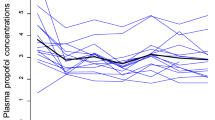
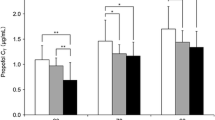
Abstract
Target-controlled infusion (TCI) is based on pharmacokinetic models designed to achieve a desired drug level in the blood. TCI’s predictive accuracy of plasma propofol levels at the end of surgery with major blood loss has not been well established. This prospective observational study included adult patients (BMI 20–35 kg/m2) undergoing surgery with expected blood loss ≥ 1500 mL. The study was conducted with the Schnider TCI propofol model (Alaris PK Infusion Pump, CareFusion, Switzerland). Propofol levels were assessed in steady-state at the end of anaesthesia induction (Tinitial) and before the end of surgery (Tfinal). Predicted propofol levels (CTCI) were compared to measured levels (Cblood). Twenty-one patients were included. The median estimated blood loss was 1600 mL (IQR 1000–2300), and the median fluid balance at Tfinal was + 3200 mL (IQR 2320–4715). Heart rate, mean arterial blood pressure, and blood lactate did not differ significantly between Tinitial and Tfinal. The median bispectral index (0–100) was 50 (IQR 42–54) and 49 (IQR 42–56) at the two respective time points. At Tinitial, median CTCI was 2.2 µmol/L (IQR 2–2.45) and Cblood was 2.0 µmol/L (bias 0.3 µmol/L, limits of agreement − 1.1 to 1.3, p = 0.33). CTCI and Cblood at Tfinal were 2.0 µmol/L (IQR 1.6–2.2) and 1 µmol/L (IQR 0.8–1.4), respectively (bias 0.6 µmol/L, limits of agreement − 0.89 to 1.4, p < 0.0001). Propofol TCI allows clinically unproblematic conduct of general anaesthesia. In cases of major blood loss, the probability of propofol TCI overestimating plasma levels increases.
Trial registration German Clinical Trials Register (DRKS; DRKS00009312).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Propofol is currently the drug of choice for intravenous general anaesthesia. Since administering constant propofol infusion according to the weight of the patient (mg/kg/h) leads to an accumulation of the drug in the body, strategies for dose reduction during long surgical procedures were proposed [1].
In the mid-1990s “target-controlled infusion” (TCI) first became available [1]. According to the patient’s age, gender, height, and weight, the propofol dosage required for anaesthesia is determined by a pharmacokinetic multi-compartment model [1]. Since the introduction of TCI, several different models have become available. However, the classic models by Marsh and Schnider continue to be the most commonly used [2].
Findings from several pharmacological studies indicate that the calculated pharmacokinetically-predicted propofol plasma levels are often sufficiently similar to chemically-measured values [3,4,5]. While this conclusion may be applied to the general adult population, it is likely that there are sub-groups in which differences exist. For instance, the most common commercially available TCI systems are not validated for severely obese patients or children [6, 7]. Furthermore, they cannot adjust to variations in patients’ characteristics such as their physical condition, chronic use of concomitant medication, or additional anaesthetic co-medication. Some investigators have reported problems with the precision and consistency of TCI models [8, 9]. It remains unclear if the predicted (calculated by the pharmacokinetic model) and measured plasma levels of propofol are similar at the end of surgical procedures with major blood loss. For this investigation, we hypothesized that the differences between the predicted and measured propofol values were greater at the end of such procedures than at the beginning.
2 Methods
This prospective observational study was conducted after obtaining approval by the ethical review committee of canton Thurgau (Switzerland; KEKTGOV2015/09), and the study was subsequently reported on the German Clinical Trials Register (DRKS; DRKS00009312). All patients were informed about the study design and provided their written consent to participate.
2.1 Study participants
We included both male and female adult patients who were undergoing a surgical procedure with an expected blood loss of at least 1500 mL. Patients had to be eligible for a propofol-based anaesthetic and scheduled for insertion of an arterial line in accordance with departmental guidelines. The exclusion criteria were patients with a body mass index (BMI) below 20 or above 35 kg/m2, preoperative haemodynamic instability, allergies to propofol or components of the preparation, known or suspected liver dysfunction, and those who were pregnant or undergoing an emergency-related procedure.
2.2 Conduct of the study
All patients were treated according to departmental guidelines and under the responsibility of the senior anaesthetist in charge. All patients entered the anaesthetic preparation area after receiving a 7.5 mg tablet of midazolam on the ward. Upon arrival, patients were connected to standard anaesthesia monitoring equipment (i.e., non-invasive blood pressure, ECG, oxygen saturation; IntelliVue MP30, Philips, Zurich, Switzerland). Peripheral venous access was then established and a radial arterial line (Haemofix Exadyn Set, B. Braun, Melsungen, Germany) was inserted. The latter served as a means for drawing blood samples to analyse the propofol plasma level. To monitor the depth of anaesthesia, a bispectral index pad was applied (BIS; Philips, Zurich, Switzerland). According to patients’ needs, a second venous access and a central line were placed.
The general anaesthetic was performed by constant infusion of propofol using a TCI system (Schnider Model; Alaris PK Infusion Pump, CareFusion, Rolle, Switzerland). The following biometric data points were entered into the system: age, gender, height, and actual weight. For induction of anaesthesia, a target effect site level (Cet) of 6 µg/mL was used. Boluses of fentanyl (1–3 µg/kg) for induction and atracurium (0.5 mg/kg) for tracheal intubation were administered. After securing the airway, Cet was reduced for maintenance of anaesthesia. BIS values between 40 and 60, blood pressure, and heart rate were used to guide the current target level. Additional inputs for maintaining anaesthesia (e.g., fentanyl and atracurium boluses; level of remifentanil infusion; addition of sevoflurane, if necessary) were carefully observed by the anaesthetist. Achieving haemodynamic stability and normovolaemia was the objective during the entire procedure, with possible volume depletion detected by integration of blood pressure values, heart rate, arterial blood gas analyses, and measurement of diuresis. The threshold for transfusion of packed red cells was usually 70 g/L, and 80 g/L under special circumstances, such as severe coronary heart disease, severe chronic obstructive pulmonary disease or unstable bleeding situation. Fluid management included the use of a CellSaver System (Elite; Haemonetics, Braintree MA, USA), if necessary. Emphasis was put on maintaining and managing body temperature.
2.3 Data collection
Assessments of propofol levels were done twice during steady-state, the first at the very end of the anaesthesia induction period, prior to surgery (Tinitial) and the second towards the end of the procedure (Tfinal) when skin closure began. ‘Steady-state (CTCI)’ was achieved when predicted plasma level (Cp), effect-site level (Ce), and Cet were all at the same value for at least 10 min. Cet represented the parameter that was changed by the anaesthetist according to patient’s needs during the course of the procedure. This was generally guided by a combination of the aforementioned clinical parameters, particularly the BIS values.
Blood samples for analyses of propofol plasma concentration (Cblood) and arterial blood gases (in combination with parameters such as pH, haemoglobin, and lactate) were taken at both time points (Tinitial and Tfinal). TCI-predicted propofol values (CTCI) were also recorded at these same points in time. The samples for propofol concentration analysis were placed in plastic tubes and immediately taken in an opaque container to be centrifuged at the hospital’s main laboratory. The plasma was safely stored in a − 70 °C freezer. The samples were then sent to an external laboratory (Institute for Forensic Medicine, University of Zurich, Switzerland). Propofol plasma levels were determined using gas chromatography coupled with mass spectrometry (GC–MS) in single ion monitoring mode (plasma initial and plasma post). Propofol was then quantified by comparison of its peak area ratio to calibration curves (accuracy > 15%).
Data on the following variables were recorded: the patient’s ASA physical status (American Society of Anesthesiologists’ classification system); age; gender; height; weight; chronic use of medication; amount of infused propofol, remifentanil, and fentanyl; all fluids given (crystalloids, colloids, packed red cells, fresh frozen plasma); administration of inotropic substances (dobutamine, epinephrine); amount of diuresis; body temperature; duration of the surgical procedure; and amount of blood loss. The fluid balance was determined by measuring the fluid administered and fluid lost (blood, urine).
2.4 Statistical analysis
At both Tinitial and Tfinal the measured plasma levels of propofol were compared to the estimated levels given by the infusion pump (CTCI). Bias and limits of agreement were calculated and graphically displayed. The relationships between fluctuations in Cblood and CTCI according to patient’s characteristics, such as fluid balance, were assessed using simple regression tests. To measure the intraoperative haemodynamic stability, comparisons of heart rate, mean arterial pressure, and lactate values were carried out using Wilcoxon signed rank test. The performance error (PE) was determined using the following formula: PE (%) = ((Cblood – CTCI)/CTCI)*100. Tests of normality were measured with the Shapiro–Wilk test. The level of statistical significance was set at < 0.05. The data were analysed using Stata version 15.1 (StataCorp, College Station, Texas, USA).
3 Results
A total of twenty-one patients undergoing gynaecological, visceral or orthopaedic surgery were included in the study between September 2015 and December 2016. Demographic and main procedure-related data are displayed in Tables 1 and 2. No patients reported chronic use of anti-epileptic drugs or known cytochrome P450-inducing medication. All patients received remifentanil and fentanyl (Table 2).
The estimated blood loss was at a median value of 1600 mL (IQR 1000–2300, range 600–3500). The median fluid balance at the end of surgery was + 3200 mL (IQR 2320–4715, range 200–6600). None of the patients received inotropic medication. All patients were in a stable haemodynamic state at the end of surgery. Heart rate, mean arterial blood pressure, and blood lactate did not differ significantly between Tinitial and Tfinal. However, the haemoglobin concentration was lower at Tfinal (Table 3). The body temperature of all patients at the end of surgery was > 35 °C (median 36.6, IQR 35.9–36.9, range 35.3–38.5).
Table 4 presents the predicted and measured propofol levels at the two study measurement points. The measured propofol level at the final measurement was significantly lower than the values predicted with the TCI. Likewise, significant differences were detected between the performance error of initial and final comparisons (Fig. 1; p = 0.0279)—initial median − 14 (− 34 to 16.7; − 60 to 61.1) and final − 36.2 (− 57.5 to − 25; − 68.8 to 52.9). Figure 2 shows the performance error at the time points of blood sampling versus the estimated blood loss.
Findings from multivariate linear regression analyses showed a moderate negative relationship between the PE final and blood loss (− 0.47, p-value = 0.04), which indicated an increased blood loss may be associated with an increased likelihood that the TCI will overestimate the amount to propofol in the plasma. No significant correlations were found among the other study variables (age, BMI, ASA, performance error at initial measurement, duration of operation).
4 Discussion
The aim of this study was to measure the predictive performance of a commercially available propofol target-controlled infusion system in the context of intraoperative blood loss by comparing it to propofol plasma levels determined in laboratory. The main finding was that, despite allowing for clinically unproblematic conduct of anaesthesia, TCI tended to overestimate propofol plasma levels, with the trend in overestimation increasing with the amount of blood loss.
Target-controlled infusion is a modern concept for dosing propofol that uses patient-specific data to direct a computer-driven infusion pump [1, 10, 11]. While propofol-based general anaesthesia has advantages over inhalational anaesthesia, it also carries some risks. In extreme cases, the propofol is not properly infused into the patient’s veins. In less extreme examples, the accuracy of propofol TCI may be impaired when the propofol distribution volume is significantly changed (even if the volume of distribution for propofol is very high in the first place, and the lipid soluble drug eventually accumulates in fatty tissue) [12]. Propofol TCI cannot take this into account, which might be the reason for overestimation of propofol plasma levels at the end of surgery in our study. It is also worth noting that the Schnider model was derived based on an effect on EEG parameters and not plasma levels [11], and was developed in the absence of surgery or major blood loss. In anaesthesia not complicated by major blood loss, Glen and Servin [13] found that the bias of the Schnider model changed during differing phases of propofol administration, underpredicting plasma levels during recovery from anaesthesia, which is the opposite of our findings. We believe the extent of the blood loss and consecutive alteration in distribution volume occurring in our patients contributed to these divergent findings.
In theory, alterations in propofol elimination could lead to false estimations of plasma levels by TCI. The Schnider propofol TCI model does not account for the effect of administered co-medication. It is known that pharmacokinetic drug interactions can alter propofol concentrations and thus cause erroneously predicted plasma concentration values. In order to explain our study’s findings, an increase in the propofol elimination rate during the surgical procedure would have been necessary. This could have been the result of a significantly higher cardiac output. However, none of our study patients were infused with inotropic medication. It was also suggested that antiepileptic drugs could increase propofol clearance because of the induction of cytochrome P450 activity [14]. Again, none of our patients received antiepileptic drugs.
In contrast, Wietasch et al. found that when remifentanil was combined with propofol, the TCI system (Marsh model) performed poorly and systematically underestimated the propofol plasma concentrations in ASA III patients undergoing routine surgery [9]. The authors speculated that a possible pharmacodynamic effect of propofol/remifentanil co-administration negatively influenced sympathetic drive, cardiac output, and hepatic blood flow. All our patients received remifentanil by infusion, with the vast majority resulting in an over- not underestimation of propofol plasma levels.
Propofol TCI is a clinically tested and proven method for administering propofol as an anaesthetic, and provided adequate and titratable anaesthesia in our patient group. The bias has been reported to be smaller than the difference between end-tidal and arterial partial pressures of inhalational anaesthetics after 15 min and 60 min of isoflurane administration [15]. Swinhoe et al. and Sepulveda et al. suggested that the performance of a TCI system is clinically acceptable if the bias is no greater than 10–20% [5, 7]. In spite of the limited precision of TCI models, the TCI rates largely remain constant [3]. Attempts to measure propofol or its metabolites in expired air of patients, which is one of the main advantages of inhalational anaesthesia, have not yet been widely recognized. Propofol point-of-care testing is available, but not widely used in clinical practice.
Since the TCI pump displays an estimation of the drug concentration and is unable to precisely gauge if the drug dose is sufficient, adequate anesthetic depth monitoring of the individually-achieved drug effect should be a prerequisite [16, 17]. All pharmacokinetic and pharmacodynamic models are inherently inaccurate since they are based on a limited number of samples drawn from a specific patient population. Once patient characteristics or circumstances differ from the “model population”, predictions will likely be imprecise. In our patient group, the BIS values stayed relatively unchanged while propofol levels halved.
Our study has its inherent weaknesses that limit the generalizability of our findings. Hemodynamic monitoring and treatment were not standardized, which means erroneous and variable predicted plasma propofol concentration could be influenced by differential hemodynamic management. Due to organisational and financial constraints, the number of patients and blood samples were limited. Any further investigation of confounders for bias should include an increased number of patients and blood samples. Second, we did not use any tracer substances or additional methods for haemodynamic monitoring to quantify the volume status of the patients. Further, a control group with patients not suffering major blood loss would have likely provided additional information.
In conclusion, dosing of propofol by target-controlled infusion ought to be supported by monitoring of haemodynamic and depth of anaesthesia parameters to allow for clinically unproblematic conduct of general anaesthesia. Target-controlled infusion may carry the risk of overestimating propofol levels after major blood loss. If exact values are important (e.g. for scientific purposes), measuring of propofol plasma levels by laboratory methods should be considered.
References
Struys MM, De Smet T, Glen JI, et al. The history of target-controlled infusion. Anesth Analg. 2016;122:56–69.
Absalom AR, Mani V, De Smet T, et al. Pharmacokinetic models for propofol–defining and illuminating the devil in the detail. Br J Anaesthesia. 2009;103:26–37.
Cowley NJ, Hutton P, Clutton-Brock TH. Assessment of the performance of the Marsh model in effect site mode for target controlled infusion of propofol during the maintenance phase of general anaesthesia in an unselected population of neurosurgical patients. Eur J Anaesth. 2013;30:627–32.
Struys MM, De Smet T, Depoorter B, et al. Comparison of plasma compartment versus two methods for effect compartment-controlled target-controlled infusion for propofol. Anesthesiology. 2000;92:399–406.
Swinhoe CF, Peacock JE, Glen JB, et al. Evaluation of the predictive performance of a ‘Diprifusor’ TCI system. Anaesthesia. 1998;53(Suppl 1):61–7.
Cortinez LI, De La Fuente N, Eleveld DJ, et al. Performance of propofol target-controlled infusion models in the obese: pharmacokinetic and pharmacodynamic analysis. Anesth Analg. 2014;119:302–10.
Sepulveda P, Cortinez LI, Saez C, et al. Performance evaluation of paediatric propofol pharmacokinetic models in healthy young children. Br J Anaesth. 2011;107:593–600.
Cortegiani A, Pavan A, Azzeri F, et al. Precision and bias of target-controlled prolonged propofol infusion for general anesthesia and sedation in neurosurgical patients. J Clin Pharmacol. 2018;58:606–12.
Wietasch JK, Scholz M, Zinserling J, et al. The performance of a target-controlled infusion of propofol in combination with remifentanil: a clinical investigation with two propofol formulations. Anesth Analg. 2006;102:430–7.
Glen JB. The development of ‘Diprifusor’: a TCI system for propofol. Anaesth. 1998;53(Suppl 1):13–21.
Schnider TW, Minto CF, Struys MM, et al. The safety of target-controlled infusions. Anesth Analg. 2016;122:79–85.
Bolkenius D, Dumps C, Halbeck E. Drugs for intravenous induction of anesthesia: propofol. Anaesthesist. 2018;67:147–62.
Glen JB, Servin F. Evaluation of the predictive performance of four pharmacokinetic models for propofol. Br J Anaesth. 2009;102:626–32.
Sahinovic MM, Eleveld DJ, Miyabe-Nishiwaki T, et al. Pharmacokinetics and pharmacodynamics of propofol: changes in patients with frontal brain tumours. Br J Anaesth. 2017;118:901–9.
Frei FJ, Zbinden AM, Thomson DA, et al. Is the end-tidal partial pressure of isoflurane a good predictor of its arterial partial pressure? Br J Anaesth. 1991;66:331–9.
Struys MM, Sahinovic MM, Lichtenbelt BJ, et al. Optimizing intravenous drug administration by applying pharmacokinetic/pharmacodynamic concepts. Br J Anaesth. 2011;107:38–47.
Nimmo AF, Absalom AR, Bagshaw O, et al. Guidelines for the safe practice of total intravenous anaesthesia (TIVA). Joint Guidelines from the Association of Anaesthetists and the Society for Intravenous Anaesthesia. Anaesthesia. 2018. https://doi.org/10.1111/anae.14428.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Mohler, T., Welter, J., Steurer, M. et al. Measuring the accuracy of propofol target-controlled infusion (TCI) before and after surgery with major blood loss. J Clin Monit Comput 34, 97–103 (2020). https://doi.org/10.1007/s10877-019-00261-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00261-8