Abstract
Purpose
Different organs have different autoregulatory capacities for blood pressure changes and/or circulatory volume changes. This study assessed the autoregulation of the stomach, liver, kidney and skeletal muscle, under baseline, hypovolemic, and post-fluid-resuscitation conditions using near-infrared spectroscopy (NIRS).
Methods
Ten pigs (bodyweight 24.5 ± 0.5 kg) were anesthetized with 2.5% isoflurane and administered 0.5, 1, 2 and 5 µg kg− 1 min− 1 of phenylephrine at 10-min intervals, followed by similar stepwise infusion of sodium nitroprusside (SNP) to induce a wide range of mean arterial pressures (MAPs). A 600-ml bleed was induced to create the hypovolemic condition, and only phenylephrine was re-administered. Hydroxyethyl starch (600 ml) was infused to create the post-fluid-resuscitation condition, and phenylephrine and SNP were re-administered. Average relationships between mean arterial pressure (MAP) and each tissue oxygenation index (TOI) were assessed, and the individual relationships were evaluated based on the correlation coefficients between MAP and TOI during each vasoactive drug infusion.
Results
Based on the evaluation using each TOI as a substitute of blood flow, the kidney autoregulation was robust, similar to muscle, but had a prominent lower limit. The stomach had weaker autoregulation than the kidney and muscle. The liver had no autoregulation. The kidney TOI showed 2-fold greater changes in response to volume condition changes than the stomach and liver TOIs.
Conclusion
In our NIRS-based assessment of autoregulatory capacity, the liver oxygenation is highly blood pressure dependent, and the kidney is highly susceptible and the skeletal muscle is highly tolerable to low blood pressure and volume loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Autoregulation plays an important role in organ blood flow homeostasis [1]. The classic concept is that blood flow is maintained at a constant level within a wide range of mean arterial pressures (MAPs), while pressure-dependent changes occur when the upper and lower limits are exceeded. However, different organs have different autoregulatory capacities [1], and the impact of changes in blood pressure and/or intravascular volume, and the impact of the applied interventions differs between organs.
Recently, organ autoregulation was assessed by near-infrared spectroscopy (NIRS) in several studies [2,3,4,5,6,7,8,9]. NIRS measures changes in oxyhemoglobin and deoxyhemoglobin in arterial, venous, and capillary blood within the field of view based on the modified Beer-Lambert law [10, 11], and determines the mixed tissue hemoglobin oxygen saturation, which is expressed as the oxygenation index. Therefore, although NIRS does not estimate blood flow, the oxygenation index could be used as a substitute noninvasive indicator of blood flow under conditions in which the oxygen demand, supply, and utilization are stable. Specifically, organ autoregulation is quantified using the correlation coefficient between the MAP and the oxygenation index, with low and high correlation coefficients indicating the presence and absence of autoregulation, respectively [2,3,4,5,6,7,8,9]. Our previous studies, which investigated cerebral [8, 9], renal [8] and spinal cord [9] autoregulation under 3 volume conditions (baseline, hemorrhage-induced hypovolemia and subsequent fluid resuscitation), elucidated that kidney and spinal cord have measurable autoregulation, but not as robust as the brain [8, 9]. Furthermore, the kidneys were susceptible to hypovolemia, and fluid resuscitation was effective for improving renal oxygenation [8], whereas the impact of circulatory volume alteration on cerebral and spinal cord (central nervous system) oxygenation was minimal [9]. These previous studies also proved that blood pressure is unreliable as an indicator to maintain microcirculation because the tissue oxygenations were quite different depending on the conditions, even at similar blood pressure [8, 9]. Although we have examined these organs with relatively robust autoregulation [1], few studies have used NIRS to investigate the autoregulation of splanchnic organs and skeletal muscle.
As a continuation of our previous studies [8, 9], the present porcine study assessed stomach, liver and skeletal muscle autoregulation simultaneously, and compared the impact of blood pressure changes, hypovolemia and fluid resuscitation on oxygenation of each tissue. We also re-assessed kidney autoregulation, for comparison. Based on our previous studies performed using the same protocol [8, 9], the present study might be able to evaluate the lower but not the upper limit of autoregulation by inducing a wide range of blood pressures using phenylephrine and sodium nitroprusside (SNP) infusions in a stepwise fashion up to 5 µg kg− 1 min− 1.
Based on the findings of previous studies investigating splanchnic autoregulation [1, 8, 12,13,14,15,16,17,18,19], we hypothesized that: stomach and liver autoregulation are weak (sloped plateau, lower limit poorly identifiable, and a strong correlation between MAP and the oxygenation index across a wide range of MAPs); and that kidney and skeletal muscle autoregulation are robust (flat to minimally sloped plateau, prominent lower limit, and a weak correlation between MAP and the oxygenation index above the lower limit). As the impact of volume changes on tissue oxygenation is different to the robustness of autoregulation, we also hypothesized that the kidney is more susceptible to hypovolemia than skeletal muscle, stomach, and liver.
2 Methods
2.1 Animal preparation
This study was approved by the Ethical Committee of the Animal Research Division at Hamamatsu University School of Medicine (approval number 2,020,036 was allocated on 29 July, 2020 by President Hiroyuki Konno). All the experiments in this study were conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines on 10 swine (2 males and 8 females, mean ± standard deviation (SD) body weight 24.5 ± 0.5 kg, range 23.8–25.5 kg, approximately 2 months old) from 26 August to 28 October 2020, and all methods were performed in accordance with the relevant guidelines and regulations. All animals were sheltered with an unrestricted water supply, but were fasted for 15 h before the experiments. General anesthesia was induced by the inhalation of 5% isoflurane and oxygen using an animal face mask, and tracheostomy was performed. Anesthesia was maintained with 2.5% isoflurane (approximately 1.2 minimum alveolar anesthetic concentration (MAC)) [20] with an oxygen-air mixture (fraction of inspiratory oxygen = 0.6) through mechanical ventilators. An IntelliVue G5-M1019A (Philips Medical Systems, Eindhoven, the Netherlands) was used to analyze the exhaled gases. The end-tidal carbon dioxide partial pressure was confirmed to be 35–45 mmHg during the animal preparation period, and the ventilator setting was continued throughout the experiment. Three cutaneous electrodes were used to monitor electrocardiographic lead II. A 14-gauge double-lumen catheter and 5 F pulmonary artery catheter (Nihon Kohden, Tokyo, Japan) were inserted via the right jugular vein, and a 16-gauge catheter was inserted into the right femoral artery for the measurement of arterial blood pressure. All sensors were connected to IntelliVue MX800 monitoring system (Philips Japan Medical Systems, Tokyo, Japan) to record macrohemodynamic data. Recorded data were downloaded and processed through RS232 USB port. All processes from tracheostomy to catheter insertions were executed using local anesthetics, under general anesthesia. Saline (100 ml h− 1) was instilled for maintenance. An electric heater and air conditioning were used to maintain the body temperature at 38.0–39.0 °C (normothermia of the pig) throughout the study.
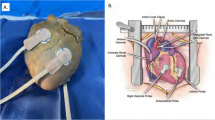
The pigs were initially placed in the prone position to expose the right kidney. The kidney was surgically exposed via a 10-cm incision in the costovertebral angle, in preparation for positioning the NIRS probe (NIRO-200; Hamamatsu Photonics, Hamamatsu, Japan) [8]. Then the pigs were placed back in the supine position. The stomach and liver were surgically exposed via a 15-cm incision in the left subcostal mammillary line. The NIRS probe was placed on the surface of each organ (gastric corpus, hepatic left lobe and center of the right kidney) and fixed using a clothespin applied with light pressure; this fixation method prevented the NIRS probe from slipping off the viscous surface of each organ without affecting the NIRS measurement (Fig. 1). The skeletal muscle probe was positioned on the surface of the left quadriceps muscle. As the NIRO-200 system has only 2 NIRS probes, we used 2 different NIRO-200 systems. The NIRO-200 system uses the modified Beer-Lambert law to measure changes in oxyhemoglobin and deoxyhemoglobin concentrations and uses spatially resolved spectroscopy to measure the absolute tissue hemoglobin oxygen saturation expressed as the tissue oxygenation index (TOI) [10, 11]. Because we used a probe with a distance of 30 mm between the emitter and receiver (Fig. 1), the depth of the oxygenation measurement site was approximately 20 mm (i.e., approximately 30 × 0.7 mm) [21]. We confirmed that the thickness of each organ was more than 20 mm, and placed each probe in a similar position on each organ in all animals. Each TOI was continuously monitored and was recorded at 10-s intervals throughout the study.
The position of each near-infrared spectroscopy (NIRS) probe. NIRS probes were placed on the center of the right kidney, the left lobe of the liver, the gastric corpus and the surface of the left quadriceps muscle, and were affixed using a clothespin between the probe and each organ, except for the quadriceps muscle (the clothespin is not shown in the figure). The probe of the NIRO-200 system consisted of an emitter and a receiver, arranged in 2 parallel photodetectors, which were set in a rubber holder to ensure a receiver-emitter distance of 30 mm
2.2 Experimental protocol (Fig. 2)
After animal preparation, 0.5 µg kg− 1 min− 1 of phenylephrine was infused for 10 min, increased to 1, 2 and 5 µg kg− 1 min− 1 at 10-min intervals, and stopped after the 5 µg kg− 1 min− 1 infusion. After 30 min, SNP was infused at 0.5 µg kg− 1 min− 1 for 10 min, increased to 1, 2 and 5 µg kg− 1 min− 1 at 10-min intervals, and stopped after 10 min. Heart rate (HR), mean arterial pressure (MAP), mean pulmonary arterial pressure (MPA), and central venous pressure (CVP) were continuously monitored and recorded at the end of exhalation before and at the end of each infusion dose as one-off values. Only MAP was recorded at 10-s intervals for the assessment of autoregulation. The CO was measured four times using 5 ml of cold 5% glucose injected into the right atrium, and the mean of the last three values was recorded before and at the end of each infusion dose. After baseline measurements, the hypovolemic condition was induced by removing 600 ml (approximately 35% of the estimated total blood volume) of blood over 15 min. After hemodynamic stabilization (defined as < 10% variation in HR and MAP during a 5-min period), phenylephrine infusion was re-started as described for the baseline condition. SNP infusion was not performed for the hypovolemic condition to avoid lethal hypotension. Finally, the hypovolemic condition was reversed by the infusion of 600 ml of hydroxyethyl starch in 15 min, creating the post-fluid-resuscitation condition. After hemodynamic stabilization, phenylephrine and SNP were re-infused as per the baseline condition. Arterial blood gases and hematocrit were measured before the phenylephrine infusion in each condition. After the completion of the experiment, animals were euthanized with a lethal dose of propofol (500 mg).
2.3 Assessment of each organ’s autoregulation
Each organ’s autoregulation was quantified using Spearman’s correlation coefficient between the MAP and each TOI [2,3,4,5,6,7,8,9], calculated by recording the MAP and each TOI every 10 s for 10 min during each infusion dose, generating 60 paired samples for each TOI. When autoregulation is intact, each correlation coefficient value approaches zero, whereas in a pressure-passive relationship, the coefficients approach + 1. Conversely, when there is a paradoxical response (TOI inversely related to MAP), correlation values approach − 1. In previous studies [2,3,4,5], the correlation coefficient thresholds to distinguish between the presence and absence of autoregulation were arbitrarily selected by the authors and were in the range of 0.25 to 0.5. In the present study, a threshold of 0.36 was chosen in accordance with our previous swine studies [8, 9]; we defined the following states: Spearman’s correlation coefficient > 0.36 (pressure-passive); 0.36 > Spearman’s correlation coefficient > − 0.36 (intact autoregulation); and Spearman’s correlation coefficient < − 0.36 (paradoxical response).
2.4 Statistical analysis
Data are shown as the mean ± SD. A repeated-measures 1-way analysis of variance (ANOVA) was used to analyze differences in hemodynamic and arterial blood gas variables, and differences in the organ TOIs at each infusion dose and for each condition. If the ANOVA indicated significant differences, a Scheffe F-test for multiple comparisons was executed. Differences in the correlation coefficient between each organ during each vasoactive drug infusion were also analyzed using ANOVA, and a Bonferroni/Dunn test was used for multiple comparisons. The linear regression equations for the correlation between the MAP and each TOI during phenylephrine or SNP infusion in each condition were calculated by simple linear regression analysis using the least squares method. P values of < 0.05 were considered to indicate statistical significance.
3 Results
All 10 pigs survived to the end of the experiment. Throughout all volume conditions, phenylephrine decreased the HR and increased the MAP and MPA, and SNP increased the HR and decreased the MAP and MPA in a dose-dependent manner (Table 1). Phenylephrine increased the CO during hypovolemia, but decreased the CO after fluid resuscitation. SNP decreased the CO. A 600-ml bleed increased the HR and decreased the MAP, MPA, CVP, and CO, while subsequent hydroxyethyl starch infusion decreased the HR and increased the MAP, MPA, CVP, and CO. Compared with before the start of the phenylephrine infusion, oxygen delivery and arterial carbon dioxide partial pressure (400 ± 62 ml min− 1 and 40 ± 3 mmHg, respectively) decreased during hypovolemia (198 ± 52 ml min− 1 and 36 ± 5 mmHg) and returned after fluid resuscitation (363 ± 50 ml min− 1 and 40 ± 4 mmHg) (P < .0001 and P = .01, respectively). Arterial oxygen partial pressure did not differ between conditions (P = .863). Hematocrit did not change from baseline after the 600-ml bleed but decreased after fluid resuscitation (P < .0001; 30 ± 2, 31 ± 2 and 19 ± 2%, respectively).
The liver TOI was lower than the other organ TOIs (P < .0001) in the baseline condition before drug infusion (Table 2). With increasing blood pressure, the stomach, liver, and kidney TOIs increased in a dose-dependent manner in all conditions; in contrast, the muscle TOI increased during the baseline and hypovolemic conditions, but decreased during the post-fluid-resuscitation condition. With decreasing blood pressure, the liver and kidney TOIs decreased during both the baseline and post-fluid-resuscitation conditions and the stomach TOI decreased during the post-fluid-resuscitation condition. There was no change in the stomach TOI during the baseline condition and no change in the muscle TOI during either the baseline or post-fluid-resuscitation conditions. The 600-ml bleed decreased the stomach, liver and kidney TOIs; the stomach and kidney TOIs increased after fluid resuscitation, but liver TOIs did not. The 600-ml bleed did not change the muscle TOI, but fluid resuscitation increased that TOI above baseline.
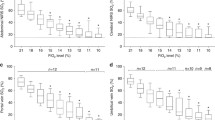
Figure 3 shows the percentage changes in each organ TOI, with each baseline value set to zero. The decrease in the kidney TOI in response to the 600-ml bleed was 2-fold greater than the decrease in the stomach TOI (P = .0045) and liver TOI (P = .0023), and the subsequent 600-ml hydroxyethyl starch infusion was 2-fold more effective at restoring the kidney TOI than the stomach TOI (P = .043) and liver TOI (P = .0200). The effect of SNP on the muscle TOI was biphasic; SNP decreased the muscle TOI at ≤ 1 µg kg− 1 min− 1 doses but increased the TOI at doses of more than 2 µg kg− 1 min− 1 during the baseline and the post-fluid-resuscitation condition. The kidney TOI decreased the most with decreasing MAP in the post-fluid-resuscitation condition (P < .0001 vs. other organs).
The stomach, liver, and kidney TOIs showed positive correlations between MAP and the TOI in all conditions (Fig. 4), whereas the muscle TOI showed positive correlations only during phenylephrine infusion in the baseline and hypovolemic conditions. The stomach and liver TOIs showed linear relationships with MAP across a wide range of MAPs, whereas the kidney TOI had a threshold around 60 mmHg (lower limit of autoregulation). The relationship between MAP and muscle TOI did not show the typical autoregulation shape.
Averaged relationships between the mean arterial pressure (MAP) and each tissue oxygenation index (TOI). All linear regression lines were statistically significant (all P values < 0.0001 to 0.0128). Note that the vertical scale in the kidney graph is 40%, whereas those in the graphs for other organs are 20%
Table 3 shows the mean values of the Spearman’s correlation coefficients between MAP and each of the TOIs, and the autoregulation patterns. The correlation coefficient of the liver was higher than those of the other organs and pressure-passive patterns were most frequent in the liver. Whereas, during decreasing blood pressure after fluid resuscitation, the correlation coefficient of the kidney was higher than those of the other organs and pressure-passive patterns were most frequent in the kidney. Negative correlation coefficients and paradoxical responses were most frequently observed in the muscle.
4 Discussion
The present study continuously monitored stomach, liver, kidney, and skeletal muscle oxygenation using NIRS, and compared the impact of vasoactive drug-induced blood pressure changes on the oxygenation of each organ under baseline, hemorrhage-induced hypovolemic, and subsequent fluid-resuscitation conditions, which are commonly encountered in the clinical setting. The effects of vasoactive drug infusion, a 600-ml bleed, and subsequent hydroxyethyl starch infusion on the hemodynamic variables were similar to the effects observed in our previous studies [8, 9]. Vasoactive drug infusion also had a similar effect on the CO, and the effect of phenylephrine on the CO differed depending on the volume condition [8, 9]. We proved that the impact of blood pressure and/or circulatory volume changes was different between organs, and that each organ’s oxygenation differed depending on the conditions even when MAP was similar, indicating that blood pressure is an unreliable index for maintaining microcirculation [22]. We also confirmed that vasopressor administration did not decrease oxygenation even with increasing dosage under any condition in any splanchnic organ, but rather improved liver and kidney oxygenation even during hypovolemia. To our knowledge, this is the first study to investigate the autoregulation of splanchnic organs and skeletal muscle using NIRS in several volume conditions.
4.1 Autoregulation of each organ
Based on the Spearman’s correlation coefficients, the correlation coefficient of the stomach was lower than that of the liver, but higher than those of the kidney and muscle in the baseline condition, indicating that the autoregulation of the stomach is better than that of the liver, but not as robust as that of the kidney and muscle. Previous studies demonstrated that gastric blood flow is not autoregulated in sympathetically innervated stomach [12, 13], although significant autoregulation was observed after sympathetic denervation, which indicates that sympathetic influences may override the intrinsic function of the stomach to regulate the gastric blood flow. We assessed organ autoregulation under 2.5% isoflurane, and the depression of sympathetic activity due to isoflurane [14] might have helped reveal the intrinsic stomach autoregulation.
Liver TOIs changed linearly with MAP across a wide range of blood pressures, and the correlation coefficients of the liver were higher than those of the other organs, except during SNP infusion in the post-fluid-resuscitation condition. Liver blood flow is controlled by the mechanisms regulating hepatic arterial blood flow, portal blood flow, and the interrelationship between the 2 flows (hepatic arterial buffer response: HABR) [15, 16]. HABR is the ability of the hepatic artery to produce compensatory flow changes in response to changes in portal blood flow. When portal blood flow reduces or increases, hepatic artery dilates or constricts. However, the portal vein has no compensatory mechanism to control hepatic arterial blood flow [15]. The portal blood flow accounts for 70–75% of total hepatic flow and collects deoxygenated blood from the splanchnic organs. The hepatic arterial blood accounts for the remaining 25–30% [15]. In fact, liver TOIs were significantly less than the TOIs for other organs. Although some studies have provided evidence of autoregulation of hepatic arterial blood flow in sympathetically denervated preparations [17], the degree of autoregulation is weak. In sympathetically innervated liver, the hepatic artery and portal venous vasculature show a linear relationship between pressure and flow, without autoregulation [18]. It was unclear the impacts of HABR, which might change the blood distribution between highly oxygenated arterial blood and less oxygenated portal blood, on our results, but liver TOIs changed linearly with MAP over a wide range of blood pressures. Our findings suggested that liver blood flow is not autoregulated, consistent with previous reports.
The present study demonstrated that the degree of autoregulation in the kidney may be higher than or similar to that of skeletal muscle during phenylephrine-induced hypertension, whereas the kidney becomes pressure-passive during hypotension. Individual responses indicated that skeletal muscle produced many paradoxical responses. Paradoxical responses do not exist in the conventional autoregulation concept but are ordinarily observed, especially in the brain in animals [8, 9] and humans [5]. As a solid vasomotor response that suppresses excessive changes in organ blood flow, arteriolar vasoconstriction or vasodilation occurs in response to excessive increases or decreases in perfusion pressure. The resulting alteration in the arteriovenous blood ratio causes the paradoxical response [23].
4.2 Impact of circulatory volume changes on oxygenation in each organ
The 600-ml bleed decreased the kidney TOI 2-fold more than it decreased the stomach and liver TOIs, and the subsequent 600-ml hydroxyethyl starch infusion was 2-fold more effective at restoring kidney TOIs. In our previous study [9], a similar degree of hemorrhage decreased cerebral, thoracic and lumbar spinal cord TOIs by 6%, 12% and 13%, and fluid resuscitation increased them by 0%, 7% and 7%, respectively. Combining these findings, the impact of circulatory volume changes on the kidney TOI is 2-fold greater than on the spinal cord, stomach and liver, and 4-fold greater than on the brain. Hypovolemia is, thus, a critical condition for the kidney. These findings are consistent with the clinical setting, as hypovolemia is the most frequent etiology of acute kidney injury in critical patients [24]. The kidney has a high oxygen demand associated with the tubular oxygen consumption necessary for solute reabsorption, and requires a high blood flow (oxygen supply) to maintain organ homeostasis [25], despite having less robust autoregulation than the brain [8].
We also found that muscle oxygenation was relatively unaffected by blood loss but showed the greatest impact of fluid resuscitation. Organ perfusion is determined not only by blood pressure but also by the distribution of CO among different organs [26, 27]. In the present study, the 600-ml bleed decreased the CO by 50%, and fluid resuscitation increased the CO by three-fold before fluid infusion. Although further investigation is required to explore the mechanism, skeletal muscles (quadriceps) might minimally lose their share of CO after hemorrhage, in contrast to the kidney, and maximally increase the oxygenation after fluid resuscitation (Fig. 3).
4.3 Study limitations
Our study had several limitations. The present study did not adopt a crossover design to administer phenylephrine and SNP, and each condition was sequentially induced. Therefore, the infusion order and/or repeated administration of vasoactive drugs might have influenced the following measurements. Furthermore, a hyperdynamic state still remained at the start of the phenylephrine infusion after fluid resuscitation because the preceding hypovolemia induced sympathetic nerve stimulation (CO was 4.2 ± 0.4 l min− 1 before phenylephrine and 2.7 ± 0.5 l min− 1 before SNP infusion). Thus, a hemodynamic steady state was not achieved during the post-fluid-resuscitation condition, and this might have influenced the results. In addition, a wide range of MAPs was induced by phenylephrine and SNP infusion under 1.2 MAC of isoflurane anesthesia. However, the results might be different if other vasoactive drugs and/or other anesthetics were used, because phenylephrine, SNP and isoflurane might have direct effect on each organ’s oxygen extraction. SNP, particularly, can increase skeletal muscle oxygenation directly, regardless of the systemic blood pressure [28]. Furthermore, in the present study, only Spearman’s correlation coefficients between the MAP and each TOI were used to assess each organ’s autoregulation, and the threshold value to distinguish between the presence and absence of autoregulation (0.36) was decided arbitrarily. Finally, the present study did not measure actual organ blood flow and used NIRS as a substitute indicator of blood flow. Therefore, NIRS might not reflect organ blood flow when the oxygen demand, supply, and utilization change during the experiments and/or when the distribution of arterial and venous blood alters such as HABR in the liver. Furthermore, the results might differ depending upon where on each organ and/or skeletal muscle the NIRO probe was attached, due to the heterogeneity in vascular flow.
In conclusion, our assessment of splanchnic organ and skeletal muscle autoregulation using NIRS indicates that the decreasing order of autoregulation robustness is: skeletal muscle, kidney, stomach, and liver. The impact of volume changes is different from the robustness of autoregulation; the kidney is most susceptible to volume loss, while skeletal muscle is most tolerant to volume loss. These findings were based on the evaluation using each organ oxygenation instead of organ blood flow, further investigations might be required to validate the results.
References
Meng L, Wang Y, Zhang L, McDonagh DL. Heterogeneity and variability in pressure autoregulation of Organ Blood Flow: Lessons learned over 100 + years. Crit Care Med. 2019;47:436–48.
Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–25.
Zweifel C, Castellani G, Czosnyka M, Carrera E, Brady KM, Kirkpatrick PJ, et al. Continuous assessment of cerebral autoregulation with near-infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke. 2010;41:1963–8.
Ono M, Joshi B, Brady K, Easley RB, Zheng Y, Brown C, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012;109:391–8.
Moerman AT, Vanbiervliet VM, Van Wesemael A, Bouchez SM, Wouters PF, De Hert SG. Assessment of cerebral autoregulation patterns with near-infrared spectroscopy during pharmacological-induced pressure changes. Anesthesiology. 2015;123:327–35.
Rivera-Lara L, Geocadin R, Zorrilla-Vaca A, Healy R, Radzik BR, Palmisano C, et al. Validation of Near-Infrared Spectroscopy for Monitoring Cerebral Autoregulation in Comatose Patients. Neurocrit Care. 2017;27:362–9.
Healy RJ, Zorrilla-Vaca A, Ziai W, Mirski MA, Hogue CW, Geocadin R, et al. Glasgow coma scale score fluctuations are inversely associated with a Nirs-based index of cerebral autoregulation in acutely comatose patients. J Neurosurg Anesthesiol. 2019;31:306–10.
Kurita T, Kawashima S, Morita K, Nakajima Y. Assessment of cerebral and renal autoregulation using near-infrared spectroscopy under normal, hypovolaemic and postfluid resuscitation conditions in a swine model: an observational study. Eur J Anaesthesiol. 2019;36:531–40.
Kurita T, Kawashima S, Morita K, Nakajima Y. Spinal cord autoregulation using near-infrared spectroscopy under normal, hypovolemic, and post-fluid resuscitation conditions in a swine model: a comparison with cerebral autoregulation. J Intensive Care. 2020;8:27.
Matcher SJ, Kirkpatrick P, Nahid K, Cope M, Delpy DT. Absolute quantification methods in tissue near-infrared spectroscopy. Proc SPIE. 1995;2389:486–95.
Suzuki S, Takasaki S, Ozaki T, Kobayashi Y. (1999) A tissue oxygenation monitor using NIR spatially resolved spectroscopy. Proc SPIE 3597.
Holm-Rutili L, Perry MA, Granger DN. Autoregulation of gastric blood flow and oxygen uptake. Am J Physiol. 1981;241:G143–9.
Kiel JW, Riedel GL, Shepherd AP. Autoregulation of canine gastric mucosal blood flow. Gastroenterology. 1987;93:12–20.
Seagard JL, Hopp FA, Bosnjak ZJ, Osborn JL, Kampine JP. Sympathetic efferent nerve activity in conscious and isoflurane-anesthetized dogs. Anesthesiology. 1984;61:266–70.
Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16:6046–57.
Lautt WW. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: hepatic arterial buffer response. Am J Physiol. 1985;249:G549–56.
Hanson KM, Johnson PC. Local control of hepatic arterial and portal venous flow in the dog. Am J Physiol. 1966;211:712–20.
Richardson PD, Withrington PG. Pressure–flow relationships and effects of noradrenaline and isoprenaline on the hepatic arterial and portal venous vascular beds of the dog. J Physiol. 1978;282:451–70.
Stainsby WN, Renkin EM. Autoregulation of blood flow in peripheral vascular beds. Am J Cardiol. 1961;8:741–7.
Kurita T, Takata K, Uraoka M, Morita K, Sanjo Y, Katoh T, et al. The influence of hemorrhagic shock on the minimum alveolar anesthetic concentration of isoflurane in a swine model. Anesth Analg. 2007;105:1639–43.
Ohmae E, Ouchi Y, Oda M, Suzuki T, Nobesawa S, Kanno T, et al. Cerebral hemodynamics evaluation by near-infrared time-resolved spectroscopy: correlation with simultaneous positron emission tomography measurements. NeuroImage. 2006;29:697–705.
Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19:8.
Ogoh S, Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH. The effect of phenylephrine on arterial and venous cerebral blood flow in healthy subjects. Clin Physiol Funct Imaging. 2011;31:445–51.
Montomoli J, Donati A, Ince C. Acute kidney injury and fluid resuscitation in septic patients: are we protecting the kidney? Nephron. 2019;143:170–3.
Hansell P, Welch WJ, Blantz RC, Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol. 2013;40:123–37.
Neutze JM, Wyler F, Rudolph AM. Changes in distribution of cardiac output after hemorrhage in rabbits. Am J Physiol. 1968;215:857–64.
Fell C. Changes in distribution of blood flow in irreversible hemorrhagic shock. Am J Physiol. 1966;210:863–8.
Pitkanen OP, Laine H, Kemppainen J, Eronen E, Alanen A, Raitakari M, et al. Sodium nitroprusside increases human skeletal muscle blood flow, but does not change flow distribution or glucose uptake. J Physiol. 1999;521:729–37.
Funding
Support was received solely from institutional and/or departmental sources.
Author information
Authors and Affiliations
Contributions
Tadayoshi Kurita contributed to the study design, data collection, analysis of data, and manuscript preparation. Shingo Kawashima contributed to the study design and data collection. Mohamed Mathar Sahib Ibrahim Khaleelullah contributed to the data collection and analysis of data. Yoshiki Nakajima contributed to the study design. The first drafts of the manuscript was written by Tadayoshi Kurita, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Statements and declarations
Competing interests: The authors have no relevant financial or non-financial interests to disclose.
Ethics approval:
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted (The Committee of the Animal Research Division at Hamamatsu University School of Medicine, approval number 2020036). This article does not contain any studies with human participants performed by any of the authors.
Consent to participate:
Not applicable.
Consent to publish:
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kurita, T., Kawashima, S., Ibrahim Khaleelullah, M. et al. Stomach, liver, kidney and skeletal muscle autoregulation evaluated by near-infrared spectroscopy in a swine model. J Clin Monit Comput 37, 899–909 (2023). https://doi.org/10.1007/s10877-022-00956-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00956-5