Abstract
Objective
To compare the accuracy of fingerstick with laboratory venous plasma glucose measurements (laboratory glucose) in medical ICU patients and to determine the factors which interfere with the accuracy of fingerstick measurements.
Participants
The study included 80 consecutive patients aged 58 ± 7 years, BMI 29.5 ± 9.0, and APACHE II score 15 ± 6 (277 simultaneous paired measurements).
Measurements
This prospective observational study compared fingerstick measurements to simultaneously sampled laboratory glucose once a day in patients in our medical ICU (twice daily if on an insulin infusion). Data recorded included patient demographics, admission diagnoses, APACHE II score, BMI, daily hematocrit, arterial blood gasses, chemistry results, concomitant medications (including vasopressors and corticosteroids), and upper extremity edema. Accuracy was defined as the percentage of paired values not in accord (> 15 mg dl–1 / 0.83 mmol–1 l–1 difference for laboratory values < 75 mg dl–1 / 4.12 mmol–1 l–1 and > 20% difference for laboratory values ≥ 75 mg/dl). Outliers (blood glucose difference > 100 mg dl–1 / 5.56 mmol–1 l–1) were excluded from the correlation and distribution analyses.
Results
Mean fingerstick glucose was 129 ± 45 mg/dl (7.2 ± 2.5 mmol/l) and mean laboratory glucose 123 ± 44 mg/dl (6.8 ± 2.4 mmol/l). The correlation coefficient between the two values was 0.9110 (Clinical and Laboratory Standards Institute threshold 0.9751). The mean difference (bias) between the two methods was 8.6 ± 18.6 mg/dl (0.48 ± 1.0 mmol/l) and limits of agreement +45.8 and –28.6 mg/dl (+2.5 and –1.6 mmol/l). Fifty-three (19%) paired measurements in 22 patients were not in accord (CLSI threshold ≤ 5%). In 44 (83%) of these paired measurements fingerstick glucose was greater than laboratory glucose.
Conclusions
The findings suggest that capillary blood glucose as measured by fingerstick is inaccurate in critically ill ICU patients and does not meet the CLSI standard. It is unclear whether the sampling method, device used, or both contributed to this inaccuracy. The wide limits of agreement suggest that fingerstick measurements should be used with great caution in protocols of tight glycemic control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2001 van den Berghe and coworkers published a landmark study which demonstrated that intensive insulin therapy improves the outcome of critically ill surgical patients [1]. Following this study tight glycemic control (target glucose 80–110 mg/dl) was rapidly adopted as the standard of care in intensive care units (ICUs) throughout the world. The study by van den Berghe and colleagues measured blood glucose on whole undiluted arterial blood using a blood gas analyzer. However, in many ICUs blood glucose is measured using capillary blood and point-of-care (POC) glucose meters (fingerstick glucose). POC glucose meters are approved for use in stable, ambulatory, diabetic patients but have not been specifically sanctioned for use in ICU patients on tight glycemic protocols. Emerging data suggest that fingerstick glucose measurements are inaccurate and tend to overestimate the actual, blood glucose levels in critically ill ICU patients [2–5]. This places the ICU patient at an increased risk of undetected hypoglycemia [2, 6]. This is a potentially serious problem as hypoglycemia is common in patients on tight glycemic protocols, and this complication has been associated with excess mortality [7].
The primary aim of this study was to determine the accuracy of fingerstick glucose measurements in critically ill ICU patients using the standards as defined by the Clinical and Laboratory Standards Institute (CLSI; formerly the National Committee for Clinical Laboratory Standards). Additionally, we sought to determine the clinical factors associated with inaccurate fingerstick glucose measurements. This study was presented in part at the Society of Critical Care Medicine's 36th Annual Critical Care Congress [8].
Methods and materials
This study was performed as part of a quality assurance project with the implementation of a computerized insulin infusion protocol in the Medical Intensive Care Unit (MICU) of Thomas Jefferson University Hospital, a 650-bed tertiary care teaching hospital in Philadelphia (USA). A computerized insulin infusion protocol was developed as a joint project between the Division of Critical Care Medicine (P.E.M.), Division of Endocrinology (S.J.), Departments of Nursing (A.C., C.A.), and Information Services. Due to concerns about the accuracy of fingerstick glucose measurements in ICU patients, the increased risk of hypoglycemia with “tight” glycemic protocols and controversy regarding the goals and benefits of tight glycemic control, we targeted a blood glucose of 100–140 mg/dl (5.6–7.8 mmol/l) [9]. In addition, patients were placed on insulin infusion only if their admission laboratory blood glucose was greater than 250 mg/dl (13.8 mmol/l), or if their blood glucose remained greater than 140 mg/dl after 24 h on a subcutaneous insulin sliding scale. This study was approved by the local institutional review board, and the insulin infusion protocol was approved by the hospital's Pharmacy and Therapeutic Committee. As this study was part of our approved insulin infusion protocol and did not involve any additional patient interventions, the need for informed consent was waived.
Over a 4-month period 80 consecutive patients were admitted to our MICU and were included in this prospective observational study (Table 1). Norepinephrine was used in 20 (25%) patients. Moderate to severe upper extremity edema was recorded in 34 (42%), and 39 (48%) were treated with vasopressors and/or had moderate/severe edema. According to ICU routine, laboratory venous plasma glucose (laboratory glucose) was measured on admission and daily (at 6 a.m.) in all ICU patients and repeated as clinically indicated. In patients on the insulin infusion protocol laboratory glucose was measured ever 12 h. The laboratory glucose is sent to the central laboratory as a “stat” specimen for immediate analysis. Our central laboratory measures plasma glucose using the glucose oxidase method (Beckman–Coulter LX-20, Brea, Calif., USA). The intra- and interassay coefficient of variation values for the test are 3% and 2%, respectively. The calibration material is traceable to standard reference materials from the United States National Institute for Standards and Technology.)
Fingerstick glucose is measured every 6 h in patients on a subcutaneous sliding scale and hourly in patients on the computerized insulin infusion protocol. During the study period fingerstick glucose was measured at the same time as venous blood draws for blood laboratory glucose. A total of 277 simultaneous fingerstick and laboratory glucose measurements were performed. Capillary blood samples were obtained from the patient's fingertip with a lancet device. Accu-Chek Comfort Curve test strips and two Accu-Check Inform (Roche Diagnostics, Mannheim, Germany) glucose monitors were used for fingerstick glucose measurements. The devices were calibrated daily; the coefficient of variation for the high control was 5.5% and that for the low control was 7.6%. Fingerstick glucose testing was performed by six medical technicians whose major clinical responsibility was that of POC glucose testing. The method of sample collection and measurement was standardized according to the recommendations of the CLSI [“Procedures and devices for the collection of diagnostic blood specimens by skin puncture,” approved standard 1999, 4th edition (document H4-A4): http://www.nccls.org, accessed 9 January 2007] and the manufacturer of the laboratory glucose meter [Roche Diagnostics, “Points to keep in mind for accurate glucose meter testing,” 2002 (document PT-358-14852)]. The POC technicians were trained and certified by our central laboratory and underwent annual recredentialing. The quality assurance program for POC testing was managed by our central laboratory who participates in the College of American Pathologists Proficiency testing program.
The following data were prospectively recorded on each patient; age, sex, admission diagnoses, body mass index (BMI), history of diabetes (type I or II), Acute Physiology and Chronic Health Evaluation (APACHE) II score (for first 24 h) [10], daily hematocrit (Hct), arterial blood gasses, serum chemistry results, concomitant medications (including vasopressors and steroids), and upper extremity edema. All routine laboratory tests were performed at 6:00 a.m. (the same time as the venous blood laboratory glucose). As norepinephrine is the predominant vasopressor used in our MICU, this was recorded as yes/no regardless of dose; corticosteroids were similarly recorded. Upper extremity edema was graded (daily) subjectively on a 0–3 scale as follows; 0 none, 1 trace/mild, 2 moderate, and 3 severe. This grading was based on the depth of depression of the skin of the forearm following uniform pressure applied by the thumb [11]. For consistency the grading was carried out by a single investigator (C.D.C.). According to the policy of the institutional review board and in compliance with privacy regulations, the data were deidentified and stored in an electronic spreadsheet (Excel 2003, Microsoft, Redmond, Wash., USA).
Data analysis
The CLSI has determined that “95% of individual results from POC laboratory glucose monitoring systems should agree within ± 15 mg/dl of the laboratory analyzer at laboratory glucose concentration below 75 mg/dl and within ± 20% of the laboratory analyzer values at laboratory glucose concentrations at or above 75 mg/dl” [“Point-of-care blood glucose testing in acute and chronic care facilities,” approved guideline 2002, 2nd edition (document C30-A2), CLSI: http://www.nccls.org, accessed 9 January 2007; “Glucose monitoring in settings without laboratory support,” approved guideline 2005, 2nd edition (vol. 25 no.12, AST4-A2), CLSI: http://www.nccls.org, accessed 9 January 2007; “Review criteria assessment of portable blood glucose monitoring in vitro diagnostic devices using glucose oxidase, dehydrogenase or hexokinase methodology,” 1997, United States Food and Drug Administration: http://www.fda.gov/cdrh/ode/gluc.html, accessed 8 January 2007]. We used these criteria to determine the percentage of paired fingerstick and laboratory glucose measurements that were in accord (difference within this range). The use of corticosteroids, vasopressors, and other medications and the presence of diabetes and a diagnosis of sepsis were recorded as 0 or 1 (binary value). Similarly, edema was classified as absent/mild (0) or moderate/severe (1).
Statistical analysis
Summary statistics were compiled to allow a description of the patient population and to compare the clinical and laboratory features of patients with results that were in accord or not in accord. Statistical analysis was performed using NCSS 2004 (Kaysville, Utah, USA). Agreement between the methods was determined using the method of Bland and Altman [12]. Bias between measurements was defined as the mean of the difference between measurements, precision as the standard deviation of the differences between measurements, and the 95% upper and lower limits of agreements as ± 2 SD from the mean bias. We used χ2 analysis to compare categorical data. Continuous data were compared using Student's t test. The Mann–Whitney U test was used for data that failed tests of normality. Pearson's correlation coefficient was used to determine the strength of association between fingerstick and laboratory measurements. The CLSI suggest that a correlation above 0.9751 is indicative of an equivalence to the laboratory standard. Outliers (blood laboratory glucose difference, > 100 mg dl– 1 5.6 mmol– 1 l– 1) were excluded from the correlation and distribution analyses.
Univariate and multivariate analyses were performed to determine the clinical and laboratory factors associated with results not in accord. The following variables were included in this analysis: age, sex, APACHE II score, sepsis (diagnosis), edema, vasopressor use, corticosteroid use, upper extremity edema, Hct, serum albumin, arterial pH, PaO2, and PaCO2. Unless otherwise stated, all data are expressed as mean ± standard deviation, with statistical significance declared for probability values of 0.05 or less.
Results
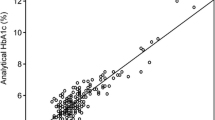
Mean fingerstick glucose was 129 ± 45 mg/dl (7.2 ± 2.5 mmol/l) and mean laboratory glucose 123 ± 44 mg/dl (6.8 ± 2.4 mmol/l) There were two outlying paired results (fingerstick glucose-laboratory glucose difference > 100 mg/dl). The correlation coefficient between the fingerstick and laboratory values was 0.911. The mean difference (bias) between the two methods was +8.6 ± 18.6 mg/dl (+0.48 ± 1.0 mmol/l), while the limits of agreement were +45.8 and –28.6 mg/dl (+2.5 and –1.6 mmol/l). Fingerstick measurements overestimated the laboratory value by 15.5 ± 12.2 mg/dl (0.86 ± 0.68 mmol/l) in 208 instances and underestimated them by 14.2 ± 19.7 mg/dl (0.79 ± 1.09 mmol/l) in 59. There were 53 (19%) paired measurements in 22 patients that were not in accord (CLSI threshold ≤ 5%). In 44 (83%) of these paired measurements the fingerstick value was greater than the laboratory value. Eleven patients had multiple discordant values.
According to Roche Diagnostics (“Points to keep in mind for accurate glucose meter testing,” 2002, document PT-358-14852) the acceptable Hct range for laboratory glucose measurements using Accu-Chek Comfort Curve test strips is between 20% and 55%. Nine paired samples in eight patients had a value below 20% (mean Hct of 16.4%); the mean difference between the measurement methods in these nine samples was +7.5 ± 5.3 mg/dl (+0.42 ± 0.29 mmol/l). None of the specimens had an Hct value greater than 55%, the highest being 49.6%.
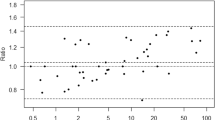
A scatterplot of the laboratory and fingerstick glucose measurements is shown in Fig. 1. A distribution plot showing the difference between the fingerstick and laboratory glucose vs. the reference standard (laboratory glucose) with NCIS accuracy thresholds is presented in Fig. 2. By univariate analysis only the use of a vasopressor agent (odds ratio 2.81, 95% confidence interval 1.5–5.4) and moderate/severe upper extremity edema (2.1, 1.05–4.19) were associated with an increased probability of results not in accord. On multivariate analysis only treatment with a vasopressor remained in the predictive equation. The correlation between fingerstick and laboratory glucose values was 0.936 in patients who were neither receiving norepinephrine nor had moderate/severe upper extremity edema.
Discussion
The results of our study demonstrate that fingerstick glucose as measured with the Accu-Chek point-of-care device is inaccurate in critically ill ICU patients and does not meet the standard as set by the CLSI. It is unclear, however, from our study whether the sampling method, device used, or both contributed to this inaccuracy. Our findings are particularly concerning as they demonstrate that fingerstick measurements tend to overestimate the reference standard. This shows that episodes of hypoglycemia may be missed in patients on tight glycemic protocols. This is particularly important as ICU patients are frequently sedated and/or intubated and may not be able to communicate symptoms of hypoglycemia. This finding is particularly important as hypoglycemia is a frequent complication in patients on tight glycemic protocols and is associated with an increased mortality [1, 6, 7].
The results of our study are supported by those Kanji et al. [2] and other investigators [3–5, 13, 14]. Kanji and colleagues compared capillary and arterial blood glucose measured with a glucose meter (Accu-Check Inform) with a reference standard in 30 ICU patients (10 vasopressor dependent, 10 with edema, and 10 postsurgical). In this study the overall agreement of capillary blood with the laboratory reference was only 56%, being 26.3% in patients with hypoglycemia. As in our study, fingerstick measurements tended to overestimate the reference standard. Furthermore, in their study the accuracy of fingerstick as compared to the reference standard was equally poor in vasopressor dependent, edematous, and postsurgical patients. In a retrospective study Finkielman and colleagues [4] compared fingerstick (SureStepFlexx; LifeScan, New Brunswick, N.J., USA) with laboratory glucose measurements in a heterogeneous group of ICU patients. In this study the bias was +7.9 mg/dl (0.43 mmol/l) and precision 17.6 mg/dl (0.98 mmol/l) with the limits of agreement being +43.1 and –27.2 mg/dl (+2.39 and –1.51 mmol/l). These results are remarkably similar to our findings.
Chakravarthy and colleagues [3] compared fingerstick measurements (Accu-Check Inform) with their laboratory reference standard in 21 hypotensive patients; fingerstick measurements were higher than laboratory values by a mean of 20.9 mg/dl (16.9%). Atkins and colleagues [13] compared fingerstick (Accu-Check II glucose monitor) with laboratory glucose measurements in 25 severely hypotensive patients; only 36% of patients had fingerstick measurements within 20% of the laboratory value. Similarly, Sylvain and colleagues [14] compared fingerstick (One Touch II glucose meter, Lifescan) with laboratory glucose in 38 patients (27 receiving vasopressor agents) with “poor tissue perfusion” and found a 30% variance between the two methods with a mean difference of 77 mg/dl.
We found that the use of a vasopressor agents (poor peripheral perfusion) and upper extremity edema (either of which were present in 48% of our patients) were associated with results not in accord. This finding is not surprising considering the effect these conditions have on the “quality” of capillary blood. However, the correlation between fingerstick and laboratory glucose measurements remained poor (0.936) after exclusion of these patients. This suggests that it is be possible to predict which patients are likely to have inaccurate fingerstick glucose measurements.
Although the measurement of glucose is one of the oldest established tests in clinical medicine, it is extremely complex and often only an approximation of the “true” level [15, 16]. Blood glucose levels measured from different sites, using different fractions of blood and different methods provide different results. Glucose measurements can be performed on whole-blood, plasma, and serum, and these may be native or deproteinized or hemolyzed in the case of capillary whole blood. Furthermore, the blood may be arterial, capillary, or venous in origin. Glucose is dissolved only in the aqueous part of the drawn specimen and not in its entire volume. This is the major reason for differing glucose concentration in plasma and whole-blood samples [15]. The glucose concentration is approx. 10% higher when measured in plasma as compared to whole blood. This difference is more marked in patient with higher Hct [17]. A number of glucose meters correct for this difference and give results as “plasma equivalent.” Other blood glucose strips retain red blood cells through a filtering process and measure glucose content in plasma in their reaction zone (e.g., Accu-Chek Comfort Curve test, Roche Diagnostics). Even the Yellow Springs Instrument's Blood Glucose analyzer, which is considered the reference gold standard, yields laboratory glucose results dependent on Hct when whole-blood samples are used [18]. Furthermore, if laboratory glucolysis is not inhibited in whole-blood specimens the laboratory glucose levels fall with delays in processing the specimen [19]. The method of glucose measurement used by POC meters in routine clinical use are based on either chromogenic or electrochemical reactions of the three enzymes glucose oxidase, dehydrogenase, and hexokinase. This gives rise to method-based specific interferences such as blood and ambient (altitude) oxygen tension, blood pH, and serum cholesterol and triglycerides levels and interfering drugs (maltose, d-xylose, icodextrin, dopamine, acetaminophen) [20, 21] (Roche Diagnostics, “Accu-Chek blood glucose monitoring systems-reminder of potential for falsely elevated blood glucose due to drug interferences,” 7 September 2006: http://www.accu-chek.com/us/rewrite/generalContent/en_US/article/ACCM_general_article_3586.htm 06-204, accessed 14 January 2007). Due to the interaction of all these factors “blood” glucose measurements performed with different sampling methods and measurement methods do not agree perfectly. The NCIS has therefore set limits by which different measurement techniques may differ; 95% of readings should agree within ± 15 mg/dl of the laboratory analyzer at glucose concentrations below 75 mg/dl and within ± 20% of the laboratory analyzer values at glucose concentrations at or above 75 mg/dl and the correlation between the two methods should be above 0.9751.
Despite the fact that the accuracy of fingerstick glucose measurements in ICU patients has not been established, this test is used in many ICUs throughout the world (Roche Diagnostics, “Points to keep in mind for accurate glucose meter testing,” 2002, document PT-358-14852). Based on the results of our study and reports by other investigators [2–5, 13, 14] we believe that fingerstick glucose measurements should be interpreted with caution in critically ill ICU patients, and that this technology may not be ideal for managing patients on tight glycemic protocols. As demonstrated by Kanji and colleagues [2], the inaccuracy of fingerstick measurements results in inappropriate adjustments of the insulin infusion rate in a large percentage of patients on a tight glycemic protocol. More importantly, inaccurate fingerstick measurements may lead to untreated hypoglycemic episodes.
Our study is limited by the fact that none of our patients had a true hypoglycemic episode (laboratory glucose < 40 mg/dl), and the performance of fingerstick measurements at this level could therefore not be assessed. However, as is evident from Fig. 2, the differences between fingerstick and laboratory values were similar across the entire range of measurements, making it unlikely that fingerstick measurements would be more accurate in the hypoglycemic range. It should be noted that our study as well as in those of Kanji et al. [2] and Chakravarthy et al. [3] used the Accu-Check Inform glucose meter for the fingerstick measurements. It is possible that different results would be obtained using other POC glucose meters. However, studies using the SureStepFlexx and One Touch II glucose meters produced results similar to those of our study [4, 14].
In conclusion, our study demonstrates that fingerstick glucose as measured with the Accu-Chek POC meter in a cohort of critically ill ICU patients did not meet the regulatory requirements for accuracy, and therefore this measurement technique and/or glucose meter should be used with great caution in patients on tight glycemic protocols. It is likely that fingerstick glucose measurements using the Accu-Chek POC meter may result in episodes of undetected hypoglycemia and inappropriate adjustments to insulin infusion rates.
References
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345:1359–1367
Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC (2005) Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med 33:2778–2785
Chakravarthy SB, Markewitz BA, Lehman C, Orme JF (2005) Accuracy of glucose measurements in critically ill patients in shock (abstract). Chest 128:220S
Finkielman JD, Oyen LJ, Afessa B (2005) Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest 127:1749–1751
Kulkarni A, Saxena M, Price G, O'Leary MJ, Jacques T, Myburgh JA (2005) Analysis of blood glucose measurements using capillary and arterial blood samples in intensive care patients. Intensive Care Med 31:142–145
Vriesendorp TM, van Santen S, DeVries JH, de Jonge E, Rosendaal FR, Schultz MJ, Hoekstra JB (2006) Predisposing factors for hypoglycemia in the intensive care unit. Crit Care Med 34:96–101
van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, van Wijngaerden E, Bobbaers H, Bouillon R (2006) Intensive insulin therapy in the medical ICU. N Engl J Med 354:449–461
Critchell C, Callahan A, Aboud C, Jabbour S, Marik PE (2006) Accuracy of bedside capillary blood glucose measurements in critically ill patients (abstract). Crit Care Med 34(Suppl A):68
Marik PE, Varon J (2007) Intensive insulin therapy in the ICU: is it now time to jump off the bandwagon? Resuscitation 2007:191–193
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–828
Szczesny G, Olszewski WL (2003) The pathomechanism of posttraumatic edema of the lower limbs. II. Changes in the lymphatic system. J Trauma 55:350–354
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet I:307–310
Atkin SH, Dasmahapatra A, Jaker MA, Chorost MI, Reddy S (1991) Fingerstick glucose determination in shock. Ann Intern Med 114:1020–1024
Sylvain HF, Pokorny ME, English SM, Benson NH, Whitley TW, Ferenczy CJ, Harrison JG (1995) Accuracy of fingerstick glucose values in shock patients. Am J Crit Care 4:44–48
Brunkhorst FM, Wahl HG (2006) Blood glucose measurements in the critically ill: more than just a blood draw. Crit Care 10:178
Wiener K (1995) Whole blood glucose: what are we actually measuring? Ann Clin Biochem 32:1–8
Sidebottom RA, Williams PR, Kanarek KS (1982) Glucose determinations in plasma and serum: potential error related to increased hematocrit. Clin Chem 28:190–192
Fogh-Anderson N, D'Orazio P (1998) Proposal for standardizing direct-reading biosensors for blood glucose. Clin Chem 44:655–659
Sazama K, Robertson EA, Chesler RA (1979) Is antiglycolysis required for routine glucose analysis? Clin Chem 25:2038–2039
Tang Z, Louie RF, Lee JH, Lee DM, Miller EE, Kost GJ (2001) Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med 29:1062–1070
Tang Z, Du X, Louie RF, Kost GJ (2000) Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol 113:75–86
Acknowledgements
The authors are indebted to the dedication of the ICU nurses and medical technicians whose dedication made this project possible. We also acknowledge Dr. Vidya Choudary who assisted with data collection. The authors have no financial interest in any of the products mentioned in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Critchell, C.D., Savarese, V., Callahan, A. et al. Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med 33, 2079–2084 (2007). https://doi.org/10.1007/s00134-007-0835-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0835-4