lines parallel to the sliding pleura or 0 to 2 B lines; (1) no fewer than 3 dispersive B lines; one or more small subpleural consolidations separated by a normal pleural line; (2) multiple coalescent B lines, multiple small subpleural consolidations separated by a thickened or irregular pleural line; (3) consolidation or subpleural consolidation of more than 1 cm × 2 cm. Lung aeration was assessed and scored separately for each scanned area.
The primary outcome was the LUS score before discharge from the PACU (T3). The secondary outcomes included the LUS score at the end of surgery (T2) and the incidence of atelectasis at T2 and T3. The SpO2 values at different time points, lengths of PACU and hospital stay and oxygenation parameters were also collected in this study.
2.5 Statistical analysis
The primary hypothesis of the study was that ultrasound-guided RMs could decrease the LUS scores before discharge from the PACU compared with conventional RMs. Based on our pilot study result, the mean LUS score at T3 in the control group would be 2.2 ± 1.1, and hypothesized that LUS-guided RMs would decrease the LUS score to 1.5 ± 0.8. To achieve a power of 90% and α error of 5%, at least 27 subjects were needed in each group. Given a dropout rate of 10%, a total of 60 patients (n = 30/each group) were included in our study.
The data were analysed using SPSS Statistics (version 23.0, IBM Corp., Armonk, New York, USA). After analysing the normality distribution using the Shapiro‒Wilk test, continuous variables were presented as the mean (standard deviation [SD]) or median [IQR]. The Student’s t test was performed to compare intergroup differences for normally distributed variables including height, weight, and BMI. The Mann–Whitney U test was used to assess non-normally distributed data such as the LUS scores, PaO2/FiO2, and PaCO2. The Wilcoxon signed-rank test was performed to compare within-group LUS scores. Categorical data were presented as numbers (%) and assessed using the χ2 test or Fisher’s exact test. P < 0.05 was considered statistically significant.
3 Results
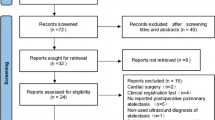
A total of 76 patients were evaluated for eligibility in our study. Seventy patients were enrolled and randomly assigned to Group UR or Group CR. Ten patients were excluded from analysis due to failure to complete at least one of three LUS examinations since ultrasound machine unavailable. Sixty patients were finally included in our data analysis, as shown in the flow diagram of patient recruitment (Fig. 3). No significant between-group differences were found with respect to demographics and clinical characteristics (Table 1). Thirty-eight patients underwent VATS on the right side (63%), while 22 (37%) on the left side. Wedge resection, segmentectomy, or lobectomy was performed in 31 (52%), 6 (10%), and 23 (38%) patients, respectively.
Before surgery, the LUS scores were comparable between Group UR and Group CR 1 min after anaesthetic induction (T1; Table 2). After surgery, lung aeration worsened even after lung RMs in both groups, especially in the dependent lungs (T2). Ultrasound-guided lung recruitment significantly reduced LUS scores compared with Group CR in the bilateral lungs (2.0 [0.8 to 4.0] vs. 8.0 [3.8 to 10.3], P < 0.01) and dependent lungs (2.0 [0.8 to 4.0] vs. 6.0 [2.8 to 10.0], P < 0.01) at the end of surgery, respectively. Before patients discharged from the PACU, lung aeration improved in both Group UR (2.0 [0.8 to 4.0] vs. 0 [0.0 to 0.3], P < 0.01) and Group CR (8.0 [3.8 to 10.3] vs. 2.0 [0 to 4.3], P < 0.01). The inter-group differences remained in the bilateral lungs (0.0 [0.0 to 0.3] vs. 2.0 [0.0 to 4.3], P < 0.01) and dependent lungs (0.0 [0.0 to 0.0] vs. 1.5 [0 to 3.0], P < 0.01) before patients were discharged from the PACU. The LUS scores of the dependent lungs were statistically higher than those of the independent lungs at T2 and T3 in Group CR.
Next, we analysed the effect of ultrasound-guided lung recruitment on atelectasis at different timepoints. Compared with that after anesthetic induction (T1), the incidence of atelectasis at the end of surgery (T2) assessed by LUS significantly increased in Group CR (53% vs. 3%; P < 0.01; Table 3) especially in the dependent lungs, whereas the incidence of atelectasis showed no differences in Group UR (7% vs. 3%; P = 1.00). Furthermore, the incidence of atelectasis was statistically lower in Group UR than in Group CR (7% vs. 53%; P < 0.01) at the end of surgery (T2). However, the incidence of atelectasis decreased after extubation in both groups, and the difference between the two groups decreased (3% vs. 23%; P = 0.05; Table 3) in the PACU (T3). The incidence of atelectasis in the dependent lungs was significantly higher than that in the independent lungs at both T2 and T3 in Group CR. Additionally, the proportion of multiple B lines (≥ 3) and atelectasis detected in the posterior lung areas was higher than that in other regions (Table 4). None of the patients underwent unstable haemodynamics during lung recruitment and severe hypoxemia (SpO2 < 90%) during OLV regardless of the surgical type.
Thirty minutes after the PACU admission, SpO2 value was significantly higher in Group UR group than that in Group CR [95.0% (92.0 to 97.0%) vs. 93.0% (92.0 to 95.0%), P = 0.04]. However, before patients were discharged from the PACU, no intergroup differences were observed in terms of PaO2 /FiO2 (P = 0.87), PaCO2 (P = 0.91), SpO2 (P = 0.19), length of PACU stay (P = 0.86), lactate level (P = 0.14), or haemoglobin level (P = 0.92) (Table 5). The length of postoperative hospital stay was similar for both groups (P = 0.34).
4 Discussion
Our study showed that among patients undergoing VATS for lung cancer resection, ultrasound-guided RMs significantly reduced the incidence of atelectasis and improved lung aeration at the end of surgery compared with conventional RMs. The difference in lung aeration between the groups remained after spontaneous breath before discharged from the PACU, as evaluated by the LUS scores. However, the ultrasound-guided RMs failed to further improve arterial oxygenation at that time. In addition, we noticed a higher incidence of atelectasis in the dependent lungs than in the independent lungs immediately after surgery and in the PACU.
With continuous advancements in surgical techniques and perioperative anaesthetic management, lung resection with VATS has been widely accepted for its less invasive and faster recovery compared with the traditional thoracotomy [15]. However, perioperative lung complications, including atelectasis, remain a major concern [16]. The RMs have been demonstrated to be effective at reducing perioperative atelectasis and lung infections, improving oxygenation and lung compliance, and reducing the need for high FiO2 in patients undergoing thoracic surgery [17, 18]. However, there is no clinical consensus on the optimal recruitment strategy for individual patient [16]. Therefore, individualized and tailored RM strategies are needed for surgical patients that take into account their physiologic differences.
Recent studies have suggested that ultrasound-guided RMs reduce atelectasis in children or adult laparoscopic surgical patients [7, 8, 12]. In our study, ultrasound-guided RMs was found to be more effective at improving lung aeration as well as reducing perioperative atelectasis in patients undergoing thoracic surgery compared with Group CR. The LUS can be applied to evaluate the extent of atelectasis and the patients’ response to lung recruitment individually and dynamically; thus, it may be helpful to perform optimal lung recruitment in surgical patients [12].
During VATS procedures, the lungs undergo a variety of stress factors, including OLV, compression and injury of the lung, positive pressure ventilation, and surgical trauma-induced inflammatory response [19]. These factors may contribute to increase the development of atelectasis, hypoxemia, and lung dysfunction during the perioperative period [20]. In the present study, we found that ultrasound-guided RMs improved SpO2 30 min after patients entering the PACU, which may be due to reduced atelectasis and improved lung aeration. However, ultrasound-guided RMs did not significantly improve the PaO2/FiO2 ratio in the early postoperative period, suggesting that some other mechanisms, such as postoperative pain, sputum excretion, and a reduction in lung volume might also influence oxygenation.
Insufficient RMs produce suboptimal lung aeration, while excessive high-pressure RMs may contribute to alveolar overdistention and barotrauma, leading to decrease in cardiac output, hypotension, and ventilator-induced lung injury [21]. Although no obvious hypotension and bradycardia were found during lung recruitment in the present study, we should pay attention to their hemodynamics when applying RMs. From the view of risk-benefit, RMs guided with real-time LUS at the bedside may be a good strategy.
A previous study demonstrated that atelectasis caused by surgery and anaesthesia rapidly resolved after extubation in the PACU [11]. Similarly, our study showed that the incidence of atelectasis decreased significantly after extubation in both groups, which may be due to lung recruitment by the cough reflex. However, our results showed that a certain degree of lung aeration deterioration still existed after extubation in Group CR, as evidenced by high LUS scores at T3, indicating that aeration loss in several lung areas remained even after the neuromuscular blockade had been completely reversed. These findings further demonstrate the advantages of LUS-guided RMs in terms of lung aeration improvement.
We also noticed a higher incidence of atelectasis in the dependent lungs than in the independent lungs in both groups after surgery and in the PACU, which may be related to the lateral decubitus position and the fact that RMs of the surgical lung were performed directly under surgeon supervision [22]. During OLV in the lateral decubitus position, the expansion of the dependent lung is usually hindered by the overlying compression of the mediastinum and abdominal organs, the raised paralysed diaphragm, and the pressure and noncompliance of the chest wall [20]. Therefore, atelectasis may readily appear in the dependent lung, resulting in a lung surface with lower ventilation and oxygenation [23]. A previous study demonstrated that the incidence of atelectasis was higher in the dependent anterior chest in the prone position after surgery. In another study, worst LUS scores were detected in the inferoposterior quadrant in the supine position during gynaecological surgery [7, 24]. In our study, atelectasis occurred mainly at the posterior lung regions of the dependent lungs, which is consistent with the idea that atelectasis primarily exists in gravity-dependent lung regions [25].
A high oxygen concentration is often used to maintain adequate oxygenation during OLV [26]. In our study, an 80% FiO2 was used during OLV, and no patient developed dangerous hypoxemia during surgery. Exposure to 100% O2 can precipitate absorption atelectasis and an overproduction of radical oxygen species and proinflammatory cytokines, ultimately aggravating lung injury during OLV [26]. A previous study suggested that if a low FiO2 can be tolerated during OLV, lung injury such as pulmonary oedema and alveolar thickening can be minimized compared with a higher FiO2 [27].
Our study had several limitations. First, while reduced atelectasis was observed with the ultrasound-guided recruitment strategy, other clinically relevant data, such as incidences of postoperative 30-day pulmonary complications and lung mechanics, were not evaluated or followed up. Therefore, the clinical implications of ultrasound-guided RMs may be limited, and these endpoints should be investigated in further studies. Second, we excluded patients with morbid obesity or potential lung diseases to minimize confounding factors. Further research is needed to assess the effects of ultrasound-guided RMs in those specific patients. Third, hyperinflation that may have occurred during our RMs were not detected on LUS. However, the peak inspiratory pressure in our study was similar to or lower than that reported previously to avoid lung hyperinflation [7].
5 Conclusions
Ultrasound-guided RMs significantly reduced atelectasis and improved lung aeration in patients undergoing thoracic surgery perioperatively, and these effects remained significant in the early postoperative period. Further studies should investigate the long-term clinical effects of ultrasound-guided RMs.
References
Monastesse A, Girard F, Massicotte N, Chartrand-Lefebvre C, Girard M. Lung Ultrasonography for the Assessment of Perioperative Atelectasis: a pilot feasibility study. Anesth Analg. 2017;124:494–504.
Bosch L, et al. Assessment of lung ultrasound for early detection of respiratory complications in thoracic surgery. Brazilian J Anesthesiology. 2022;72:128–34.
Naik BI, et al. Value of the oxygenation index during 1-lung ventilation for predicting respiratory complications after thoracic surgery. J Crit Care. 2017;37:80–4.
i PNi, Belda J, Ferrando C, Garutti I. The effects of an Open-Lung Approach during one-lung ventilation on postoperative pulmonary complications and driving pressure: a descriptive, Multicenter National Study. J Cardiothorac Vasc Anesth. 2018;32:2665–72.
O’Gara B, Talmor D. Perioperative lung protective ventilation. BMJ. 2018;362:k3030.
Acosta CM, et al. Lung recruitment prevents collapse during laparoscopy in children: a randomised controlled trial. Eur J Anaesthesiol. 2018;35:573–80.
Park SK, et al. Ultrasound-guided versus conventional lung recruitment manoeuvres in laparoscopic gynaecological surgery: a randomised controlled trial. Eur J Anaesthesiol. 2021;38:275–84.
Lee JH, et al. Effect of an ultrasound-guided lung recruitment manoeuvre on postoperative atelectasis in children: a randomised controlled trial. Eur J Anaesthesiol. 2020;37:719–27.
Grichnik KP, Clark JA. Pathophysiology and management of one-lung ventilation. Torac Surg Clin. 2005;15:85–103.
Zhu C, Zhang S, Dong J, Wei R. Effects of positive end-expiratory pressure/recruitment manoeuvres compared with zero end-expiratory pressure on atelectasis in children: a randomised clinical trial. Eur J Anaesthesiol. 2021;38:1026–33.
Genereux V, et al. Effects of positive end-expiratory pressure/recruitment manoeuvres compared with zero end-expiratory pressure on atelectasis during open gynaecological surgery as assessed by ultrasonography: a randomised controlled trial. Br J Anaesth. 2020;124:101–9.
Song IK, et al. Effects of an alveolar recruitment manoeuvre guided by lung ultrasound on anaesthesia-induced atelectasis in infants: a randomised, controlled trial. Anaesthesia. 2017;72:214–22.
L S, et al (2020) Lung ultrasound evaluation of incremental PEEP recruitment maneuver in children undergoing cardiac surgery. Pediatr Pulmonol 55:1273–81.
Xie C, et al. Lung ultrasound and diaphragmatic excursion assessment for evaluating perioperative atelectasis and aeration loss during video-assisted thoracic surgery: a feasibility study. Annals Palliat Med. 2020;9:1506–17.
Sihoe ADL. Video-assisted thoracoscopic surgery as the gold standard for lung cancer surgery. Respirol (Carlton Vic). 2020;25(Suppl 2):49–60.
Unzueta C, Tusman G, Suarez-Sipmann F, Böhm S, Moral V. Alveolar recruitment improves ventilation during thoracic surgery: a randomized controlled trial. Br J Anaesth. 2012;108:517–24.
Hu MC, et al. Recruitment maneuvers in patients undergoing thoracic surgery: a meta-analysis. Gen Thorac Cardiovasc Surg. 2021;69:1553–9.
Tusman G, Bohm SH, Suarez-Sipmann F. Alveolar recruitment maneuvers for one-lung ventilation during thoracic anesthesia. Curr Anesthesiology Rep. 2014;4:160–9.
Lohser J. Managing hypoxemia during minimally invasive thoracic surgery. Anesthesiol Clin. 2012;30:683–97.
Campos JH, Feider A. Hypoxia during one-lung Ventilation-A review and update. J Cardiothorac Vasc Anesth. 2018;32:2330–8.
Hartland BL, Newell TJ, Damico N. Alveolar recruitment maneuvers under general anesthesia: a systematic review of the literature. Respir Care. 2015;60:609–20.
Tusman G, et al. Alveolar recruitment strategy increases arterial oxygenation during one-lung ventilation. Ann Thorac Surg. 2002;73:1204–9.
Peel JK, Funk DJ, Slinger P, Srinathan S, Kidane B. Positive end-expiratory pressure and recruitment maneuvers during one-lung ventilation: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2020;160(4):1112–1122e1113.
Jang YE, et al. Effect of regular alveolar recruitment on intraoperative atelectasis in paediatric patients ventilated in the prone position: a randomised controlled trial. Br J Anaesth. 2020;124:648–55.
Wu L, et al. Modified lung Ultrasound examinations in Assessment and Monitoring of positive end-expiratory pressure-Induced Lung Reaeration in Young Children with congenital Heart Disease under General Anesthesia. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and. Crit Care Soc. 2019;20:442–9.
Choi YS, et al. Effects of Alveolar Recruitment and positive end-expiratory pressure on oxygenation during one-lung ventilation in the Supine position. Yonsei Med J. 2015;56:1421–7.
Xu Z, et al. Oxygenation, inflammatory response and lung injury during one lung ventilation in rabbits using inspired oxygen fraction of 0.6 vs. 1.0. J Biomedical Res. 2016;31:56–64.
Acknowledgements
This work was supported by Shanghai Natural Science Foundation from Municipal Science and Technology Committee (To Jun Zhang, No. 22Y11904200).
Funding
This work was supported by Shanghai Natural Science Foundation from Municipal Science and Technology Committee (To Jun Zhang, No. 22Y11904200). The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. J.Z. and L.W. contributed to all aspects of this manuscript, including study design and methodology, and manuscript preparation. L.Y. and L.W. were involved in conduct of this study and manuscript revision. X.W. was involved in data validity extracting and assessing and manuscript revision. Y.Y. contributed to statistical analysis acquisition and analysis of the data. All authors provided contributions to this work and approved the final manuscript for submission.
Corresponding authors
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of the Fudan University Shanghai Cancer Center (No.220825810; date of approval: August 5, 2022).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publication
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 2a.
Competing interests
The authors declare no competing interests.
Suggesting reviews
Mazhong Zhang, MD, PhD, Department of Anesthesiology and Pediatric Clinical Pharmacology Laboratory, Shanghai Children’s Medical Center Affiliated to School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 1678 Dongfang Road, Pudong, 200127 Shanghai, China. Email: zmzscmc@shsmu.edu.cn.
Yan Wang, MD, Department of Ultrasound in Medicine, Affiliated Sixth People’s Hospital of Shanghai Jiao Tong University, Shanghai Institute of Ultrasound in Medicine, 600 Yishan Road, Shanghai 200233, China. Email: yannanfly@126.com. A link to the publication record: https://pubmed.ncbi.nlm.nih.gov/34804877/.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, L., Yang, L., Yang, Y. et al. Ultrasound-guided versus conventional lung recruitment manoeuvres in thoracic surgery: a randomised controlled study. J Clin Monit Comput 38, 731–739 (2024). https://doi.org/10.1007/s10877-024-01134-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-024-01134-5