Abstract
Blood glucose and its variability of is a major prognostic factor associated with morbidity. We hypothesized that intravenous microdialysis incorporated in a central venous catheter (CVC) would be interchangeable with changes in blood glucose measured by the reference method using a blood gas analyzer. Microdialysis and central venous blood glucose measurements were simultaneously recorded in high-risk cardiac surgical patients. The correlation between absolute values was determined by linear regression and the Bland–Altman test for repeated measurements was used to compare bias, precision, and limits of agreement. Changes in blood glucose measurement were evaluated by four-quadrant plot and trend interchangeability methods (TIM). In the 23 patients analyzed, the CVC was used as part of standard care with no complications. The correlation coefficient for absolute values (N = 99) was R = 0.91 (P < 0.001). The bias, precision and limits of agreement were − 9.1, 17.4 and − 43.2 to 24.9 mg/dL, respectively. The concordance rate for changes in blood glucose measurements (N = 77) was 85% with the four-quadrant plot. The TIM showed that 14 (18%) changes of blood glucose measurements were uninterpretable. Among the remaining 63 (82%) interpretable changes, 23 (37%) were interchangeable, 13 (20%) were in the gray zone, and 27 (43%) were not interchangeable. Microdialysis using a CVC appears to provide imprecise absolute blood glucose values with risk of insulin misuse. Moreover, only one third of changes in blood glucose measurements were interchangeable with the reference method using the TIM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Blood glucose level is a prognostic factor involved in morbi-mortality in surgical and intensive care setting [1], reflecting various mechanisms, including inflammation and insulin-resistance. The glycemic control concept was developed more than 15 years ago [2], and was initially associated with a reduction of mortality but also with a risk of hypoglycemia [1]. Indeed, an effective glycemic control results from the combination of a dynamic insulin administration protocol, repeated glucose concentration measurements, and nurse acceptance.
Capillary blood glucose monitoring is the most common method used at bedside, but this method could be inaccurate [3], time consuming and invasiveness. A subcutaneous continuous glucose measurement has been developed using completed automated closed-loop glucose control with contrasting results [4, 5]. Some studies demonstrated that subcutaneous continuous glucose device did not perform with satisfactory accuracy and feasibility in comparison with blood glucose measurements as reference method in intensive care patients [6,7,8], probably because the tissue perfusion can be altered.
While new intravascular sensors have been developed through a peripheral venous catheter; to date no system was available in routine care [9], because of difficulties in regulatory approval, thrombotic events at the sensor tip, unexpected drug interferences, and inconsistent manufacturer support [9]. Recently, the Eirus system (Maquet Critical Care, Solna, Sweden) was proposed using a central venous catheters (CVCs) and a microdialysis device with encouraging results [10,11,12,13,14,15]. Before recommending a wider used of this new monitoring for bedside, its accuracy must be further validated in independent work, with the trend interchangeability statistic method (TIM), as recently reported [16].
The objective of the present study, conducted in selected high-risk cardiac surgical patients for hyperglycemia, was to compare intravascular microdialysis blood glucose measurements with central venous blood sample measurements as the reference method. We tested the hypothesis that changes in blood glucose values determined by intravascular microdialysis using the TIM would be interchangeable with those measured by the reference method.
2 Methods
2.1 Compliance with ethical standards
2.1.1 Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
2.1.2 Informed consent
In agreement with French law for clinical research, as data were collected during routine care administered according to standard procedures currently used in our institution, the need for written informed consent was waivered. However, preoperative verbal consent was obtained from all study participants before surgery.
2.1.3 Ethical approval and registration
This study was approved by the Local Ethics Committee. (Reference A14-D36-VOL.22, CPP Nord Ouest III, Caen University Hospital, France (Chairman Dr. C. Gourio) on 21 October 2014). The study was registered in https://ClinicalTrials.gov (NCT02296593), and the study methodology complied with the STROBE Statement [17] and GRRAS guidelines [18]. All procedures performed in this study involving human participants were in accordance with the Ethical Standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
2.2 Study population
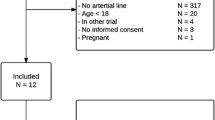
From January 2015 to July 2015, all consecutive adult high-risk cardiac surgical adult patients (i.e., with high-risk of stress hyperglycemia) were included preoperatively in the case of left ventricular ejection fraction < 30% and/or high-risk cardiac surgery (aortic dissection, double valve replacement, left ventricular assist device, left ventriculoplasty, endocarditis). An appointed anaesthesiologist acquired all data. Patients without high surgical risk, off-pump cardiac surgery (predictable short operating time), or for whom the investigator was not available, were not included. Patients with CVC misplacement (not positioned with the tip in the superior vena cava above the right atrium confirmed by echocardiography) were excluded from the study. As recommended by the manufacturer, blood glucose values higher than 360 mg/dL were not analyzed, as the Eirus device V2.0 cannot provide accurate values in this range (the monitor simply displays “> 360 mg/dL”).
2.3 Study procedure
In the operating room, after induction of general anesthesia, a radial arterial catheter and a right jugular CVC with an integrated microdialysis function (Eirus, Maquet Critical Care AB, Solna, Sweden) were placed by ultrasound guidance in all patients included in the study. The CVC was then connected to a dedicated monitor (Eirus V2.0, Maquet Critical Care AB, Solna, Sweden) via a specific microdialysis sensor, which converts biochemical data into numerical data (glucoseEirus). This specific five-lumen CVC used two dedicated lumens for the microdialysis system, and 3 lumens for usual drug administration. After automatic rinsing of the system with 0.9% sodium chloride solution, venous blood glucose measurements were calibrated with a blood sample obtained from the CVC (glucoseref) using a blood gas analyzer (ABL 800 Flex, Radiometer Medical, Copenhagen, Denmark), located adjacent to the operating room, with a biochemical method (oxygen electrode oxidation). Continuous blood glucose monitoring was then displayed on the monitor with a 5-min delay (corresponding to the transit time for microdialysis fluid from the microdialysis membrane to the microdialysis sensor). Only one calibration was performed because the study period was shorter than the calibration interval (8 h, required by the monitor). All patients were intubated, ventilated (volume-controlled regimen) and sedated with propofol and remifentanil to maintain a bispectral index between 40 and 60 during surgery.
2.4 Data processing
Demographic data (sex, age, height, weight, preoperative LVEF, history of systemic hypertension and/or diabetes mellitus and/or chronic obstructive pulmonary disease, EuroSCORE) [19] and type of surgery were recorded. One hour following calibration, the blood glucose measurement displayed on the monitor was recorded by a dedicated investigator (CG) 5 min after each central venous sample to take into account to the microdialysis time lag, and measurements were repeated every 30–60 min during surgery. The number of measurements was dependent on the surgical procedure and differed for each patient.
The repeatability of the blood gas analyzer, previously determined on 30 sets of measurements for one sample, based on the mean of three different samples, was calculated to be 2%.
2.5 Statistical analysis
Data are expressed as mean (standard deviation) (SD) or median (interquartile range) for not normally distributed variables (Kolmogorov–Smirnov test) or number (percentage), as appropriate. Correlations between absolute values of glucoseref and glucoseEirus during surgery were determined by linear regression, with calculation of the corresponding correlation coefficient. The bias, precision (SD of bias) and limits of agreement [bias (1.96 SD)] were evaluated using multiple observations per subject [20].
Changes in blood glucose measurements were evaluated with a four-quadrant plots with a calculated concordance rate (ratio of the number of changes in the same directions (i.e., in the top right and bottom left quadrants/all changes) [21], and the trend interchangeability method (TIM), recently described [16]. Briefly, this new method was designed to objectively define the interchangeability of each change of a variable. The first step of the TIM is to determine whether or not each variation is interpretable. A variation is considered as interpretable if the confidences intervals of the reference values (reference value ± reference value multiplied with the repeatability coefficient) of the two measurements (time 1 and time 2) do not overlap. A change between points 1 and 2 would then be interpretable (1 and 2 with non-overlapping confidences intervals) or uninterpretable (1 and 2 with overlapping confidences intervals). The second step of the TIM is to assess each change in measurements between two methods of measurement as interchangeable (the second point was in the interchangeability zone defined by using interchangeability lines), in a gray zone (only the repeatability of the second point was in the interchangeability zone), or not interchangeable (neither the second point nor its repeatability was not in the interchangeability zone) [16]. An interchangeability rate can then be calculated by the number of interchangeable changes divided by the total number of interpretable changes. A laboratory tolerance limit > 90% was a priori considered to be appropriate for the trend interchangeability rate between changes in glucoseref and glucoseEirus.
In line with the primary objective, we empirically considered that at least 80 changes in blood glucose measurements were needed to calculate a trend interchangeability rate. With at least 4 blood glucose changes per patient during the study period, a sample size of 20 patients was required. We decided to include an additional four patients to allow for possible technical problems.
A P-value less than 0.05 was considered statistically significant, and all P-values were two-tailed. Statistical analysis was carried out using MedCalc® Software bvba version 14.10.2 (Ostend, Belgium), and Excel version 14.4.8 (Microsoft Corporation, Redmond, Washington).
3 Results
Among the 288 screened patients during the study period, 39 were enrolled (239 did not have surgical high-risk surgery as previously defined, and 10 patients have off-pump cardiac surgery). Fifteen patients were not included because the investigator was not available, and 1 patient was excluded because of catheter misplacement. For the remaining 23 patients, the CVC was used for standard perioperative care. After CVC removal, no blood clotting was observed on the microdialysis membrane, and no CVC-related complications were reported. The study population is presented in Table 1.
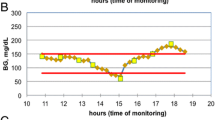
A total of 99 pairs of absolute blood glucose values were recorded, without pairs higher than 360 mg/dL. The mean value for the pairs of measurements was 4 ± 2 per patient (range 1–9). Mean glucoseref and glucoseEirus values ± SD [range] were 142.0 ± 39.2 [75.6–259.2] mg/dL and 151.1 ± 42.2 [76.0–257.1] mg/dL, respectively (P = 0.940). The correlation coefficient was r = 0.91 (95% CI 0.87–0.94) (P < 0.001), and the regression equation was Y = 11.45 + 0.98X (Fig. 1). The bias, precision and limits of agreement were − 9.1, 17.4 and − 43.2 to 24.9 mg/dL, respectively (Fig. 2). Two examples of individual measurements of glucoseref and glucoseEirus during the study period are showed in Fig. 3.
Seventy-seven pairs of changes of blood glucose measurements were collected with a mean of 3.5 ± 1.9 changes per patient (range 0–8). The four-quadrant plots presented changes of blood glucose values (Fig. 4). The calculated concordance rate was 95%. Using the TIM, 14 (18%) changes of blood glucose measurements were uninterpretable. Among the remaining 63 (82%) interpretable changes, 23 (37%) were interchangeable, 13 (20%) were in the gray zone, and 27 (43%) were not interchangeable (Figs. 4, 5).
Four-quadrant graphical representation between changes in absolute values of glucoseref and glucoseEirus (77 paired of data points) using TIM. A specific color is applied to each change: uninterpretable (blue), non-interchangeable (red), in the gray zone of interpretation (orange), and interchangeable (green). Ref reference method
4 Discussion
The main findings of this observational study conducted in high-risk cardiac surgical patients are as follows: (i) absolute blood glucose measurements obtained with the Eirus device were well-correlated with those obtained with the reference method with moderate agreement, (ii) although changes in blood glucose measurements as assessed with Eirus and the reference method showed an excellent concordance rate, changes between the two methods were poorly interchangeable with the TIM.
Blood glucose monitoring is useful in high-risk surgical and critically ill patients, and it has been described as an accurate marker closely correlated with morbidity [22, 23]. Elevated blood glucose can be the result of releasing of stress hormones (epinephrine, cortisol, glucagon), abdominal hormones (GLP-I and GIP), or central nervous system failure [24]. Some previous validation studies in humans compared static blood glucose values obtained via the microdialysis method and the reference method and reported similar results in terms of the correlation coefficient. Clinicians should be aware about a risk of over or under-measurement of blood glucose using Eirus device in comparison with reference method, because this can lead to a misuse of insulin with a risk of hypoglycaemia with deleterious effects.
Previous studies did not assess changes in blood glucose measurements, expect one using Clarke error grid [14], which has been described as an inaccurate method [25]. Because variation between two blood glucose measurements are informative about the efficacy of treatment, the present study compared variations in successive blood glucose measurements between microdialysis compared and the reference method. Repeated blood glucose measurements are recommended to avoid hypoglycaemia, and to reduce blood glucose variability during surgery or in ICU [24, 26]. Indeed, high glucose variability is associated with worse outcome [27]. In the present study, we used the TIM, a new method to objectively and specifically study the changes recorded by two techniques. The TIM complies with the GRRAS guidelines and appears to be more rigorous than previously published methods [15]. By analysing the repeatability of the reference method, we classified each change as uninterpretable or interpretable and then as either non-interchangeable, in the gray zone or interchangeable. An interchangeability rate (number of interchangeable changes divided by the total number of interpretable changes) can then be calculated. The TIM has also been described as a plug-and-play method by using free tools available online [16]. In contrast with the four-quadrant plots method or the Clarke error grid for changes, the TIM showed that microdialysis was interchangeable in one-third of interpretable blood glucose changes, which would appear to be insufficient for clinical practice. One explanation could be that the device was not sufficiently precise to follow the blood glucose variations and cannot be used at bedside. Another explanation could be related to specific technical limitations of the present study. Indeed, the validity of the present study is limited by the intraoperative period, and some specific factors of this type of surgery, such as CPB, mechanical contact of the microdialysis catheter and CPB canula, priming, reperfusion, or hypothermia could influence glucose measurements.
Some limitations of this study should be discussed. First, the study population consisted of few selected high-risk cardiac surgical patients, with only 77 changes in blood glucose measurements (which is under the 80 changes calculated a priori for the power of the present study). Other studies must be conducted during other types of surgery to assess the clinical value of microdialysis before recommending more extensive use of this technique. In particular, patients with unstable diabetes mellitus should be evaluated during the perioperative period. Second, we used venous blood for reference blood glucose measurements. The investigators considered this approach to be more reliable, as the CVC was inserted in the internal jugular vein and the comparison between microdialysis values and arterial blood samples could be considered to constitute a bias, which would decrease the internal validity of the study. Moreover, arterial and central venous blood glucose has been reported to be interchangeable [28]. Third, high blood glucose measurements (> 360 mg/dL) were not evaluated in this study, although the assessment of high blood glucose levels could be more clinically relevant. Last, phase three clinical utility/outcome studies are required to evaluate blood glucoseEirus monitoring and validate its clinical value for glycemic control or close-loop device using both insulin and dextrose.
In conclusion, microdialysis with an invasive CVC during the intraoperative period for high-risk cardiac surgery appears to provide imprecise absolute blood glucose values with risk of insulin misuse. Moreover, only one-third of changes in blood glucose measurements were interchangeable with the reference method, which means that this method must be used with caution. Further studies are mandatory before recommending this new device in routine clinical practice for high-risk surgical patients or critically ill patients.
References
NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY-S, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97.
van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67.
Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med. 2007;33:2079–84.
Yatabe T, Yamazaki R, Kitagawa H, et al. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive care unit patients. Crit Care Med. 2011;39:575–8.
Leelarathna L, English SW, Thabit H, et al. Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial. Crit Care 2013;17:R159.
Wollersheim T, Engelhardt LJ, Pachulla J, et al. Accuracy, reliability, feasibility and nurse acceptance of a subcutaneous continuous glucose management system in critically ill patients: a prospective clinical trial. Ann Intensive Care 2016;6:70.
van Hooijdonk RTM, Leopold JH, Winters T, et al. Point accuracy and reliability of an interstitial continuous glucose-monitoring device in critically ill patients: a prospective study. Crit Care 2015;19:34.
Boom DT, Sechterberger MK, Rijkenberg S, et al. Insulin treatment guided by subcutaneous continuous glucose monitoring compared to frequent point-of-care measurement in critically ill patients: a randomized controlled trial. Crit Care 2014;18:453.
Smith JL, Rice MJ. Why have so many intravascular glucose monitoring devices failed? J Diabetes Sci Technol. 2015;9:782–91.
Schierenbeck F, Owall A, Franco-Cereceda A, Liska J. Evaluation of continuous blood glucose monitoring system using a central venous catheter with an integrated microdialysis function. Diabetes Technol Ther. 2013;15:26–31.
Schierenbeck F, Franco-Cereceda A, Liska J. Evaluation of continuous blood glucose monitoring system using central venous microdialysis. J Diabetes Sci Technol. 2012;6:1365–71.
Blixt C, Rooyackers O, Isaksson B, Wererman J. Continuous on-line glucose measurement by microdialysis in a central vein. A pilot study. Crit Care 2013;17:R87.
Schierenbeck F, Franco-Cereceda A, Liska J. Accuracy of 2 different continuous glucose monitoring systems in patients undergoing cardiac surgery: intravascular microdialysis versus subcutaneous tissue monitoring. J Diabetes Sci Technol. 2017;11:108–16.
Leopold JH, van Hooijdonk RTM, Boshuizen M, et al. Point and trend accuracy of continuous intravenous microdialysis-based glucose-monitoring device in critically ill patients: a prospective study. Ann Intensive Care 2016;6:68.
Klonoff DC, Lias C, Vigersky R, et al. The surveillance error grid. J Diabetes Sci Technol. 2014;8:658–72.
Fischer MO, Diouf M, de Wilde RBP, Dupont H, Hanouz JL, Lorne E. Evaluation of cardiac output by 5 arterial pulse contour techniques using trend interchangeability method. Medicine (Baltim) 2016;95:e3530.
von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7.
Kottner J, Audigé L, Brorson S, et al. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64:96–106.
Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–22–23.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–82.
Perrino AC, Harris SN, Luther M. Intraoperative determination of cardiac output using multiplane transoesophageal echocardiography. A comparison to thermodilution. Anesthesiology 1998;89:350–7.
Ouattara A, Lecomte P, Le Manach Y, et al. Poor intraoperative blood glucose control is associated with a worsened hospital outcome after cardiac surgery in diabetic patients. Anesthesiology 2005;103:687–94.
Thiele RH, Hucklenbruch C, Ma JZ, et al. Admission hyperglycemia is associated with poor outcome after emergent coronary bypass grafting surgery. J Crit Care 2015;30:1210–6.
SFAR, SRLF. Formal recommendations by the experts. Glycemic control in intensive care unit and during anaesthesia. Ann Fr Anesth Reanim. 2009;28:410–5.
Wentholt IM, Hoekstra JB, Devries JH. A critical appraisal of the continuous glucose-error grid analysis. Diabetes Care 2006;29:1805–11.
Krinsley JS, Chase JG, Gunst J, et al. Continuous glucose monitoring in the ICU: clinical considerations and consensus. Crit Care 2017;21:197.
Bansal B, Carvalho P, Mehta Y, et al. Prognostic significance of glycemic variability after cardiac surgery. J Diabetes Complicat. 2016;30:613–7.
Skjaervold NK, Aadahl P. Comparison of arterial and mixed venous blood glucose levels in hemodynamically unstable pigs: implications for location of a continuous glucose sensor. Acta Diabetol. 2012;49:489–91.
Acknowledgements
The authors would like to thank Maquet Critical Care AB (Solna, Sweden) and especially Emmanuel Cheyron for providing all devices and monitoring equipment. The manufacturer provided no input to the design or conduct of the study or in the decision to submit the manuscript for publication.
Funding
This study was a standard care study and was not funded by any institution. All furniture (monitoring device and single-use material) was provided free of charge by Maquet Critical Care AB. The authors performed the present work in the course of their normal duties as full-time employees of public-sector healthcare institutions.
Author information
Authors and Affiliations
Contributions
Conception and design: MOF, JLF, SF, JLH, JLG; Data collection: CG, MOF; Data analysis: MOF, VS; Drafting the manuscript: CG, MOF, JLF and Revision of the manuscript after critical review: all authors.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fischer, MO., Gouëzel, C., Fradin, S. et al. Assessment of changes in blood glucose concentration with intravascular microdialysis. J Clin Monit Comput 32, 1135–1142 (2018). https://doi.org/10.1007/s10877-018-0111-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-018-0111-x