Abstract
Two proton-conductive complexes based-on decorated Keggin-type clusters, {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (1) and {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (2) (where dmphen is 4,7-dimethyl-1,10-phenanthroline and DMF is N,N-dimethylformamide), were simply synthesized by the reaction of H4SiW12O40·24H2O, CuCl2·6H2O/ZnCl2 and dmphen at room temperature. The products were structurally characterized by elemental analyses, infrared spectroscopy, and single-crystal X-ray diffraction analyses. Single-crystal X-ray diffraction analyses at 293 K revealed that two complexes both crystallized in the monoclinic space group C2/c and presented two three-dimensional supramolecular networks with one-dimensional hydrophilic channels constructed by decorated Keggin-type clusters and solvent water molecules via the hydrogen-bonding interactions. The results of thermogravimetric analyses suggest that two complexes have good water holding in one-dimensional hydrophilic channels in the temperature range 20–100 °C. Two Complexes exhibit good proton conductivities (over 10−5 S cm−1 for 1 and over 10−4 S cm−1 for 2) at 100 °C in the relative humidity range 35–98%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been continuous investigation on developing new organic–inorganic hybrid material with extended planar aromatic ligands to enhance noncovalent binding to transition metal ions [1–24]. In this respect, ligands such as 4,4′-dimethyl-2,2′-bipyridine (dmbipy) and 4,7-dimethyl-1,10-phenanthroline (dmphen) as bidentate nitrogen donor ligands are very suitable organic building blocks in organic–inorganic hybrid materials [1–5]. As a result of rotational flexibility around the carbon–carbon single bond bridging the two pyridine rings of dmbipy, the two pyridine planes adopt either a coplanar or tilted conformation in coordination with the metal centers [6–9]. However, such rotational flexibility is not possible for dmphen ligand, which remains coplanar even after metal-coordination [22–24]. In addition, there has been particular emphasis on developing new organic–inorganic hybrid material based–on decorated Keggin-type cluster and extended planar aromatic ligands [20, 21]. Recently, we have chosen dmbipy to construct proton-conductive organic–inorganic hybrid materials based–on decorated Keggin-type clusters [20, 21]. Heterocyclic dmphen containing two heteroatoms possesses various applications and is important due to its synthetic flexibility, selectivity and sensitivity towards the metal ions [22–24]. In addition, dmphen has two hydrophobic methyl groups, suggesting that dmphen is a good organic building block for constructing organic–inorganic hybrid materials with the phase separation of hydrophilic/hydrophobic domains. To better understand the influence of the decoration of Keggin-type polyanions based-on such an organic building block as dmphen on the resultant structures and proton conductivities of organic–inorganic hybrid materials, herein, Cu2+/Zn2+ ion, dmphen, and Keggin-type [SiW12O40]4− polyanions were chosen to construct proton-conductive organic–inorganic hybrid materials based–on decorated Keggin-type clusters. We synthesized two proton-conductive complexes based–on decorated Keggin-type clusters, {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (1) and {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (2), by the reaction of a-H4SiW12O40·24H2O, CuCl2·6H2O/ZnCl2 and 4,7-dimethyl-1,10-phenanthroline at room temperature. Two complexes both presented three-dimensional supramolecular network structures with the phase separation of hydrophilic/hydrophobic domains constructed by {[M(dmphen)(DMF)2(H2O)]2[SiW12O40]}(M = Cu and Zn) clusters and solvent water molecules. Interestingly, two coordinated water molecules are bound to open coordination sites of two Cu2+/Zn2+ ions in the {[M(dmphen)(DMF)2(H2O)]2[SiW12O40]}(M = Cu and Zn) cluster. Binding substantially increases the acidity of the incorporated water molecule, enabling it to donate a proton to solvent water molecules and thereby rendering conductive the network of hydrogen-bonded solvent molecules filling in the one-dimensional hydrophilic channels. The title complexes show proton conductivities across a wide range of temperature and RH and achieves proton conductivities over 10−5 S cm−1 for 1 and over 10−4 S cm−1 for 2 at 100 °C in the RH range 35–98%. Here, we report their crystal structures and proton conductivities as a function of temperature and RH.
Experimental
Materials and Methods
All organic solvents and materials used for synthesis were of reagent grade and used without purification. Elemental analyses (C, H, and N) were carried out on a Perkin Elmer 240C analyzer. X-ray powder diffraction was performed on a Bruker D8 Avance Instrument using Cu-Kα radiation and a fixed power source (40 kv, 40 mA). IR spectra were recorded on a VECTOR 22 Bruker spectrophotometer with KBr pellets from 400 to 4000 cm−1 at room temperature. Thermogravimetric analysis was performed on a Perkin Elmer thermal analyzer under nitrogen at a heating rate of 10 °C min−1. For an electrical conductivity study, the powdered crystalline samples were compressed to 0.8–1.0 mm in thickness and 12.0 mm in diameter under a pressure of 12–14 MPa. AC impedance spectroscopy measurement was performed on a chi660d (Shanghai chenhua) electrochemical impedance analyzer with copper electrodes [2, 3] (the purity of Cu/Zn is more than 99.8%) over the frequency range from 105 to 1 Hz. The conductivity was calculated as σ = (1/R) × (h/S), where R is the resistance, h is the thickness, and S is the area of the tablet.
Synthesis of {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (1)
The copper heteropolyacid salts(Cu2SiW12O40·nH2O) was prepared by mixing α-H4SiW12O40·24H2O (300 mg, 0.1 mmol) and CuCl2·6H2O (34 mg, 0.2 mmol) in water about 4 ml, and then drying the solution at 80 °C in a water bath. A buffer layer of a solution (6 mL) of methanol/DMF/water (3:2:1, v/v/v) was carefully layered over a DMF/water (3:1, v/v) solution (6 mL) of the resultant copper heteropolyacid salts (30 mg, 0.01 mmol). Then, a methanol solution (6 mL) of dmphen (4.2 mg 0.02 mmol) was carefully layered over the buffer layer. Light-green crystals were formed after about 6 weeks. Yield: 22 mg, 73.3% based on heteropolyacid salts. Anal. Calcd (%) for C40H68N8O52Cu2W12Si (%): C, 12.46; H, 1.78; N, 2.91. Found (%): C, 12.37; H, 1.75; N, 2.85. IR (KBr) ν, cm−1 (Fig. S1): 797 ν(W–Oc),886 ν(W–Ob), 977 ν(W–Ot), 923 ν(Si–Oa), 3440 ν(=C–H), 1627 ν(C=N).
Synthesis of {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (2)
Complex 2 was prepared in the same way as for 1, except using ZnCl2 (27 mg, 0.2 mmol) to replace CuCl2·6H2O (34 mg, 0.2 mmol). Light-pink crystals were formed after about 6 weeks. Yield: 20 mg, 67.7% based on heteropolyacid salts. Anal. Calcd (%) for C40H68N8O52Zn2W12Si (%): C, 12.45; H, 1.78; N, 2.91. Found (%): C, 12.53; H, 1.82; N, 2.98. IR (KBr) ν, cm−1 (Fig. S1): 799 ν(W–Oc),887 ν(W–Ob), 978 ν(W–Ot), 925 ν(Si–Oa), 3443 ν(=C–H), 1622 ν(C=N).
Crystal Structure and Determination
Intensity data of complexes 1 and 2 were collected on a Siemens SMART-CCD diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073) using SMART and SAINT [25]. The structure was solved by direct methods and refined on F 2 using full-matrix least-squares with SHELXTL version 5.1 [26]. Non-hydrogen atoms were refined anisotropically, except for disordered O atoms in polyanion and solvent water molecules. Hydrogen atoms of organic molecules were localized in their calculated positions and refined using a riding model. Hydrogen atoms of coordinated water molecules were localized by difference Fourier maps and refined by fixing the isotropic temperature factors 1.2 times that of mother atoms attached. Hydrogen atoms of solvent water molecules were not treated. The crystal parameters, data collection, and refinement results are summarized in Table 1.
Results and Discussion
Structure Description
Complexes {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (1) and {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (2) were simply synthesized by the reaction of the copper/zinc heteropolyacid salts (Cu2SiW12O40·nH2O/Zn2SiW12O40·nH2O) and 4,7-dimethyl-1,10-phenanthroline at room temperature. X-ray diffraction analysis at 293 K revealed that two complexes both crystallized in the monoclinic space group C2/c, and then presented similar three-dimensional supramolecular network structures with one-dimensional hydrophilic channels constructed by {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]} or {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]} clusters and solvent water molecules. There are the hydrogen-bonding interactions among coordinated water molecules, solvent water molecules and O atoms from heteropolyanions.
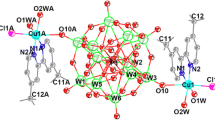
As shown in Fig. 1a, the {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]} cluster in complex 1 is a decorated Keggin-type cluster based-on Cu(II) ion and dmphen molecule. In the cluster, two [Cu(dmphen)(DMF)2(H2O)]2+ ions are located on symmetric sides of the [SiW12O40]4− anion. In the [Cu(dmphen)(DMF)2(H2O)]2+ ion, dmphen acts as a bidentate nitrogen donor ligand in the chelating fashion. Because the evident Jahn–Teller distortion of Cu2+ ion in the crystal field leads to the elongation of the Cu–O distances, there are Cu–O weak coordination interactions [Cu(1)–O(22): 2.608(7) Å] in this complex. This distance [Cu(1)–O(22): 2.608(7) Å] in complex 1 is comparable to that distance [Cu(1)–O(10): 2.554(10) Å] in complex {[Cu(dmbipy)(H2O)2Cl0.5]2[PW12O40]}·7H2O. In addition, this distance [Cu(1)–O(22): 2.608(7) Å] is close to the sum of the van der waals radii (2.5–2.6 Å), which is thus indicative of the weak secondary bonding [20, 21, 27–29]. As a result, the [SiW12O40]4− anion in the {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]} cluster acting as a bidentate ligand coordinated to two Cu2+ ions from two adjacent [Cu(dmphen)(DMF)2(H2O)]2+ ions. The coordination number of the Cu2+ ion is six (two N atoms from the dmphen ligand, two O atoms from two DMF molecules, an O atoms from a water molecule, and an O atom from the [SiW12O40]4− anion). The coordination environment of the Cu2+ ion can be described as octahedral geometry, as shown in Fig. 1a. This is one of the common coordination geometries for complexes with a coordination number of 6.
a The {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]} cluster in complex 1. Selected atom…atom distances [Å]: Cu(1)–O(22) 2.608(7), Cu(1)–N(1) 2.002(9), Cu(1)–N(2) 1.998(8), Cu(1)–O(1W) 2.26(2), Cu(1)–O(24) 2.004(19), Cu(1)–O(25) 1.984(12). b The {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]} cluster in complex 2. Selected atom…atom distances [Å]: Zn(1)–O(22) 2.441(6), Zn(1)–N(1) 2.079(11), Zn(1)–N(2) 2.096(8), Zn(1)–O(1W) 2.096(9), Zn(1)–O(24) 2.042(7), Zn(1)–O(25) 2.055(8)
As shown in Fig. 1b, the {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]} cluster in complex 2 is a decorated Keggin-type cluster based-on Zn(II) ion and dmphen molecule. In the cluster, two [Zn(dmphen)(DMF)2(H2O)]2+ ions are located on symmetric sides of the [SiW12O40]4− anion. In the [Zn(dmphen)(DMF)2(H2O)]2+ion, dmphen acts as a bidentate nitrogen donor ligand in the chelating fashion. Because the Jahn–Teller distortion of Zn2+ ion in the crystal field leads to the elongation of the Zn–O distances, there are Zn–O weak coordination interactions [Zn(1)–O(22): 2.441(6) Å] in this complex. In addition, this distance [Zn(1)–O(22): 2.441(6) Å] is smaller than the sum of the van der waals radii (2.9–3.0 Å), which is thus indicative of the weak secondary bonding [29, 30]. As a result, the [SiW12O40]4− anion in the {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]} cluster acting as a bidentate ligand coordinated to two Zn2+ ions from two adjacent [Zn(dmphen)(DMF)2(H2O)]2+ ions. The coordination number of the Zn2+ ion is six (two N atoms from the dmphen ligand, two O atoms from two DMF molecules, an O atoms from a water molecule, and an O atom from the [SiW12O40]4− anion). The coordination environment of the Zn2+ ion can be described as octahedral geometry, as shown in Fig. 1b.
Polyoxometalates present a wide range of intriguing topologies and structures, and whose spherical surfaces give an opportunity for forming coordination bonds or hydrogen bonds with organic or inorganic moieties. In complexes 1 and 2, [SiW12O40]4– anions form coordination bonds with [Cu(dmphen)(DMF)2(H2O)]2+ and [Zn(dmphen)(DMF)2(H2O)]2+ hybrid moieties. In the [SiW12O40]4− unit, the central Si atom is surrounded by a cube of eight oxygen atoms with each oxygen site half-occupied. These eight oxygen atoms are all crystallographically disordered, and this case can be found in many compounds. The Si–O and W–O distances in complex 1 are in a range of 1.56(2)–1.73(3) and 1.50(4)–2.48(3) Å, respectively. The Si–O and W–O distances in complex 2 are in a range of 1.562(13)–1.625(12) and 1.590(15)–2.455(12) Å, respectively. They are respectively comparable to those in the 3D porous polyoxometalates-based organic–inorganic hybrid materials with Keggin anions as guests or ligands [18–21]. In addition, the O–Si–O angles are in a range of 105.2(13)°–115.9(13)° for 1 and in a range of 105.9(6)°–114.9(7)° for 2. All these results indicate that the [SiW12O40]4− units have normal Keggin structures in the {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]} and {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]}clusters.
As shown in Fig. 2, the assembly of the {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]} clusters in complex 1 form a three-dimensional network structure based-on the hydrogen-bonding interactions between coordinated water molecules O(1W) and the O(13) centers of the [SiW12O40]4− units [O(1W)···O(13A): 2.794 Å, A: −x − 1/2, y − 1/2, −z + 1/2]. So, the whole packing arrangement of the {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]} clusters and solvent water molecules results in the phase separation of hydrophilic/hydrophobic domains and the large size of the hydrophilic domain (Fig. 3). The inner surface of each one-dimensional hydrophilic channel contains all coordinated water molecules and some terminal or bridging oxygen atoms from the polyanions. The solvent water molecules just fill in the channels. Just like complex 1, complex 2 also forms a three-dimensional network structure depending on the assembly of the {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]} clusters based-on the hydrogen-bonding interactions between coordinated water molecules O(1W) and the O(13) centers of the [SiW12O40]4− units [O(1W)···O(13A): 2.703 Å, A: −x − 1/2, y − 1/2, −z + 1/2].
Views of the three-dimensional hydrogen-bonding networks in 1 and 2 down the a c axis and b a axis formed by the {[Cu(dmphen)(H2O)2]2[SiW12O40]} and {[Zn(dmphen)(H2O)2]2[SiW12O40]} clusters based on the hydrogen-bonding interactions between coordinated water molecules (O1W) and the O(13) centers of the [SiW12O40]4− units
In complex 1, two coordinated water molecules are bound to open coordination sites of two Cu2+ ions in the {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]} cluster. In complex 2, two coordinated water molecules are bound to open coordination sites of two Zn2+ ions in the {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]} cluster. Binding substantially increases the acidity of the incorporated water molecules, enabling it to donate a proton to solvent water molecules and thereby rendering conductive the network of hydrogen-bonded solvent molecules filling in the one-dimensional hydrophilic channels. Thus, two complexes might realize such two approaches in the hydrophilic channels used to engender proton conductivity as (1) a poor proton donor (H2O) being bound to an otherwise open coordination site of a metal cation and (2) a continuous water phase being immobilized in the hydrophilic channels. Moreover, those terminal or bridging oxygen atoms from polyanions being at the inner surface of the hydrophilic cavities would contribute to the motion of protons between Keggin units, which is essential to the process of proton conduction and may impact catalytic properties as well [18–21]. Two complexes are insoluble in water and have pronounced hydrophobic/hydrophilic separation in network structures. In addition, based on coordinated and electrostatic interactions, [SiW12O40]4− anions are not easy dissociated from the hybrid networks of the title complexes. All these results indicate that two complexes can potentially be good proton-conducting materials.
Thermogravimetric Analysis
Thermal analysis of the powder of the crystalline sample of complex 1 in an atmosphere of N2 reveals that there is a weight loss of about 2.95% in the temperature range 20–120 °C, corresponding to the loss of all solvent water molecules (2.54%), and there is a weight loss of about 8.25% in the temperature range 120–340 °C, corresponding to the loss of H2O and DMF ligands (8.52%). The weight loss in the temperature range 340–700 °C corresponds to the loss of dmphen ligands and the disrupting of the structural skeletons of the [SiW12O40]4− anion (Fig. S2). Thermal analysis of the powder of the crystalline sample of complex 2 in an atmosphere of N2 reveals that there is a weight loss of about 2.91% in the temperature range 20–130 °C, corresponding to the loss of all solvent water molecules (2.53%), and there is a weight loss of about 8.45% in the temperature range 130–380 °C, corresponding to the loss of H2O and DMF ligands (8.50%). The weight loss in the temperature range 380–700 °C corresponds to the loss of dmphen ligands and the disrupting of the structural skeletons of the [SiW12O40]4− anion (Fig. S2). The weight losses are about 1.02% for 1 and 0.83% for 2 at 100 °C, suggesting that some uncoordinated water molecules still are hold in the hydrophilic channels at 100 °C. The results indicate that two complexes have good water holding in the temperature range 20–100 °C.
Proton Conductivity
The proton conductivities of complexes 1 and 2 at 25 °C in the RH range 35 to approximately 98% were evaluated by the ac impedance method using a compacted pellet of the powdered crystalline sample. The phase purity of the bulk materials was confirmed by powder X-ray diffraction (PXRD) experiments, which are in good agreement with the simulated PXRD patterns (Fig. S3). At 25 °C under a relative humidity of 35 to approximately 98%, complex 1 showed proton conductivities of 5.57 × 10−6–6.71 × 10−6 S cm−1 and complex 2 showed proton conductivities of 5.07 × 10−5–5.71 × 10−5 S cm−1. Figure 4 shows the log σ (S cm−1) versus RH plots of two complexes at 25 °C under a relative humidity of 35 to approximately 98%. Its conductivities increase with RH at 25 °C. The proton conductivities were also measured at 100 °C in the RH range 35 to approximately 98% by a complex-plane impedance method. Notably, two complexes showed proton conductivities of 2.90 × 10−5–3.18 × 10−5 S cm−1 for 1 and 1.22 × 10−4–1.30 × 10−4 S cm−1 for 2 under a relative humidity of 35 to approximately 98% (Fig. 4). Their low conductivities at low RH might result from the slow water equilibration between the one-dimensional hydrophilic channels and traces of water vapour. The increase of the RH makes water molecules be more easily uptaken into the one-dimensional hydrophilic channels, facilitating the proton transport to give larger proton conductivities.
Single-crystal X-ray diffraction analyses reveal two complexes might realize proton conductivity as of a continuous water phase being immobilized in the hydrophilic channels. But the results of thermogravimetric analyses suggest that some water molecules in two complexes have been lost when the temperature up to 100 °C. Thus, the fact that two complexes exhibit good proton conductivities at 100 °C in the RH range 35–98% is indicative of a high carrier concentration resulting from the dissociating processes of protons from coordination water molecules [11, 21, 28]. In addition, there is the possibility of hydrolysis of two complexes when they are held at 100 °C with a RH higher than 98%. The powder X-ray diffraction data in Fig. S3 suggested that the powder samples after the proton-conductive measurement have the same networks as those of two complexes, respectively. Thus, the structural descriptions of the one-dimensional hydrophilic channels are valid for the proton conductivities of two complexes even at 100 °C under 35–98% RH. We have reported a proton-conductive complex based-on decorated Keggin-type cluster, {[Cu(dmbipy)(H2O)2Cl0.5]2[PW12O40]}·7H2O, showing the proton conductivity values (10−6–10−4 S cm−1) below 100 °C under 98% RH. Two title complexes have similar structures with {[Cu(dmbipy)(H2O)2Cl0.5]2[PW12O40]}·7H2O. The proton conductivity values (10−6–10−5 S cm−1 for 1 and 10−5–10−4 S cm−1 for 2) below 100 °C under 98% RH are comparable to {[Cu(dmbipy)(H2O)2Cl0.5]2[PW12O40]}·7H2O (10−6–10−4 S cm−1).
The proton conductivities of two complexes under 98% RH increase on a logarithmic scale with temperature range from 25 to 100 °C (Fig. 5). Figure 6 shows their Arrhenius plots of the proton conductivities in the temperature range of 25–100 °C under 98% RH. Such a variable-temperature impedance study might allow the activation energy of the proton transfer in the title complexes to be extracted on the basis of an Arrhenius relationship. The lnσT increases almost linearly with temperature range from 25 to 100 °C, and the corresponding activation energy (E a) of conductivity was estimated to be 0.22 eV for 1 and 0.14 eV for 2. These E a values are close to those of pure heteropolyacids (0.25–0.4 eV) [31]. In two complexes, water molecules as poor proton donors being bound to open coordination sites of Cu2+/Zn2+ cations. The hydrophilic environment in the interior of the one-dimensional channel, created by the coordinated water molecules and terminal or bridging oxygen atoms from polyanions, could provide favorable pathways for proton conduction. Thermal analyses of two complexes indicated that a majority of solvent water molecules are not lost below 100 °C. These facts suggest that the mechanism of proton conduction of two complexes is expected to be similar to that of the Grotthus mechanism [32]. The theory of hard and soft acids and bases is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways. The gist of this theory is that soft acids react faster and form stronger bonds with soft bases, whereas hard acids react faster and form stronger bonds with hard bases. In coordination chemistry soft–soft and hard–hard interactions exist between ligands and metal centers. As a hard base, water has greater selectivity for less acidic metal ions such as Zn2+ over highly acidic metal ions such as Cu2+ [33]. Though two title complexes have the same structure, two coordinated water molecules in complex 1 are bound to open coordination sites of two Cu2+ ions with the distance of Cu(1)–O(1W) being 2.26(2) Å and two coordinated water molecules in complex 2 are bound to open coordination sites of two Zn2+ ions with the distance of Zn(1)–O(1W) being 2.096(9) Å. Thus, binding between Zn2+ and O(1W) increases the acidity of the incorporated water molecules over binding between Zn2+ and O(1W), resulting in the proton conductivity of complex 2 is 10 times higher than complex 1.
Conclusions
Two proton-conductive complexes with the phase separation of hydrophilic/hydrophobic domains based on a-H4SiW12O40·24H2O, CuCl2·6H2O/ZnCl2 and dmphen have been constructed. Two similar decorated Keggin-type clusters, {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O and {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O, were formed. Based on such two approaches used to engender proton conductivity as water molecules as poor proton donors being bound to open coordination sites of Cu2+/Zn2+ cations and a continuous water phase being immobilized in the hydrophilic channels, two complexes exhibit good proton conductivities of over 10−5 S cm−1 for 1 and 10−4 S cm−1 for 2 at 100 °C in the relative humidity range 35–98%. These complexes make us better understand the influence of the decoration of Keggin-type polyanions based-on such an organic building block as dmphen on the resultant structures and proton conductivities of organic–inorganic hybrid materials.
Supplementary Material
CCDC-1513030 and 1513031 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. IR spectra of 1 and 2. The curves of the thermogravimetric analyses for complexes 1 and 2. The powder X-ray diffraction data of 1 and 2.
References
H. Mansouri-Torshizi, M. Saeidifar, A. Divsalar, and A. A. Saboury (2010). Spectrochim. Acta A 77, 312.
G. Faraglia, S. Sitran, and D. Montagner (2005). Inorg. Chim. Acta 258, 971.
C. A. Johns, G. M. Golzar-Hossain, K. M. Abdul-Malik, S. Zahir-Haider, and U. K. Rowzatur-Romman (2001). Polyhedron 20, 721.
M. J. Plater, M. R. Foreman, M. S. Skakle, and R. A. Howie (2002). Inorg. Chim. Acta 332, 135.
A. M. Thumas, A. D. Naik, M. Nethaji, and A. B. Chakravarty (2004). Inorg. Chim. Acta 357, 2315.
R. E. Shepherd, Y. Chen, R. A. Kortes, and M. S. Ward (2000). Inorg. Chim. Acta 303, 30.
Y. Wang and N. Okabe (2005). Inorg. Chim. Acta 358, 3407.
V. Amani, N. Safari, B. Notash, and H. R. Khavasi (2009). J. Coord. Chem. 62, 1939.
R. D. Willett, G. Pon, and C. Nagy (2001). Inorg. Chem. 40, 4342.
Q. X. Han, C. He, M. Zhao, B. Qi, J. Y. Niu, and C. Y. Duan (2013). J. Am. Chem. Soc. 135, 10186.
M. L. Wei, X. X. Wang, and X. Y. Duan (2013). Chem. Eur. J. 19, 1607.
C. M. Roch, E. Ayrault, L. Lisnard, J. Marrot, F. X. Liu, and F. Secheresse (2006). J. Clust. Sci. 17, 283.
Y. W. Liu, S. M. Liu, X. Y. Lai, J. Miao, D. F. He, N. Li, F. Luo, Z. Shi, and S. X. Liu (2015). Adv. Funct. Mater. 25, 4480.
E. L. Zhou, C. Qin, P. Huang, X. L. Wang, W. C. Chen, K. Z. Shao, and Z. M. Su (2015). Chem. Eur. J. 21, 11894.
D. Y. Du, J. S. Qin, S. L. Li, Z. M. Su, and Y. Q. Lan (2014). Chem. Soc. Rev. 43, 4615.
E. L. Zhou, C. Qin, X. L. Wang, K. Z. Shao, and Z. M. Su (2015). Chem. Eur. J. 21, 13058.
T. Dong, J. B. Du, M. Cao, and C. W. Hu (2010). J. Clust. Sci. 21, 155.
M. L. Wei, H. H. Li, and X. X. Wang (2012). J. Clust. Sci. 23, 325.
C. Y. Duan, M. L. Wei, D. Guo, C. He, and Q. J. Meng (2010). J. Am. Chem. Soc. 132, 3321.
X. Wang, C. Y. Kong, J. J. Lai, and M. L. Wei (2016). J. Clust. Sci. 27, 645.
X. Wang, C. Y. Duan, C. Y. Kong, and M. L. Wei (2016). J. Coord. Chem. 69, 779.
C. R. De Silva, J. R. Maeyer, R. Wang, G. S. Nichol, and Z. Zheng (2007). Inorg. Chim. Acta 360, 3534.
I. Warad, M. Al-Ali, B. Hammouti, T. B. Hadda, R. Shareiah, and M. Rzaigui (2013). Res. Chem. Intermed. 39, 2451.
V. Singh, D. Singh, R. Kumar, S. Lata, and M. Sharma (2010). Asian J. Chem. 22, 5482.
SMART and SAINT Area Detector Control and Integration Software (Siemens Analytical X-ray Systems Inc, Madison, 1996).
G. M. Sheldrick SHELXTL Version 5.1, Software Reference Manual (Bruker AXS Inc, Madison, 1997).
I. D. Brown and D. Altermatt (1985). Acta Crystallogr. Sect. B Struct. Sci. 41, 244.
M. L. Wei, J. J. Sun, and X. Y. Duan (2014). Eur. J. Inorg. Chem. 2014, 345.
S. C. Nyburg and C. H. Faerman (1985). Acta Crystallogr. Sect. B Struct. Sci. 41, 274.
H. Strasdeit, I. Büsching, S. Behrends, W. Saak, and W. Barklage (2001). Chem. Eur. J. 7, 1133.
O. Nakamura, T. Kodama, I. Ogino, and Y. Miyake (1979). Chem. Lett. 1, 17.
K. D. Kreuer, A. Rabenau, and W. Weppner (1982). Angew. Chem. Int. Ed. 21, 208.
R. D. Hancock and A. E. Martell (1989). Chem. Rev. 89, 1875.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21171050 and 21501047).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kong, CY., Duan, XY., Lai, JJ. et al. Syntheses, Structures and Proton Conductivities of Two Complexes Based on Decorated Keggin-Type Clusters: {[M(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (M = Cu and Zn; dmphen = 4,7-dimethyl-1,10-phenanthroline). J Clust Sci 28, 1407–1420 (2017). https://doi.org/10.1007/s10876-016-1144-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1144-2