Abstract
An end-tidal CO2 monitor (capnometer) is used most often as a noninvasive substitute for PaCO2 in anesthesia, anesthetic recovery, and intensive care. Additionally, the wide spread on-site use of portable capnometers in emergency and trauma situations is now observed. This study was conducted to compare PaCO2 measurement between the EMMA™ portable-capnometer and sidestream capnometry. End-tidal CO2 (portable capnometer: EMMA™ capnograph, side stream capnometry module: Datex-Ohmeda S5 Anesthesia Monitor) levels were recorded at the time of arterial blood gas sampling of patients undergoing general anesthesia. Data were compared using the Bland and Altman method, and by evaluating the clinical significance performed by calculating the percent error (%). A total of 100 data were obtained from 35 patients. The bias of PaCO2 and portable capnometer was 6.0 mmHg, where the upper and lower limits of the agreement were 11.8 and 0.3 mmHg, respectively. The percent error was 18.0 %. The bias of side stream capnometry and portable capnometer was 2.2 mmHg, where the upper and the lower limits of the agreement were 6.0 and −1.6 mmHg, respectively. The percent error was 13.0 %. Significant differences between the PETCO2 and PaCO2 values of the EMMA™ portable-capnometer were not observed for patients undergoing general anesthesia.
ClinicalTrials.gov identifier NCT02184728.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
End-tidal CO2 (PETCO2) measurement is a continuous, non-invasive method for estimating blood carbon dioxide levels . It has been widely used in operating rooms, and is now also used to evaluate patients’ respiration in intensive care units (ICU) and emergency rooms [1–3]. The 2010 CPR Guidelines of the American Heart Association (AHA) also recommended PETCO2 monitoring for the confirmation of tracheal intubation, as well as to assess the appropriateness of thoracic compression [4]. Capnometers are generally included in anesthesia machines, but not in the ventilators used in ICU or emergency rooms. It is more challenging to monitor the PETCO2 values in patient in the wards or outside of hospitals because they are expensive and cumbersome [5, 6]. Therefore, there is a need for light capnometers that can be easily used in ICU, emergency rooms, and outside of hospitals. EMMA™ capnograph (Masimo, danderyd, Sweden) is easy to carry around as they are small and light (5.2 × 3.9 × 3.9 cm and 59.5 g with batteries); also, a routine calibration is not required before the device is used and the device can be warmed up in a lesser amount of time. Also it detects and display PETCO2 values and capnogram from a single respiration [1], and only require 15 s to present accurate PETCO2 values and respiratory rates. Moreover, it can easily be connected to endotracheal tubes or facial masks, which can be used mainly in a variety of situation at patient wards, recovery rooms, ICU and emergency rooms. Despite this usefulness, only a few studies have focused on verifying the accuracy of transportable capnometers [1, 7]. We therefore compare the PETCO2 measured with an EMMA™ capnograph (EMMA) and arterial carbon dioxide values in patients receiving mechanical ventilation during general anesthesia.
2 Methods
The study enrolled 35 consecutive patient classified as American Society of Anesthesiologists physical status 1–2 who were scheduled for general anesthesia, with arterial catheter inserted to analysis arterial blood gas. The patients with pulmonary disease or undergoing lung surgery or laparoscopic surgery were excluded. The research was approved by the clinical research ethics committee of the hospital, and written consent was obtained from all patients after informing them of the purpose of the study. In the operating room, the patients were monitored with an ECG, NIBP (non-invasive blood pressure), and pulse oximetry. The patients were premedicated with 0.004 mg/kg glycopyrrolate intramuscularly 30 min before induction of the anaesthesia. To induce anesthesia a 1.5–2 mg/kg dose of propofol and 0.5–1 mg/kg/min dose of remifentanil were used. For muscle relaxation, 0.6 mg/kg of rocuronium were administered before the endotracheal intubation, after inducing the anesthesia, a 22G catheter was inserted for arterial blood sampling and blood pressure monitoring in the radial artery after performing the Modified Allen’s test. The anesthesia was maintained with sevoflurane 1–2 vol%, and 0.05–2 μg/kg/min of remifentanil were continuously infused.
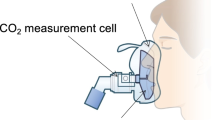
The EMMA was connected between the endotracheal tube and Y piece, and a sidestream sample line was connected to the Y-piece as the capnometry module (Datex-Ohmeda S/5™ Anesthesia Monitor, Datex-Ohmeda, Finland). Both monitors were confirmed to continuously show the capnogram and PETCO2 values. A fully-charged battery was used for the EMMA.
When analyzing the arterial blood gas during the surgery, we first confirmed that the capnogram and PETCO2 values on both monitors remained stable for at least 5 min, then recorded the PETCO2 values and collected the arterial blood at the same time in order to record the PaCO2 value. The vital signs were maintained during the sampling period. All the blood gas analyses were performed immediately, using a nearby blood gas analyzer (GEM Premier 3000, Instrumentation Laboratory, MA, USA).
2.1 Statistical analysis
The data were compared using the Bland and Altman method and an evaluation of the clinical significance by calculating the percent error (%). The Bland–Altman analysis was used to evaluate the degree of agreement between the measured CO2 values provided by the two monitors [8]. The evaluation of the clinical significance of the results obtained by the Bland–Altman analysis was performed through calculation of the percent error (%) based on Critchley and Critchley’s formula [9], which doubles the standard deviations (SD) of the difference between the two measurements and divides this value by the mean value of the two measurements, as follows: Percentage error = (2 × SD/mean CO2) × 100.
The results of the two methods were considered to be equivalent when the percent error was <30 %. All the statistical analyses were performed using the MedCalc software package, version 14.8.1 (MedCalc Software, Ostend, Belgium) for Windows®.
3 Results
The demographic data for the 35 patients are presented in Table 1. A total of 100 measurements were collected for each method (the results, according to the time of the operation, are: 7 patients, 4 samples; 12 patients, 3 samples; and 18 patients, 2 samples). The mean value for each measurement method was as follows: PaCO2: 35.1 ± 3.8 mmHg, EMMA: 29.1 ± 2.5 mmHg, and side stream: 31.3 ± 2.4 mmHg. The Bland–Altman analysis produced the graphs shown in Figs. 1, 2 and 3. The bias of the PaCO2 and EMMA measurements was 6.0 mmHg, with an upper agreement limit of 11.8 mmHg and a lower agreement limit of 0.3 mmHg. The percent error was 18 %. The bias of the PaCO2 and side stream measurements was 3.8 mmHg, with an upper agreement limit of 10.2 mmHg and a lower agreement limit of −2.5 mmHg. The percent error was 20 %. The bias of the side stream and EMMA measurements was 2.2 mmHg, with an upper agreement limit of 6.0 mmHg and a lower agreement limit of −1.6 mmHg. The percent error was 13 %. (Table 2).
4 Discussion
In our study, EMMA represents reliable PETCO2 values compared to PaCO2 in patients undergoing general anesthesia. The mean bias for the PaCO2 and PETCO2 values measured by the EMMA was 6.0 mmHg. It might appear to be tolerated, as the difference between the PaCO2 and PETCO2 in normal respiration is generally around 2–5 mmHg [10]. Moreover, the PETCO2 values collected by the EMMA could be regarded as significantly similar to those of the PaCO2, with a percent error of 18 %. The mean bias for the PaCO2 and PETCO2 values measured by the side stream was 3.8 mmHg, with a percent error of 20 %, exhibiting high similarity. Both capnometers could therefore be used to predict the PaCO2 values with regard to the general difference between the PaCO2 and PETCO2. Furthermore, the side stream and EMMA showed high similarity, with a mean bias of 2.2 mmHg and a percent error of 13 %, indicating that these two monitors represented appropriate alternatives to one another. They can also be used to predict the PaCO2 values in operating rooms and postoperative care units when a sidestream capnometer is unavailable.
CO2 measurement by the EMMA is based on the fact that different gas components absorb infrared light at specific wavelengths. A beam of infrared light passes through the airway adapter, some of the light is absorbed by the gas mixture. The amount of absorbed light is measured by a miniaturized two-channel spectrometer positioned to receive the infrared light beam. The spectrometer converts the light beam into an electrical signal, which is converted into a digital value [11]. The measured absorption of CO2 can, however, be altered by cross-interference and collision broadening due to the presence of gases such as nitrogen, nitrous oxide, and oxygen. Cross-interference, the overlapping of the absorption bands of other gases, can occur with nitrous oxide due to the presence of strong absorption bands that slightly overlap both edges of the carbon dioxide band, while collision broadening is a complex function of the total pressure and presence of other gases [12]. An infrared-based device with a selective narrow-band filter is therefore used to reduce the impact of cross-interference and, if the concentration of the interfering gases is measured, a contemporary multi-gas analyzer can automatically compensate for the effect of collision broadening [13]. The design of the EMMA consists of narrow-band optical filters and automatic-compensation technology for the management of cross-interference and broadening effects (manufacturer’s homepage: http://www.masimo.com/capnography/emma-capnograph.htm).
Mainstream capnometers such as the EMMA used in this study do not represent certain disadvantages of sidestream capnometers (e.g., the accumulation of water vapor in the measuring chamber after the vapor is first condensed in the sample tubing; response-time delays caused by the gas samples forcibly traveling to the measuring cell through the sample tubing; the diffusion of CO2 outside of the sample tubing; and gas leakages at the tubing-connection site) [14]. However, mainstream capnometers are prone to expanding the endotracheal dead space due to their airway adaptors and to causing endotracheal tube movements, or to increasing the direct weight on the airways due to the direct connection to the endotracheal tube [15]. The dead space created by the EMMA used in this study was 6 ml, which does not generally represent a threat to adult patients respiration. However, due to the lack of pediatric patient-specific adaptors, the use of these capnometers on pediatrics should be performed with caution (note: there are infant-specific adaptors). The weight of the capnometer (59.5 g) is heavy enough to slant the intubated endotracheal tube to one side. Therefore, the endotracheal tubes must be firmly fixed and the connection and disconnection of the capnometers should be performed with caution in order to prevent airway injury or unintentional extubation. The display of the EMMA is attached to the device, but it may be difficult to confirm the displayed values in, for example, a head-surgery situation. During long uses, there have been incidences of capnogram display errors caused by an accumulation of moisture on the airway adapter windows. In those situations, the adapters should therefore be changed to new, dry adapters. Furthermore, the risk of adapter windows becoming clouded by secretions should also be considered.
5 Limitations
The data used in this study were only collected in situations of stable and normal respiration under general anesthesia. Due to an increase in the difference between the PETCO2 and PaCO2 values in situations where dead space, a pulmonary ventilation/perfusion ratio, or a pulmonary shunt is present, it is therefore difficult to predict the PaCO2 values accurately through the PETCO2 values for patients with severe pulmonary or major systemic diseases [16]. It should thus be noted that the limitations affecting the prediction of the PaCO2 values are in accordance with the condition of the patient. This represents a limitation, as the accuracy of capnometers in cases of severe hypercapnia or hypocapnia, or of severe reduction of the cardiac output, is therefore inconclusive from this study’s findings. In addition, because same-sized adapters used for both adults and children, further research regarding application in children is therefore necessary. Also, it is necessary to verify the accuracy according to the remaining battery level, as these capnometers are likely to be used outside hospitals.
6 Conclusion
EMMA capnometers are a viable and lighter weight alternatives to legacy capnometers, as they produce capnographs and reliable PETCO2 values rapidly despite their small size and light weight.
References
Heines SJH, Strauch U, Roekaerts PMHJ, Winkens B, Bergmans DCJJ. Accuracy of end-tidal CO2 capnometers in post-cardiac surgery patients during controlled mechanical ventilation. J Emerg Med. 2013;45:130–5.
Tyburski JG, Collinge JD, Wilson RF, Carlin AM, Albaran RG, Steffes CP. End-tidal CO2-derived values during emergency trauma surgery correlated with outcome: a prospective study. J Trauma. 2002;53:738–43.
Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337:301–6.
Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S640–56.
Varon AJ, Morrina J, Civetta JM. Clinical utility of a colorimetric end-tidal CO2 detector in cardiopulmonary resuscitation and emergency intubation. J Clin Monit. 1991;7:289–93.
Singh S, Allen WD Jr, Venkataraman ST, Bhende MS. Utility of a novel quantitative handheld microstream capnometer during transport of critically ill children. Am J Emerg Med. 2006;24:302–7.
Lindström V, Svensen CH, Meissl P, Tureson B, Castrén M. End-tidal carbon dioxide monitoring during bag valve ventilation: the use of a new portable device. Scand J Trauma Resusc Emerg Med. 2010;18:49.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15:85–91.
Fletcher R, Jonson B. Deadspace and the single breath test for carbon dioxide during anaesthesia and artificial ventilation. Effects of tidal volume and frequency of respiration. Br J Anaesth. 1984;56:109–19.
Hildebrandt T, Espelund M, Olsen KS. Evaluation of a transportable capnometer for monitoring end-tidal carbon dioxide. Anaesthesia. 2010;65:1017–21.
Raemer DB, Calalang I. Accuracy of end-tidal carbon dioxide tension analyzers. J Clin Monit. 1991;7:195–208.
Ehrenwerth J, Eisenkraft JB, Berry JM. Anesthesia equipment, principles and applications (expert consult: online and print), 2: anesthesia equipment. Elsevier Health Sciences; 2013. p 198.
Ronald DM, Lars IE, Lee AF, Jeanine PW, William LY. Miller’s anesthesia. 7th ed. Amsterdam: Elsevier; 2009.
Block FE, McDonald JS. Sidestream versus mainstream carbon dioxide analyzers. J Clin Monit. 1992;8:139–41.
Razi E, Moosavi GA, Omidi K, Khakpour Saebi A, Razi A. Correlation of end-tidal carbon dioxide with arterial carbon dioxide in mechanically ventilated patients. Arch Trauma Res. 2012;1:58–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Financial disclosure
The authors do not have any financial relationship.
Rights and permissions
About this article
Cite this article
Kim, K.W., Choi, H.R., Bang, S.R. et al. Comparison of end-tidal CO2 measured by transportable capnometer (EMMA™ capnograph) and arterial pCO2 in general anesthesia. J Clin Monit Comput 30, 737–741 (2016). https://doi.org/10.1007/s10877-015-9748-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9748-x