Abstract
An increasing number of NIRS devices are used to provide measurements of peripheral tissue oxygen saturation (StO2). The aim of the present study is to test the hypothesis that despite technological differences between devices, similar trend values will be obtained during a vascular occlusion test. The devices compared are NIRO-200NX, which measures StO2 and oxyhemoglobin by spatially resolved spectroscopy and the Beer–Lambert law, respectively, and INVOS 5100C and Foresight Elite, which both measure StO2 with the Beer–Lambert law, enhanced with the spatial resolution technique. Forty consenting adults scheduled for CABG surgery were recruited. The respective sensors of the three NIRS devices were applied over the brachioradial muscle. Before induction of anesthesia, 3 min of ischemia were induced by inflating a blood pressure cuff at the upper arm, whereafter cuff pressure was rapidly released. Tissue oxygenation measurements included baseline, minimum and maximum values, desaturation and resaturation slopes, and rise time. Comparisons between devices were performed with the Kruskal–Wallis test with post hoc Mann–Whitney pairwise comparisons. Agreement was evaluated using Bland–Altman plots. Oxyhemoglobin measured with NIRO responded faster than the other NIRS technologies to changes in peripheral tissue oxygenation (20 vs. 27–40 s, p ≤ 0.01). When comparing INVOS with Foresight, oxygenation changes were prompter (upslope 311 [92–523]%/min vs. 114[65–199]%/min, p ≤ 0.01) and more pronounced (minimum value 36 [21–48] vs. 45 [40–51]%, p ≤ 0.01) with INVOS. Significant differences in tissue oxygen saturation measurements were observed, both within the same device as between different devices using the same measurement technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Near-infrared spectroscopy (NIRS) is increasingly utilized to monitor peripheral tissue oxygenation and to assess microvascular reactivity [1]. Currently available NIRS devices use different technologies and computational algorithms to generate NIRS values [2]. Although it is acknowledged that values obtained by different devices are not interchangeable, it is expected that similar trend values are yielded [3, 4]. However, to date, a direct comparison of trend values obtained with the different technologies is absent.
NIRO (Hamamatsu Photonics, Tokyo, Japan) provides registration of tissue oxygen saturation (TOI, Tissue Oxygenation Index) and concentration change in oxyhemoglobin (O2Hb) by simultaneously integrating spatially resolved spectroscopy (SRS) and the modified Beer–Lambert law (MBL). The SRS technique is based on gathering the light signals at multiple closely spaced detectors. Since the depth of photon penetration is proportional to the source-detector distance, analyzing the differential signal as a function of this distance provides assessment of changes in oxygen saturation occurring in deep tissue layers (presented as TOI, expressed in %).
The MBL law states that the quantity of light absorbed by a substance is directly proportional to the absorption coefficient of the substance, the concentration of the substance, and the path length of the light, with a correction factor for scatter loss. By means of the MBL technology, NIRO provides a measurement of the concentration change in oxyhemoglobin relative to an arbitrary initial value. This value is presented as O2Hb and expressed in µM.
INVOS (Covidien, Mansfield, MA) and Foresight (CAS Medical Systems, Branford, CT, USA) also use MBL technology to assess tissue oxygen saturation (StO2). However, in addition, INVOS and Foresight account for superficial tissue by using differentially spaced light detectors which incorporate the principle of spatial resolution (SR). The closer receiver will measure more superficial tissue while the distal optode measures both superficial and deeper tissue. After subtraction of the interference from superficial tissues, oxygenation in the deeper tissues is derived.
The aim of the present investigation is to compare the assessment of tissue oxygen saturation changes using different NIRS technologies (SRS vs. MBL vs. MBL/SR). In order to explore this research question, changes in StO2 were induced in a standardized manner by means of a vascular occlusion test (VOT). Our hypothesis was that despite the technological differences, similar trend values would be obtained.
2 Materials and methods
This is a prospective, observational study. The data were acquired as part of a research project investigating the effects of anesthesia and cardiopulmonary bypass on microvascular reactivity.
After approval by the Institutional Ethics Committee of the Ghent University Hospital (ref: 2013/1085) and after obtaining their written informed consent, 40 adult patients (33 males/7 females, mean age 66 ± 9 year) scheduled for elective coronary artery bypass grafting (CABG) surgery were recruited. Exclusion criteria were a left ventricular ejection fraction <25%, diabetes, renal insufficiency (blood creatinine >2.0 mg/dl), significant hepatic disease (liver function tests >3× upper limit of normal), history of cerebrovascular disease, significant carotid artery stenosis (>60%), perioperative use of corticosteroids, and need for vasopressor or inotropic therapy before surgery.
All subjects needed to fasten at least 6 h prior to anesthesia and were asked to refrain from nicotine. On the morning of surgery, patients were allowed to take their routine medication, except for angiotensin-converting enzyme inhibitors and angiotensin II antagonists. Patients were premedicated with oral diazepam (5–10 mg). Standard monitoring included ECG, pulse oximetry and bispectral index (BIS XP A-2000™, Covidien, Mansfield, MA, USA). Arterial blood pressure was recorded continuously via a right radial artery fluid-filled catheter. Three disposable NIRS sensors (Foresight Elite, INVOS 5100C, and NIRO-200NX) were applied to the left forearm in a circumferential orientation (over the brachioradialis muscle, ~5 to 10 cm distal from the proximal head of the radius).
Before induction of anesthesia, changes in tissue oxygenation were induced in a standardized manner by performing a VOT. Arterial occlusion was achieved by inflating a standard blood pressure cuff (EH50U, Siemens) at the left upper arm to a pressure of 50 mmHg above the individual systolic pressure of each subject. The cuff was automatically inflated in less than 2 s to the pressure needed for arterial occlusion. After 3 min of ischemia, cuff pressure was rapidly released and StO2 response was recorded until it stabilized at the baseline value.
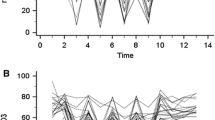
The following variables of the VOT were used for analysis (Fig. 1) [5]:
Graphic representation of changes in tissue oxygen saturation (StO2) during the vascular occlusion test. BL baseline. Modified from Bezemer et al. [5]
-
1.
Baseline StO2 (%),
-
2.
Downslope, desaturation rate from baseline until nadir
-
3.
Minimum StO2 (%),
-
4.
Maximum StO2 (%),
-
5.
Upslope, resaturation rate from minimum until maximum value,
-
6.
Rise time (s), time from cuff release to maximum value.
The baseline, minimum and maximum values are considered as static parameters. The downslope, upslope and rise time are time-related dynamic parameters.
2.1 Statistics
Statistical analysis was performed using the statistical software SPSS Statistics 23 (SPSS Inc., Chicago, IL). The raw data were tested for normality using the Shapiro–Wilk test. All data were non-parametric, and are presented as median [IQR].
O2Hb reflects a concentration change relative to an arbitrary initial value and is expressed in µM (=10−6 mol/l), whereas the TOI and StO2 variables are expressed in %. To adjust for the difference in the raw scale units of measurements between devices, the raw unit measures of each data point were converted to standard deviation units (Z-scores), according to the formula:
All data are then normalized to a common scale with an average of zero and standard deviation of 1, allowing the probability estimation that two different categories of data are significantly related.
Comparisons between devices were performed with the Kruskal–Wallis test. Pairwise differences were examined by the Mann–Whitney U-test. Bland–Altman plots were constructed, and linear regression analysis of differences on averages was performed in order to test the presence of proportional bias between measurement methods. The level of statistical significance was set at 2-sided p value 0.015, considering a post hoc Bonferroni correction with denominator 3.
3 Results
All 40 consenting patients were included in the study. Data of seven patients were incomplete for Foresight (n = 33), of two patients for INVOS (n = 38) and of one patient for NIRO (n = 39).
3.1 Comparison between values obtained by NIRO with SRS (TOI) versus MBL (O2Hb)
The values of TOI and O2Hb are presented in Table 1. Since O2Hb reflects a concentration change relative to an arbitrary initial value, no absolute data are provided for the static parameters of O2Hb. All dynamic variables significantly differed between the two measurement technologies (Table 1, statistical differences between TOI and O2Hb are indicated with *). As apparent from the rise time, O2Hb is a faster responder to oxygenation changes than TOI (p < 0.001).
For the downslope and upslope variables, the raw unit measures were converted to Z-scores to allow analysis of the Bland–Altman plot. The plots revealed wide limits of agreement (Fig. 2).
3.2 Comparison between values obtained by MBL/SR (StO2 by Foresight/INVOS) versus SRS (TOI by NIRO)
The values obtained by the different devices are presented in Table 1. Statistical differences between StO2 and TOI are indicated with § (Table 1). The downslope and upslope were significantly faster and the minimum StO2 value was significantly lower for INVOS compared to TOI. The rise time is significantly slower for Foresight.
Bland–Altman analysis demonstrated clinically acceptable differences for the downslope, however with wide limits of agreement (bias −2.0 ± 4.9%/min, limits of agreement −12 to 8%/min for Foresight vs. NIRO, and bias 3.6 ± 5.2%/min, limits of agreement −7 to 14%/min for INVOS vs. NIRO). For the upslope, the bias was clinically unacceptable with wide limits of agreement (bias −48 ± 121%/min, limits of agreement −286 to 190%/min and bias 196 ± 262%/min, limits of agreement −318 to 710%/min for Foresight vs. NIRO and INVOS vs. NIRO, respectively) (Fig. 3).
3.3 Comparison between values obtained by MBL/SR (StO2 by Foresight/INVOS) versus MBL (O2Hb by NIRO)
The dynamic data were all significantly different when comparing O2Hb (measured by NIRO) with StO2 measured with Foresight, whereas only the upslope was significantly different between NIRO and INVOS (Table 1, statistical differences between StO2 and O2Hb are indicated with #).
The Bland–Altman plots (converted to Z-scores) showed wide limits of agreement (Fig. 4).
3.4 Comparison between values obtained by MBL/SR: Foresight versus INVOS
Despite having the same measurement technology, INVOS and Foresight differed significantly in all variables, except the baseline and maximum value (Table 1, statistical differences are indicated with $).
Bland–Altman plots are presented in Fig. 5. Linear regression analysis of differences on averages was performed in order to test the presence of proportional bias between the two measurement methods. With the exception of the maximum value and the rise time, a proportional bias was revealed in all variables, that is, the difference in values changed in proportion to the change in average values (Fig. 5). The proportional bias in the baseline and minimum plots indicates that the range of StO2 values is significantly larger with INVOS compared to Foresight. For the downslope and upslope plots, the difference between Foresight and INVOS increases when the slopes become faster, indicating that INVOS responds more rapidly to changes in StO2 than Foresight.
4 Discussion
In the present study, three different NIRS measurement technologies (SRS, MBL, MBL/SR) were compared during changes in peripheral tissue oxygenation induced by a vascular occlusion test. In contrast to our hypothesis, different data were obtained, both when compared within the same device, as when compared within similar measurement technologies. O2Hb measured with NIRO demonstrated to be the fastest responder to oxygenation changes. The response to oxygenation changes was prompter and more pronounced for INVOS compared to Foresight, despite having identical measurement technology.
The differences in acquired values might be explained by several factors.
First of all, NIRS devices use different optical technologies to generate data. INVOS 5100 features two, NIRO 200-NX features three and Foresight Elite features five wavelengths of near-infrared light. Theoretically, more wavelengths should lead to greater accuracy and enhanced tissue recognition. Also the penetration depth of the light might differ depending on the wavelength and intensity of the emitted light, the sensitivity of the light detector and the spacing between the light emitter and light detectors [5, 8].
Secondly, the applied computational algorithms to derive the oxygen saturation values differ between the devices. Algorithmic formulae are complex and it is known that their validity is contingent on the assumptions made [9]. It should be reminded that all NIRS methods to evaluate tissue oxygenation are indirect, requiring various assumptions that may or may not hold true in various conditions, and none are free from error. Clarifying this issue would require access to the raw optical data and exact algorithms and calibrations which are now kept secret by the different companies.
And last but not least, the measurement technologies between devices differ. Although it might be expected that despite different measurement technologies, similar trend values would be obtained, the present study indicates that this doesn’t hold true.
We compared measurements simultaneously obtained with the same NIRS device (NIRO), but with either the SRS (TOI) or MBL (O2Hb) technology. Since the measurements were obtained from the same sample site, the same inter-optode spacing, the same light emitters, and the same light wavelengths, a number of confounding factors were excluded. Nevertheless, our data revealed that oxygenation changes were detected more promptly with the MBL technology than with SRS. Previous studies have compared SRS and MBL by focusing on blood volume indicators [6, 7]. These studies demonstrated that SRS was more sensitive to changes occurring in deep tissue layers, whereas MBL was more affected by cutaneous circulation.
In the INVOS and Foresight devices, the MBL technology is enhanced with SR, to correct for superficial tissue. When comparing the MBL (O2Hb) with the MBL/SR (INVOS and Foresight) technology, again oxygenation changes were reflected more quickly with MBL than with MBL/SR. Although intuitively one might suggest that a faster rate of oxygenation changes implies better detection, it has to be considered that this might as well indicate a more pronounced cutaneous contamination. MBL does not correct for superficial tissue, in contrast to the SRS and MBL/SR technologies, and therefore it could be assumed that the fast responses of O2Hb compared to TOI and StO2 reflect responses in the vasomotor tone of the skin rather than in the muscle capillary beds, which are the regions of interest. Especially in situations with substantial alterations in skin blood flow, such as with temperature changes or anesthetic/vasoactive drug use, this limitation should be acknowledged by clinical care givers.
In the present study, measurements obtained with the same technology (MBL/SR), but with different devices (Foresight and INVOS) were also compared. Despite having the same measurement technology, the data differed significantly and a distinct proportional bias was observed. The proportional bias in the downslope and upslope plots indicates that INVOS responds faster to changes in tissue oxygenation than Foresight. This is in accordance with one of our previous findings, where it was demonstrated that the response of INVOS to acute hemodynamic alterations was prompter and more pronounced compared to Foresight [10]. Also comparisons with other devices during VOT have reported more rapid changes with INVOS [11, 12]. The actual sources of these differences remain to be elucidated. It could be argued that INVOS data show a greater variability due to less accurate measurement technology or due to more pronounced cutaneous contamination, or alternatively, Foresight shows less variability because of a more marked signal attenuation technology, providing tissue oxygenation values that do not readily reflect true physiological changes. Since no real reference value exists for StO2, it is currently not possible to state if one measurement is more valid than another.
As can be noted from Fig. 1 and Table 1, maximum values after release of the cuff are higher than baseline values. This phenomenon is known as the hyperemic response or post-occlusive reactive hyperemia (PORH). Inducing a short period of ischemia releases endogenous nitric oxide from the microvascular endothelium, dilating precapillary arterioles [13]. Subsequent reperfusion (after release of the cuff) favours opening of previously closed capillaries (recruitment) and increases blood flow in previously patent capillaries, creating a transient increase in blood flow to a level higher than at resting state. This phenomenon is typical for VOT and can be used as a surrogate for assessment of microvascular integrity [14]. As expected, all patients had a PORH response (Table 1).
Although we did not check the repeatability of the measurements in the present study, other studies have [3, 15]. They tend to show better repeatability for Foresight and NIRO compared to INVOS. However, as discussed before, also in repeated measurements the question remains if a good repeatability implies a more accurate measurement technology or on the contrary, a more pronounced signal attenuation technology.
The measurements in the present study were performed on the forearm. We prefer this measurement site because physiologically the forearm is a predominant place for vasoconstriction in case of circulatory distress. So the vascular response will be altered sooner and more intensely [16]. However, in clinical practice, VOT measurements are also often applied on the deltoid muscle or the thenar muscle. It is not clear how the findings from the present study can be translated to other measurement sites.
To conclude, significant differences in absolute values and dynamic measurements were revealed both within the same device as between different devices using the same measurement technology. The results from the present study indicate that NIRS data are not only dependent on the measurement technology per se, but that the generated NIRS values are also determined by other factors, such as the optical technology and the computational algorithm. The respective extent of each of these components in the calculation of the NIRS data remains to be elucidated. Until standardized NIRS technologies and algorithms are used, NIRS devices cannot be used interchangeably.
References
Scheeren TW. Journal of Clinical Monitoring and Computing 2015 end of year summary: tissue oxygenation and microcirculation. J Clin Monit Comput. 2016;30:141–6.
Moerman A, Wouters P. Near-infrared spectroscopy (NIRS) monitoring in contemporary anesthesia and critical care. Acta Anaesth Belg. 2010;61:185–94.
Hyttel-Sorensen S, Hessel TW, Greisen G. Peripheral tissue oximetry: comparing three commercial near-infrared spectroscopy oximeters on the forearm. J Clin Monit Comput. 2014;28:149–55.
Hyttel-Sorensen S, Sorensen LC, Riera J, Greisen G. Tissue oximetry: a comparison of mean values of regional tissue saturation, reproducibility and dynamic range of four NIRS-instruments on the human forearm. Biomed Opt Express. 2011;2:3047–57.
Bezemer R, Lima A, Myers D, Klijn E, Heger M, Goedhart PT, Bakker J, Ince C. Assessment of tissue oxygen saturation during a vascular occlusion test using near-infrared spectroscopy: the role of probe spacing and measurement site studied in healthy volunteers. Crit Care. 2009;13(Suppl 5):S4.
Messere A, Roatta S. Influence of cutaneous and muscular circulation on spatially resolved versus standard Beer–Lambert near-infrared spectroscopy. Physiol Rep. 2013;1(e00179):1–10.
Canova D, Roatta S, Bosone D, Micieli G. Inconsistent detection of changes in cerebral blood volume by near infrared spectroscopy in standard clinical tests. J Appl Physiol. 2011;110:1646–55.
Mansouri C, L’Huillier JP, Kashou NH, Humeau A. Depth sensitivity analysis of functional near-infrared spectroscopy measurement using three-dimensional Monte Carlo modelling-based magnetic resonance imaging. Lasers Med Sci. 2010;25:431–8.
Metz AJ, Biallas M, Jenny C, Muehlemann T, Wolf M. The effect of basic assumptions on the tissue oxygen saturation value of near infrared spectroscopy. Adv Exp Med Biol. 2013;765:169–75.
Moerman A, Vandenplas G, Bové T, Wouters P, De Hert S. Relation between mixed venous oxygen saturation and cerebral oxygen saturation measured by absolute and relative near-infrared spectroscopy during off-pump coronary artery bypass grafting. Br J Anaesth. 2013;110:258–65.
Lee JH, Park YH, Kim HS, Kim JT. Comparison of two devices using near-infrared spectroscopy for the measurement of tissue oxygenation during a vascular occlusion test in healthy volunteers (INVOS® vs. InSpectra™). J Clin Monit Comput. 2015;29:271–8.
Fellahi JL, Butin G, Fischer MO, Zamparini G, Gérard JL, Hanouz JL. Dynamic evaluation of near-infrared peripheral oximetry in healthy volunteers: a comparison between INVOS and EQUANOX. J Crit Care. 2013;28:881.e1–6.
Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270:H1435–40.
Tripodaki ES, Tasoulis A, Koliopoulou A, Vasileiadis I, Vastardis L, Giannis G, Argiriou M, Charitos C, Nanas S. Microcirculation and macrocirculation in cardiac surgical patients. Crit Care Res Pract. 2012;2012:654381.
Pocivalnik M, Pichler G, Zotter H, Tax N, Müller W, Urlesberger B. Regional tissue oxygen saturation: comparability and reproducibility of different devices. J Biomed Opt. 2011;16(5):1–5.
Scheeren TWL, Schrober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput. 2012;26:279–87.
Acknowledgements
This work was supported by the Department of Anesthesiology, Ghent University Hospital, Ghent, Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.M. has received lecture fees from Medtronic (INVOS) and Sorin (NIRO). None of the other authors have a conflict of interest.
Rights and permissions
About this article
Cite this article
Steenhaut, K., Lapage, K., Bové, T. et al. Evaluation of different near-infrared spectroscopy technologies for assessment of tissue oxygen saturation during a vascular occlusion test. J Clin Monit Comput 31, 1151–1158 (2017). https://doi.org/10.1007/s10877-016-9962-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9962-1