Abstract
Measurement of intracranial pressure (ICP) is necessary in many neurological and neurosurgical diseases. To avoid lumbar puncture or intracranial ICP probes, non-invasive ICP techniques are becoming popular. A recently developed technology uses two-depth Doppler to compare arterial pulsations in the intra- and extra-cranial segments of the ophthalmic artery for non-invasive estimation of ICP. The aim of this study was to investigate how well non-invasively-measured ICP and invasively-measured cerebrospinal fluid (CSF) pressure correlate. We performed multiple measurements over a wide ICP span in eighteen elderly patients with communicating hydrocephalus. As a reference, an automatic CSF infusion apparatus was connected to the lumbar space. Ringer’s solution was used to create elevation to pre-defined ICP levels. Bench tests of the infusion apparatus showed a random error (95 % CI) of less than ±0.9 mmHg and a systematic error of less than ±0.5 mmHg. Reliable Doppler signals were obtained in 13 (72 %) patients. An infusion test could not be performed in one patient. Thus, twelve patients and a total of 61 paired data points were studied. The correlation between invasive and non-invasive ICP measurements was good (R = 0.74), and the 95 % limits of agreements were −1.4 ± 8.8 mmHg. The within-patient correlation varied between 0.47 and 1.00. This non-invasive technique is promising, and these results encourage further development and evaluation before the method can be recommended for use in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multimodal measurement of physiological data is a cornerstone in neuro-intensive care. The most-used neuro-specific parameter is intracranial pressure (ICP) [1], most often measured through intra-parenchymal pressure sensors, ventriculostomy or lumbar pressure. A drawback of these methods is that they are invasive. An easy-to-use non-invasive technique that provides high accuracy and precision for ICP assessment would be of great value in medical care outside the intensive care unit. Traumatic brain injury, subarachnoid haemorrhage, intra-cerebral haemorrhage, combat-related injuries, brain tumours, hydrocephalus, pseudotumor cerebri and meningitis are conditions where non-invasive ICP could be used for diagnosis, monitoring and selection of patients for invasive ICP registrations.
Many bed-side methods for correlation-based non-invasive approaches to ICP measurement have been tested. Among them are intraocular pressure assessment [2], tympanic membrane displacement [3], ultrasonography [4, 5] and measurement of intracranial blood flow [6–9]. In the individual, these methods have shown a fairly high correlation between non-invasive and invasive ICP assessments (Table 1). However, the limits of agreement in the patient groups are usually very wide, making the non-invasive methods impossible for clinical use. For instance, the Gosling pulsatility index measured in the middle cerebral artery by transcranial Doppler sonography (TCD) reportedly has a high correlation with ICP [10]. However, some have reported a poor and clinically unusable relationship between pulsatility index and ICP [6], indicating the need for calibration of non-invasive techniques. However, patient-specific non-invasive calibration is impossible.
So far, no non-invasive technique has proven accurate and precise enough to replace invasive procedures (Table 1). Besides agreement between techniques, a general ICP method must fulfil specifications such as age and disease independence, operator independence and informative without patient-specific calibration.
A new non-invasive ultrasonic device (Vittamed 205®) for ICP measurement has been developed [8]. Without the need for patient-specific calibration, it measures ICP in absolute values (mmHg) using the equilibrium between blood flow pulsations in the intracranial (IOA) and extra-cranial (EOA) segments of the ophthalmic artery (OA). Such equilibrium is automatically identified by two-depth trans-orbital Doppler technology. A recent study [8] of a young group of neurological patients showed that the method provides clinically acceptable accuracy (systematic error of less than 1.0 mmHg) and precision (random error SD of 2.3 mmHg). The study was conducted in the limited interval of ICP values between 5.0 and 24 mmHg, and only one patient had an ICP above the critical threshold of 20 mmHg.
Measures of ICP via a lumbar puncture corresponds well with the intra-parenchymal pressure if the cerebrospinal fluid (CSF) system is communicating [11]. The objective of this study was to assess ICP measurements obtained using a Vittamed 205® and compare them with those obtained via an invasive Celda® technique, which accurately and precisely measures ICP via the lumbar route and automatically increases ICP to a pre-determined level through infusion of Ringer’s solution [12].
2 Materials and methods
2.1 Non-invasive ICP measurements
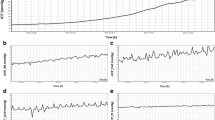
The non-invasive ICP absolute value measurement (ICPNon-Inv) was based on two-depth Doppler ultrasound monitoring of pulsations in the blood flow velocity in the IOA and EOA. The rationale is that the OA is a natural ICP sensor wherein the ICP compresses the IOA, and the blood flow velocity waveform in the IOA depends on the ICP. The waveform in the EOA depends on the surrounding intra-orbital pressure. This non-invasive ICP measurement procedure was automatic after manually adjusting the position of the ultrasonic transducer on the closed eyelid. The non-compressible tissues surrounding an eyeball were subjected to an externally applied pressure, Pext, which was automatically increased in six steps of 4 mmHg and held on each level for 40 s (Fig. 1). The ICP meter’s software determined at which Pext level the best match between IOA and EOA waveforms was achieved. Therefore, ICPNon-Inv was identified when ICP = Pext for that level. A direct comparison between ICP and Pext eliminated the need for patient-specific calibration in this proposed method. The six steps in Pext were used to minimize the time needed to produce the measurement. To achieve greater precision, Pext can be increased in steps of 3.0, 2.0 or 1.0 mmHg pressure, but this extends the duration of “snapshot” measurements. Together with a torus-shaped pressure chamber, the scanning ultrasonic TCD transducer attached to the eyelid was mounted into a mechanical head frame. Mechanical and electronic scanning modes of the ultrasonic transducer were used during the search for OA segments.
Time diagrams of the infusion test caused step-by-step mean ICP (t) changes (gray line), and externally applied pressure Pext (t) caused step-by-step changes (black lines): A. B. C initial steps of Pext (t) in three repeatable non-invasive absolute value ICP measurements. B A . B B . B C balance points, where ICPA.B.C. = (Pext)A.B.C. Δt is the duration of the transient infusion processes. ΔPext is the sampling step of Pext (t) and was equal to 4.0 mmHg (six steps in each snapshot of ICPNon-Inv measurement cycle, A, B or C). T is the duration of each snapshot ICPNon-Inv measurement cycle. TM is the duration of ICPNon-Inv value averaging during snapshot measurement in cycle C, equal to 40 s
Insonation of the OA through the orbit is a standard and safe procedure in Doppler examination of the blood flow in orbital vessels. The special two-depth TCD device meets all international safety standards for intra-orbital ultrasonic measurements. The acoustic output parameters of the device are: spatial peak temporal average intensity = 49.48 mW/cm2, spatial peak pulse average intensity = 1.335 W/cm2, maximal pressure of pulse intensity integral = 0.2147 MPa, output ultrasonic pulse duration = 3.705 µs and mechanical index = 0.1518.
2.2 The reference system
The infusion investigations were performed using an invasive device for measuring and controlling ICP via lumbar pressure (Celda®; Likvor AB, Umeå, Sweden). Two 18-G lumbar needles were inserted into the CSF space at the L3–L4 interspace. The needles were connected to the device via a disposable tubing set that included two pressure transducers (Celda® Tools) and separate Ringer’s solution in a bag. One needle was used for ICP registration and the other for infusion and withdrawal of Ringer’s solution with a built in peristaltic tubing pump that was set to automatically adjust and stabilize the patient’s ICP. Measures of ICP using the Celda® have previously been compared with those taken by a probe placed in the brain parenchyma, showing good agreement [11].
A specially-designed bed with a rectangular hole at the lumbar level allowed for placement of two needles while the patient maintained a sitting position and subsequent investigation with the patient in the supine position. The set-up was mounted on an electrically maneuvered pillar to set a zero pressure level. A built-in horizontal laser line was used for zero level alignment.
The CSF dynamic investigations began with a baseline registration of ICP followed by infusion to predetermined pressure levels (Fig. 1).
2.3 Bench test evaluation of reference system
Pressure measurements obtained by the Celda® system were evaluated using a bench test and a hydrostatic column open to the air in a 50-mL syringe. The syringe was movably mounted on a stable stand and placed at seven different levels to produce hydrostatic columns between −0.1 m and 0.5 m. Eleven Celda® tubing sets were tested, and measures were taken six times at each level for each set.
2.4 The investigation protocol
On the day prior to the planned infusion test, the accessibility and normality of both the IOA and EOA Doppler signals was confirmed using the TCD of the non-invasive ICP device. If a reliable signal could not be detected, no further non-invasive measurements were performed on that patient.
At 8.30 a.m. after 12 h of bed rest, the needles were placed while the patient was in the sitting position. A minimum leakage of CSF was strived for. The patient was then placed in the supine position, and the zero-pressure reference level of the infusion apparatus was placed at the centre of the auditory meatus using the horizontal laser line.
The Celda® investigation protocol started with a 15-min recording of baseline pressure. This was followed by pressure-regulated infusion performed using a pressure-controlled pump. Six ICP levels were strived for in each patient. The pressure levels were approximately 5–20 mmHg above baseline pressure (Fig. 1) with 3-mmHg intervals. Each level was stabilized as much as possible and held for at least 7 min. The expert operator (1 person) chose the order of the elevated pressure levels, which differed between patients and was blind to the operator. In the non-invasive method, two expert operators with extensive experience with Doppler investigations were informed of the expected ICP interval (excluding the resting ICP), which was either 4–24, 10–30 or 15–35 mmHg. This was done only to minimize the time needed for the non-invasive measurement; it was deemed unacceptable to sustain a high ICP for a long duration. A manual command, “Start non-invasive ICP measurement,” was given by the Celda® operator to the Vittamed 205® operator at the beginning of each new ICP pressure level.
When it was difficult to withdraw fluid from the CSF space, a modified protocol in which pressure control with “only inflow” infusion or a constant flow infusion was used. When the “only inflow” infusion was used, the protocol was limited to four elevated pressure levels, but when the constant flow protocol was used, the number of elevated ICP levels was limited to one or two.
The investigation was performed in a private room that was kept as silent as possible. Experienced investigators performed all measurements.
2.5 Patients
The internal review board at Umeå University approved the study (2011-256-31M). Written informed consent was obtained from all participants. Eighteen patients (seven female) evaluated at the neurological ward for the suspicion of normal pressure hydrocephalus (i.e., ventriculomegaly, gait disturbance and/or cognitive decline) were included. They were aged 73 ± 8 years (mean ± SD), and their mini-mental state estimation was 25 ± 4 points. One patient had simplex glaucoma, and three had cataracts.
2.6 Analysis and blinding procedure
The mean ICPInvasive was automatically calculated for each of the 7-min pressure levels by the Celda® apparatus. The ICPNon-Inv was assessed in the same time interval. For baseline pressures and in a few special measurements (e.g., constant infusion or pump stop), ICPInvasive was manually calculated as the mean pressure over the same 7-min time period during which ICPNon-Inv was measured. Depending on the infusion protocol and success of ICP measurement, two to seven independent pairs of ICP measurements were produced for each patient. After each patient, the operators of the non-invasive technique, still blinded to the reference ICP, made a post hoc analysis of the waveform data and calculated the ICPNon-Inv estimates. Hydrostatic errors caused by the vertical distance between the point of invasive ICP measurement, defined by the auditory meatus, and the point of the IOA segment were eliminated. The standard Celda® investigation report from the infusion investigation, which included the ICPInvasive values, was placed in a sealed envelope together with a written report from the non-invasive operator, which included the ICPNon-Inv estimates. The envelopes were opened after patient enrolment was completed at a meeting in which the authors were present. The crew groups were thus blinded to each other’s results throughout patient enrolment. The calculated values in the envelopes are the primary ICP data presented in this paper. The Introduction and Methods sections of this manuscript were agreed upon by all authors before envelopes were opened, thus un-blinding the study.
2.7 Statistics and validation criteria
We hypothesized that values of ICPNon-Inv will reflect values of ICPInvasive, allowing a general linear model with ICPNon-Inv as the dependent variable of the two. Pearson’s method was used for correlation analysis.
Differences (ICPInvasive − ICPNon-Inv) were compared to means (ICPInvasive + ICPNon-Inv)/2 in a Bland–Altman plot [13]. This plot reveals ICP-dependent differences and exposes systematic and random errors and thus the agreement between the two methods. The criteria for acceptable accuracy in ICPNon-Inv measurement was that the paired ICP data points in the plot should be within ±5 mmHg (the error corridor) with a confidence level of 95 %.
This validation criteria for ICPNon-Inv agrees with the results of a prospective clinical assessment using a young group of neurological patients [8]. In that study, the Vittamed 205® had the following specifications: ICP ranged from 0 to 50 mmHg, an SD for random error was ±2.3 mmHg (95 % CI) and a systematic error was less than 1.0 mmHg (95 % CI). This gives an uncertainty (2SD) close to 5.0 mmHg. Each patient’s ICP agreement is presented by plotting ICPNon-Inv against ICPInvasive along with the line of equality.
3 Results
Comparisons between the Celda® ICP measurement and the hydrostatic water column yielded a random error (95 % CI) of less than ±0.9 mmHg; the systematic error was less than ±0.5 mmHg.
Eighteen patients were screened, and reliable Doppler signals for estimation of ICPNon-Inv were obtained in 13 (72 %). For one patient, the infusion test was aborted because the patient fainted at the lumbar puncture. Thus, the study population consisted of twelve patients. From each of these, we obtained 2–8 simultaneous assessments of ICP using both methods, giving 61 data points. For three ICP measurements, the scanning interval of Pext did not include, or were at the limits of, the mean ICPInvasive. For these measurements, the ICPNon-Inv assessment was questionable, and they were excluded from the statistical calculations.
The concordance between the two ICP measures is shown in Fig. 2, and the correlation was R = 0.74 (p < 0.001, n = 58). A Bland–Altman plot (Fig. 3) shows that the 95 % limits of agreements were −1.4 ± 8.8 mmHg, and regression analysis showed that there was a trend (R = −0.32; p = 0.016, n = 56), indicating a larger underestimation of ICPNon-Inv at higher ICPs.
Bland–Altman plot showing the difference (ICPInvasive − ICPNon-Inv) plotted against the mean measured ICP value (ICPInvasive + ICPNon-Inv)/2. The solid line shows the systematic difference between the two, and dotted lines represent the 95 % CI of agreement. Square markers same as in Fig. 2. The limits of agreement between ICPInvasive and ICPNon-Inv including all data was −1.8 ± 9.4 mmHg
Within any individual, the two methods yielded correlation coefficients between 0.47 and 1.00. The slopes of the correlations were between 0.34 and 0.87 (Table 2).
To investigate possible biases from the 20 mmHg limitation in the Pext range, we performed a simulation in which the pressure within the given range of each interval was randomised and compared with ICPInvasive. Thirty simulations with new randomised pressures yielded an average correlation of 0.38 ± 0.09 and limits of agreement of ±15 mmHg. This shows an expected bias from interval selection, but it was significantly (p < 0.001) lower than the correlation found in the study and had much larger limits of agreement. Post hoc analysis revealed that if we used only the measurements taken when the Pext interval was 10–30 mmHg (n = 32), the correlation was 0.44 (p = 0.013), and the limits of agreement was 2.1 ± 8.4 mmHg.
4 Discussion
This study is important because most patients prefer ICP measurement without the use of needles. There is a growing commercial market for this type of device, and it is important that new products are tested before they are incorporated into clinical practice.
We compared a bedside non-invasive absolute ICP measurement method using an invasive method by taking multiple measurements in an elderly group of patients over a wide ICP span. When compared to previously suggested non-invasive methods, this method appears promising. Unlike other techniques, it offers the advantage of an estimation of the absolute value of ICP, rather than an indirect index that changes with ICP. Additionally, it does not need patient-specific calibration.
Other bedside methods have previously failed to show reliable results. Using TCD for assessing flow velocity pulsatility index in the middle cerebral artery has been suggested as a correlation-based non-invasive technique, and while Bellner et al. [10] found very promising results, they have not been reproduced by others (Table 1). A recent large study by Zweifel et al. [14] showed that the TCD pulsatility index for non-invasive assessments of ICP is very limited. Tympanic membrane displacement (TMD) from the stapedius reflex was shown to correlate with ICP but with a wide ICP prediction interval [15]. Another audiological approach was based on the evoked distorsion product otoacoustic emissions, which correlates with ICP [16, 17], but it has not been thoroughly evaluated against invasive measurements. Optic nerve sheath diameter increases with ICP [18]. Most studies evaluated correlation (Table 1) and/or the ability to detect ICPs exceeding 20 mmHg. Sekhon et al. [19] showed that the optic nerve sheath diameter had a prediction interval against invasive ICP of ±12 mmHg. Correlation between intraocular pressure and ICP has been studied, and a meta-analysis from 12 studies revealed a significant but low correlation (Table 1). A number of suggested and evaluated methods for predicting ICP use blood flow velocity from TCD and arterial blood pressure curves as inputs to mathematical models. Schmidt et al. [20] suggested a black box system that was evaluated on a group of hydrocephalus patients during infusion and showed promising results [21]. It was also evaluated on 145 severely head-injured patients, and they concluded that the method could be used to provide a noninvasive and continuous assessment of the state of cerebral autoregulation [22]. Kim et al. [23] used similar inputs and an assessment using a nonlinear mapping function. The same group later added electrocardiogram as an input, developed a method with semi-supervised learning and demonstrated that it was promising for detection of intracranial hypertension [24]. In two recent studies, Cardim et al. compared four methods used for non invasive ICP estimation using both arterial blood pressure and flow velocity as inputs. In a cohort of traumatic brain-injured patients [25] and in a group of patients investigated with infusion tests [26], limits of agreements were larger than ±9 mmHg in all four methods.
This study shows that the method presented here could assess ICP with limits of agreement of ±8 mmHg on the studied patients with only a small systematic underestimation. According to our selected validation criteria, this is not sufficient for recommending its routine clinical use. The mean value of ICP in healthy individuals is 10 mmHg, and the upper normal limit is around 15 mmHg [27]. The definition of idiopathic intracranial hypertension and normal pressure hydrocephalus is above and below 18 mmHg, respectively [28]. A common limit for initiating a therapy to lower ICP in traumatic brain injuries is about 20 mmHg [29]. Consequently, if the clinical purpose is to classify and treat wide age groups of patients according to pre-set thresholds, ICP assessment should have an accuracy that discriminates between these. Therefore, we suggest that to be clinically useful, a non-invasive ICP method should have limits of agreement comparable to that found in invasive ICP (no more than ±5 mmHg). As yet, none of the suggested methods, including the one we investigated, fulfill this criteria. However, when comparing our results to those obtained using other suggested methods (see Table 1), the limits of agreement found here (±8.8 mmHg) is promising.
When using the Bland–Altman plot to determine whether the difference (ICPInvasive − ICPNon-Inv) was pressure-dependent, we found that underestimation of ICPNon-Inv was greater at higher pressures. This was indicated both by the significant negative correlation found in the Bland–Altman plot and by the generally flat (less than 1) slopes found by plotting (ICPNon-Inv against ICPInvasive) for each individual patient (Fig. 3). A reduction in slope is partly caused by the ordinary least square regression. Random errors in both variables yields an underestimation of the slope [30]. It also indicates a trend in the method, suggesting that lower Pext is needed at higher pressure levels to produce the best concordance. It is possible that the diastolic pressure in the ICP waves, rather than the mean ICP, is detected by non-invasive methods. The underestimation would then be small at normal ICPs because the ICP pulse amplitude is small, but it would be larger at higher ICPs because the ICP amplitudes are greater and the difference between diastolic ICP and mean ICP then becomes significant [31].
There are sources that contribute to the difference between ICPNon-Inv and ICPInvasive. One is the sampling error, which depends on the Pext steps of 4.0 mmHg. Another is the methodological limitation of how well the change in measured IOA and EOA waveforms reflects the concordance between Pext and ICPInvasive. A third source is from the amount of time needed; in practical clinical use, six levels of Pext are examined over 7 min to produce a single estimate of ICPNon-Inv. Natural variations in ICP during these 7 min can contribute to errors in ICPNon-Inv. When using the non-invasive method, it is crucial to get high-quality Doppler signals from the EOA and IOA. This can be technically demanding for non-trained investigators.
However, in special circumstances, when the clinical purpose is to non-invasively verify that ICP is significantly elevated, the method could be useful.
The method is also promising for following an individual in situations where invasive methods are not available, such as investigating visual impairment intracranial pressure syndrome in astronauts in space [32]. However, the within-patient variation was similar to the total variation (Table 2); therefore, precision greater than ± 8 mmHg can not be expected in elderly patients. A recent report using our non-invasive method showed promising results in a younger population, but only a few patients had ICPs above 20 mmHg [33].
Clinical studies using this non-invasive ICP measurement method have not demonstrated relevant patient risk. The applied Pext is limited to 50 mmHg, corresponding to 0.68 m under water. When the pressure applied to the tissues surrounding the eyeball is this low, it guarantees safety. The two-depth TCD device used in this study conforms to all international safety standards for ultrasonic Doppler devices.
Though the Vittamed 205® was used by experts, 28 % of the patients could not be investigated because reliable Doppler signals were impossible to trace. A previous study [8] that used younger patients indicated a higher success rate, suggesting age-related effects. The method’s age-related usefulness must be investigated. When using expert investigators, inter-operator studies are needed. To develop into a useful clinical tool, a universal ICP assessment method should be easy to use, age independent and functional irrespective of what caused ICP to increase. Parts of the deviations between methods seen in this study depend on patient-specific errors, such as poor cooperation, moving, speaking or other unacceptable interruptions during Doppler measurements. Also, an actual clinical situation is likely to be noisier and more stressful.
The bench test of the Celda® showed that invasive pressure measurement was reliable both within a tubing set and between tubing sets, supporting its use in invasive methods for obtaining accurate ICP assessment. It also offers the advantage of monitoring the dynamic ICP variation rather than taking a snapshot of the ICP. While ICP is not a fixed value, it is described by a curve that is continuously moving as a result of physiological variations such as arterial pulsations, vasomotion, cerebrovascular autoregulatory activity and respiratory waves. A limitation in using the Vittamed 205® is that these variations will not be captured because at least 7 min are needed to produce each ICP reading.
The limitation of 7 min restricted total infusion time, produced a required reduction in the Pext sweep interval to 20 mmHg (Fig. 1). Although the study was blinded in its design, the maximum interval that could be scanned in each 7-min interval was 20 mmHg. Therefore, the Vittamed operators had to have a limited amount of knowledge of the expected ICP interval, and this was found to yield a bias. Using only those measurements taken over a Pext interval of 10–30 mmHg, we found a correlation of 0.44 and no change in the range for limits of agreement. As seen in three ICP assessments, there was a risk that the Pext interval did not cover the invasively-obtained ICP, resulting in questionable ICPNon-Inv estimates. Because all such estimates were at high pressures and deviated substantially (Fig. 2), the validity of the non-invasive method at high pressures should be further investigated so that outliers are avoided when the correct Pext interval is used.
We have shown that a non-invasive method for obtaining ICP as an absolute value measurement using this technology is likely in the near future. This is important in situations where a snapshot and rapid screening of ICP is necessary, such as in the emergency room. In addition, ICP can be followed at intervals in patients with different neurological and neurosurgical diseases when a continuous measurement is not possible or needed. However, the prototype Vittamed 205® non-invasive ICP device, or any other non-invasive ICP method, can not yet be recommended for routine use in the assessment of ICP in a neurological or neurosurgical setting.
In conclusion, non-invasive attempts to measure ICP seem to be rising in popularity. This is not surprising because patients want comfort and a minimal risk of complications. We explored the relationship between non-invasively and invasively measured ICP. The results are promising for the non-invasive two-depth Doppler technique, and continuing with additional development and evaluation is encouraged and necessary before the method can be recommended for use in clinical practice.
References
Andrews PJ, Citerio G, Longhi L, Polderman K, Sahuquillo J, Vajkoczy P, et al. NICEM consensus on neurological monitoring in acute neurological disease. Intensive Care Med. 2008;34:1362–70.
Sheeran P, Bland JM, Hall GM. Intraocular pressure changes and alterations in intracranial pressure. Lancet. 2000;355:899.
Samuel M, Burge DM, Marchbanks RJ. Tympanic membrane displacement testing in regular assessment of intracranial pressure in eight children with shunted hydrocephalus. J Neurosurg. 1998;88:983–95.
Klingelhöfer J, Conrad B, Benecke R, Sander D, Markakis E. Evaluation of intracranial pressure from transcranial Doppler studies in cerebral disease. J Neurol. 1988;235:159–62.
Petkus V, Ragauskas A, Jurkonis R. Investigation of intracranial media ultrasonic monitoring model. Ultrasonics. 2002;40:829–33.
Behrens A, Lenfeldt N, Ambarki K, Malm J, Eklund A, Koskinen L-O. Transcranial Doppler pulsatility index: not an accurate method to assess intracranial pressure. Neurosurgery. 2010;66:1050–7.
Kristiansson H, Nissborg E, Bartek J Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25:372–85.
Ragauskas A, Matijosaitis V, Zakelis R, Petrikonis K, Rastenyte D, Piper I, et al. Clinical assessment of noninvasive intracranial pressure absolute value measurement method. Neurology. 2012;78:1684–91.
Kashif FM, Verghese GC, Novak V, Czosnyka M, Heldt T. Model-based noninvasive estimation of intracranial pressure from cerebral blood flow velocity and arterial pressure. Sci Transl Med. 2012;4:129ra44.
Bellner J, Romner B, Reinstrup P, Kristiansson K-A, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004;62:45–51; discussion 51.
Lenfeldt N, Koskinen LO, Bergenheim AT, Malm J, Eklund A. CSF pressure assessed by lumbar puncture agrees with intracranial pressure. Neurology. 2007;68:155–8.
Malm J, Sundström N, Cesarini KG, Edsbagge M, Kristensen B, Leijon G, et al. Implementation of a new CSF dynamic device: a multicenter feasibility study in 562 patients. Acta Neurol Scand. 2012;125:199–205.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10.
Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012;71:853–61.
Shimbles S, Dodd C, Banister K, Mendelow AD, Chambers IR. Clinical comparison of tympanic membrane displacement with invasive intracranial pressure measurements. Physiol Meas. 2005;26:1085–92.
Voss SE, Horton NJ, Tabucchi TH, Folowosele FO, Shera CA. Posture-induced changes in distortion-product otoacoustic emissions and the potential for noninvasive monitoring of changes in intracranial pressure. Neurocrit Care. 2006;4:251–7.
Bershad EM, Urfy MZ, Pechacek A, McGrath M, Calvillo E, Horton NJ, et al. Intracranial pressure modulates distortion product otoacoustic emissions: a proof-of-principle study. Neurosurgery. 2014;75:445–54; discussion 454–5.
Galetta S, Byrne SF, Smith JL. Echographic correlation of optic nerve sheath size and cerebrospinal fluid pressure. J Clin Neuroophthalmol. 1989;9:79–82.
Sekhon MS, Griesdale DE, Robba C, McGlashan N, Needham E, Walland K, et al. Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 2014;40:1267–74.
Schmidt B, Klingelhöfer J, Schwarze JJ, Sander D, Wittich I. Noninvasive prediction of intracranial pressure curves using transcranial Doppler ultrasonography and blood pressure curves. Stroke. 1997;28:2465–72.
Schmidt B, Czosnyka M, Schwarze JJ, Sander D, Gerstner W, Lumenta CB, et al. Evaluation of a method for noninvasive intracranial pressure assessment during infusion studies in patients with hydrocephalus. J Neurosurg. 2000;92:793–800.
Schmidt B, Czosnyka M, Raabe A, et al. Adaptive noninvasive assessment of intracranial pressure and cerebral autoregulation. Stroke. 2003;34:84–9.
Kim S, Scalzo F, Bergsneider M, Vespa P, Martin N, Hu X. Noninvasive intracranial pressure assessment based on a data-mining approach using a nonlinear mapping function. IEEE Trans Biomed Eng. 2012;59:619–26.
Kim S, Hamilton R, Pineles S, Bergsneider M, Hu X. Noninvasive intracranial hypertension detection utilizing semisupervised learning. IEEE Trans Biomed Eng. 2013;60:1126–33.
Cardim D, Robba C, Donnelly J, Bohdanowicz M, Schmidt B, Damian M, et al. Prospective study on noninvasive assessment of intracranial pressure in traumatic brain-injured patients: comparison of four methods. J Neurotrauma. 2016. doi:10.1089/neu.2015.4134
Cardim D, Czosnyka M, Donnelly J, Robba C, Cabella BC, Liu X, et al. Assessment of non-invasive ICP during CSF infusion test: an approach with transcranial Doppler. Acta Neurochir (Wien). 2016;158:279–87.
Malm J, Jacobsson J, Birgander R, Eklund A. Reference values for CSF outflow resistance and intracranial pressure in healthy elderly. Neurology. 2011;76:903–9.
Corbett JJ, Mehta MP. Cerebrospinal fluid pressure in normal obese subjects and patients with pseudotumor cerebri. Neurology. 1983;33:1386–8.
Pickard JD, Czosnyka M. Management of raised intracranial pressure. J Neurol Neurosurg Psychiatry. 1993;56:845–58.
Ludbrook J. Comparing methods of measurements. Clin Exp Pharmacol Physiol. 1997;24:193–203.
Farahmand D, Qvarlander S, Malm J, Wikkelsø C, Eklund A, Tisell M. Intracranial pressure in hydrocephalus: impact of shunt adjustments and body positions. J Neurol Neurosurg Psychiatry. 2015;86:222–228.
Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118:2058–69.
Ragauskas A, Bartusis L, Piper I, Zakelis R, Matijosaitis V, Petrikonis K, et al. Improved diagnostic value of a TCD-based non-invasive ICP measurement method compared with the sonographic ONSD method for detecting elevated intracranial pressure. Neurol Res. 2014;36:607–14.
Brandi G, Béchir M, Sailer S, Haberthür C, Stocker R, Stover JF. Transcranial color-coded duplex sonography allows to assess cerebral perfusion pressure noninvasively following severe traumatic brain injury. Acta Neurochir (Wien). 2010;152:965–72.
Wakerley BR, Kusuma Y, Yeo LLL, Liang S, Kumar K, Sharma AK, et al. Usefulness of transcranial Doppler-derived cerebral hemodynamic parameters in the noninvasive assessment of intracranial pressure. J Neuroimaging. 2015;25(1):111–6.
Geeraerts T, Merceron S, Benhamou D, Vigué B, Duranteau J. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. 2008;34:2062–7.
Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15:201–4.
Soldatos T, Karakitsos D, Chatzimichail K, Papathanasiou M, Gouliamos A, Karabinis A. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care. 2008;12:R67.
Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. NCC. 2011;15:506–15.
Geeraerts T, Newcombe VFJ, Coles JP, Abate MG, Perkes IE, Hutchinson PJA, et al. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care. 2008;12:R114.
Alperin NJ, Lee SH, Loth F, Raksin PB, Lichtor T. MR-Intracranial pressure (ICP): a method to measure intracranial elastance and pressure noninvasively by means of MR imaging: baboon and human study. Radiology. 2000;217:877–85.
Yavin D, Luu J, James MT, Roberts DJ, Sutherland GR, Jette N, et al. Diagnostic accuracy of intraocular pressure measurement for the detection of raised intracranial pressure: meta-analysis: a systematic review. J Neurosurg. 2014;121:680–7.
Funding
The study is partially sponsored by European Commissions FP7 Project TBI care, NASA (US), and the Swedish National Space Board.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Koskinen received an honorarium for lecturing from DePuy Ltd (Codman Inc). Dr. Malm and Dr. Eklund are listed as inventors on a patent regarding the Celda infusion method, for which they received royalties from Likvor AB. Dr. Eklund has received an honorarium for lecturing from DePuy Ltd (Codman Inc). Dr. Ragauskas is an inventor of patented (US, EU and others) non-invasive ICP measurement technology. He consults Vittamed Corporation (Boston US) and its subsidiary, Vittamed UAB (Kaunas, Lithuania) on R&D and D&D of non-invasive ICP measurement technology.
Rights and permissions
About this article
Cite this article
Koskinen, LO.D., Malm, J., Zakelis, R. et al. Can intracranial pressure be measured non-invasively bedside using a two-depth Doppler-technique?. J Clin Monit Comput 31, 459–467 (2017). https://doi.org/10.1007/s10877-016-9862-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9862-4