Abstract
“This porridge is too hot!” she exclaimed. So, she tasted the porridge from the second bowl. “This porridge is too cold,” she said. So, she tasted the last bowl of porridge. “Ahhh, this porridge is just right,” she said happily and she ate it all up. While this describes the adventures of Goldilocks in the classic fairytale “The Story of Goldilocks and the Three Bears,” it is an ideal analogy for the need for balanced signaling mediated by phosphatidylinositol-3-kinase (PI3K), a key signaling hub in immune cells. Either too little or too much PI3K activity is deleterious, even pathogenic—it needs to be “just right”! This has been elegantly demonstrated by the identification of inborn errors of immunity in key components of the PI3K pathway, and the impact of these mutations on immune regulation. Detailed analyses of patients with germline activating mutations in PIK3CD, as well as the parallel generation of novel murine models of this disease, have shed substantial light on the role of PI3K in lymphocyte development and differentiation, and mechanisms of disease pathogenesis resulting not only from PIK3CD mutations but genetic lesions in other components of the PI3K pathway. Furthermore, by being able to pharmacologically target PI3K, these monogenic conditions have provided opportunities for the implementation of precision medicine as a therapy, as well as to gain further insight into the consequences of modulating the PI3K pathway in clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphatidylinositol-3-Kinase (PI3K) Is a Key Signaling Node in Immune Cells
PI3Ks are lipid kinases with critical roles in cell biology [1,2,3]. There are three classes of PI3K (Class IA, B; II; and III) with Class IA PI3K being the predominant type involved in lymphocyte signaling [1,2,3]. Class IA PI3Ks (hereafter referred to as PI3K) are heterodimers, comprising a regulatory (p85α, p85β, p55) and catalytic (p110α, β or δ) subunit. While p110α and β are ubiquitously expressed, p110δ is largely restricted to leukocytes [1,2,3]. PI3K is activated following engagement of the BCR and TCR, and can be enhanced by co-engagement of other receptors. In B cells, CD19 is the main mediator of PI3K activity downstream of the BCR; however, BAFF-R and CD40 also induce or amplify PI3K signaling [1,2,3,4,5]. Similarly, CD28 and ICOS are key co-receptors involved in PI3K-mediated T cell activation [1, 6, 7]. Cytokines can also activate PI3K in lymphocytes [1, 8]. Just like Newton’s Third Law of Physics, the action of PI3K is antagonized by opposing forces in the form of the lipid phosphatases PTEN and SHIP [1,2,3]. Thus, the coordinated and balanced functions of PI3K and PTEN regulate major signaling pathways downstream of Ag and co-stimulatory receptors in lymphocytes critical for survival, growth, differentiation, and metabolism.
The Role of PI3K Signaling in B and T Cells: Evidence from Gene-Targeted Mice
B Cells
Analysis of mice that lack p85α or p110δ or express catalytically inactive p110δ confirmed the importance of PI3K in B cell development and function. These mice have ~ 50% fewer follicular B cells, and a severe reduction in marginal zone (MZ) and B1 cells [9,10,11,12,13,14]. More striking though were the dramatically reduced humoral immune responses following immunization with T-dependent (TD) Ags, consistent with impaired germinal center (GC) formation, proliferation, and survival of mutant B cells [9,10,11,12,13,14]. Thus PI3K-dependent signaling is required for B cell development, survival, and eliciting Ab responses.
PTEN deficiency resulted in increased B cell numbers, especially B1 and MZ cells, increased serum IgM, and increased survival and proliferation in vitro [15, 16]. However, paradoxically, PTEN deficiency also resulted in poor GC and TD Ab responses in vivo [15,16,17,18]. These mice also revealed that PI3K inhibits class switch recombination (CSR) by suppressing induction of Aicda [19, 20], encoding activation-induced cytidine deaminase (AID), or Ig germline transcripts [17, 18]. The comparable findings for reduced or heightened PI3K signaling provided the first insights into the necessity for balanced signaling via PI3K for qualitatively and quantitatively robust humoral immune responses.
T and NK Cells
T cell development in mice lacking functional p110δ or p85α is largely intact [10, 12]. However, proliferation and induction of Th1, Th2, and Th17 effector CD4+ T cells were reduced in cells lacking functional p110δ [12] and increased by deletion of PTEN [21,22,23]. T follicular helper (Tfh) cell generation was also blocked in mice lacking p110δ, or expressing a mutant form of ICOS unable to recruit PI3K [7, 24]. Thus, PI3K is critical for CD4+ T cell differentiation. NK cell development and cytotoxicity were also greatly impaired in the absence of p85 or p110δ [25, 26]. Collectively, PI3K is fundamental for the development, migration, expansion, survival, and differentiation of adaptive immune cells.
Early Clues from Human: p85 Deficiency due to Homozygous PIK3R1 Mutation
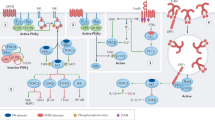
In 2012, the first report of human inborn errors in PI3K signaling was described. Conley and colleagues identified one patient with agammaglobulinemia, B cell lymphopenia, and autoimmunity due to a homozygous nonsense mutation in PIK3R1, encoding p85α, p55, and p50 [27] (Fig. 1). Since p85 stabilizes p110δ, p85 deficiency greatly reduced levels of p110δ [27]. Recently, another two patients (siblings) were reported with recessive PIK3R1 mutations who also presented with agammaglobulinemia and B-lymphopenia [55]. In contrast to B cells, T cell development and differentiation were unaffected by p85 deficiency [27, 55]. These findings demonstrated the non-redundant function of PI3K in human B cell ontogeny (Fig. 1).
Strength of PI3K signaling impacts immunity: the “Goldilocks” effect. Recessive loss of function mutations in PIK3R1 or PIK3CD that attenuate PI3K signaling result predominantly in immune deficiency due to impaired B cell development and agammaglobulinemia. Dominant mutations in PIK3CD or PIK3R1 causing hyperactive PI3K signaling result in immune dysregulation (immune deficiency, autoimmunity, malignancy). Mutations in PTEN also manifest as immune dysregulation but generally not as severe as dominant PIK3CD or PIK3R1 mutations. See Table 1 for more detailed clinical information. AR autosomal recessive, AD autosomal dominant, JIA juvenile idiopathic arthritis, IBD inflammatory bowel disease, AIHA autoimmune hemolytic anemia, ITP idiopathic thrombocytopenia
Activating Mutations in PIK3CD Cause a Novel Immune Dysregulatory Condition
In 2013 and 2014, groups from the UK/Europe and USA independently identified germline heterozygous mutations in PIK3CD, encoding the p110δ catalytic subunit of PI3K, in 31 individuals from 14 unrelated families [56, 57]. Since then, > 200 patients with such PIK3CD mutations have been identified [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Affected individuals presented with recurrent sinopulmonary infections (mostly due to S pneumonia and H influenza), lymphadenopathy, splenomegaly, viremia (EBV, CMV, VZV), and EBV-induced disease. Some patients also developed lymphoproliferation, autoimmunity (cytopenias, colitis, enteropathy, thyroiditis, arthritis), and/or B-lymphoma [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. The condition presents initially with recurrent respiratory tract infections, followed by lymphoproliferation, gastrointestinal disease, and then autoimmune manifestations [38]. Patients also have lymphopenia, normal/elevated levels of IgM, variable levels of serum IgG (characteristically reduced IgG2), and low-normal IgA. However, Ag-specific Abs against polysaccharide-containing vaccines were low/undetectable in all patients, while those against protein Ags were reduced in ~ 50–70% of cases [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] (Table 1, Fig. 1). Overall survival is ~ 80%, but event-free survival is < 40% [32, 43].
Remarkably, the same mutation—c.3061G > A, resulting in a p.E1021K substitution at a highly conserved residue in the C-terminal lobe of p110δ—was found in all patients reported by Angulo et al. [56] and 6/14 patients reported by Lucas et al. [57]. Overall, ~ 80% of all patients identified harbor the E1021K mutant [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Importantly, mutations identified in either the C-terminal lobe (E1025G) or other functional domains of p110δ (E81K, G124D, N334K, R405C, C416R, E525K, E525A, R929C) result in similar clinical features as E1021K [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43, 56,57,58] indicating limited if any genotype/phenotype correlation.
Comparison of germline PIK3CD mutations to somatic mutations identified in PIK3CA (encoding p110α) in different cancers predicted them to be activating or gain of function (GOF) [57]. This was confirmed by biochemical characterization of PIK3CD mutations [39, 56,57,58,59]. Thus, PIK3CD GOF mutations cause a novel human disorder of immune dysregulation involving immunodeficiency, lymphoproliferation, organomegaly, and autoimmunity (Table 1), thereby highlighting the complexities of PI3K signaling. This condition has been termed activated PI3K delta syndrome 1 (APDS1).
From an historical perspective, it is worth noting that the E1021K variant was actually first reported in 2006 in a single Taiwanese patient suffering from recurrent upper respiratory tract infections, extensive varicella infection, pneumonia, bronchiectasis, B-lymphopenia, and hypogammaglobulinemia [60]. While these investigators predicted that this variant was likely pathogenic, no studies were performed to determine the impact of the E1021K substitution on PI3K function [60]. Despite this, this report was clearly the first clinical description of a patient with APDS1.
Treatment
Several therapeutic modalities have been employed to treat the major symptoms of APDS1. Thus, patients have received Ig replacement and antibiotics to control recurrent infections, and immunosuppression (corticosteroids, azathioprine mycophenylate) to treat autoimmune manifestations. B cell depletion (Rituximab) and splenectomy have also been used to manage autoimmune hemolytic anemia, splenomegaly, and lymphoproliferation in some patients [32, 38, 42, 43].
A key downstream target of PI3K is Akt, which activates mTOR [1,2,3]. The finding of hyper-active PI3K signaling in APDS1 enabled gene-targeted therapy in the form of mTOR inhibitors [57]. Rapamycin is effective at ameliorating disease in > 65% of patients, resulting in moderate/good responses, especially for lymphoproliferation [32, 35, 38, 61]. Importantly, rapamycin enabled a reduction in the dose of, or need for treatment with, steroids [38]. However, gastrointestinal disease and cytopenias responded less well to rapamycin, and treatment was discontinued in some patients due to adverse events [32, 43]. Furthermore, a significant proportion of patients experienced no benefit from rapamycin therapy [38].
More recently, efficacy of targeted therapy by the p110δ-specific inhibitor leniolisib has been trialed in six APDS1 patients [62]. Leniolisib was well tolerated over a 12-week period, with no reports of significant adverse events [62]. Leniolisib greatly reduced lymphadenopathy and splenomegaly, and ameliorated autoimmune cytopenias [62]. Cellular immune dysregulation was also improved by leniolisib, with reductions in proportions of transitional B cells and senescent T cells, reduced levels of serum IgM and inflammatory mediators (CXCL10, IFNγ, TNFα), and increased frequencies of naïve B cells [62]. All of these readouts correlated with greatly improved patient well-being, and a reduced requirement for Ig replacement in three of the six treated patients. The effect of leniolisib on B cell lymphoma in APDS1 patients is unknown. Despite the limited trial and small number of patients tested, this study established the potential of directly inhibiting p110δ as precision medicine for APDS1. However, p110δ inhibitors have been used to treat human B cell malignancies, and serious adverse events have been reported [3]. Thus, further trials are needed to establish potential deleterious effects of p110δ inhibitors in the treatment of APDS1.
While these interventions can treat symptoms of APDS1, the only cure is hematopoietic stem cell transplant (HSCT). To date, two studies have provided detailed reports on outcomes of HSCT in 20 APDS1 patients [42, 43]; outcomes for an additional 13 patients have been included in cohort reports [32, 38]. Indications for HSCT were lymphoproliferation and recurrent infections. Survival post-HSCT for all patients was ~ 80%; this was accompanied by significantly reduced infections, lymphoproliferation, hypogammaglobulinemia, enteropathy and cytopenias, and cessation of immunosuppression and Ig replacement [32, 38, 42, 43]. While these findings are similar to those for HSCT in other PIDs, HSCT was associated with complications, including frequent graft failure, variable donor chimerism, and hemophagocytic syndrome, in ~ 90% of APDS1 patients [42, 43]. Despite this, most patients achieved donor engraftment. Thus, HSCT represents a promising cure for APDS1; however, studies detailing outcomes from more transplanted patients will enable optimization of conditioning regimes to improve survival rates and reduce post-HSCT complications.
Cellular Defects in Humans due to PIK3CD GOF Mutations
To provide a cellular basis for immune dysregulation in APDS1, several studies have examined the impact of PIK3CD GOF mutations on the development and function of B cells, T cells, and NK cells.
B Cells
B cells egress from the bone marrow (BM) as transitional cells and undergo further maturation in the periphery to become naïve B cells, which can then differentiate into memory and plasma cells. Peripheral blood of APDS1 patients contains low/normal proportions of circulating B cells; however, there is an accumulation of transitional B cells [28, 35, 36, 39, 56, 57, 63], predominantly of a very immature phenotype [63]. Naïve B cells in these patients also appear to be at a less mature stage of development than naïve B cells in healthy donors, suggestive of a defect in B cell development [63]. Indeed, analysis of BM from PIK3CD GOF patients revealed significant increases in pre-BII and immature B cells, and fewer circulating mature B cells, compared to healthy donors [36, 41, 63]. PIK3CD GOF patients also have few memory B cells [28, 35, 36, 39, 56, 57, 63], and the contracted memory population lacked IgG+ and IgA+ cells [36, 57, 63]. Interestingly, despite the reduction in CSR, somatic hypermutation (SHM) was largely normal [36, 56], suggesting that the role of AID in CSR versus SHM is differentially influenced by the magnitude of PI3K signaling. Thus, hyperactive PI3K signaling not only impacts B cell development in the BM, arresting development at the pre-B II/immature → transitional stage, but also impedes the generation of total and class switched memory B cells in response to infection or vaccination (Fig. 2a).
Defects in generating memory cells and poor humoral immunity in PIK3CD GOF patients could result from cell intrinsic or extrinsic defects. To test this, we examined responses of PIK3CD GOF B cells to in vitro stimulation. While proliferation was normal [57, 63], generation of IgG-secreting cells from naïve B cells, and IgG- and IgA-secreting cells from transitional B cells, was greatly reduced by PIK3CD GOF mutations [57, 63]. PIK3CD GOF transitional B cells were unable to acquire expression of a suite of genes associated with plasma cell differentiation [63], suggesting that hyperactive PI3K signaling intrinsically interferes with the molecular machinery required for terminal B cell differentiation (Fig. 2a).
CD4+ T Cells
Approximately 50% of PIK3CD GOF patients have CD4+ T cell lymphopenia due to marked reductions in naïve cells [31, 32, 56, 57]. Within the memory population, there is a selective increase in circulating Tfh (cTfh) cells, defined by a CD4+CD45RA−CXCR5+PD1hi phenotype [31, 39, 64] (Fig. 2b). However, the distribution of cTfh subsets is skewed in PIK3CD GOF patients, with a disproportionate increase in CXCR3+CCR6− Th1-type and a corresponding decrease in CCR6+CXCR3− Th17-type Tfh cells [31, 64]. Functional analysis confirmed exaggerated IFNγ and reduced Th17 cytokine production by PIK3CD GOF cTfh cells compared to healthy donors [31]. CCR6+ cTfh cells are the predominant B-helper cTfh subset, while CXCR3+ Tfh cells are poor inducers of B cell differentiation [65,66,67]. Thus, while the observation of increased cTfh cells in PIK3CD GOF patients contradicts the clinical findings of B cell immunodeficiency, this discrepancy can be explained by the predominance of IFNγ+ cTfh cells, which are less effective at promoting B cell function, and the diminution in “helpful” Th17-cTfh cells. Thus, impaired humoral immunity in APDS1 patients due to intrinsic B cell defects would be compounded by expansion of dysfunctional Tfh cells incapable of supporting the generation of memory and plasma cells responsible for long-lived serological memory (Fig. 2b).
PIK3CD GOF memory CD4+ T cells and cTfh cells also exhibited marked elevations in IL-4, IL-5, and IL-13 production compared to healthy donors [31] (Fig. 2b). Interestingly, ~ 25–50% of patients displayed Th2-related pathologies (asthma, eosinophilic esophagitis, eczema/atopic dermatitis, urticaria, rhinoconjunctivitis; Table 1); these were not accompanied by increased IgE or allergies [31, 38]. These findings suggest that dysregulated Th2 cytokine production by PIK3CD GOF CD4+ T cells, together with previous findings that PI3K signaling inhibits IgE production by B cells [68], explains the high incidence of non-IgE-mediated Th2-type diseases in APDS1 (Fig. 2b). These functional aberrations in PIK3CD GOF CD4+ T cells provide mechanisms to explain hypogammaglobulinemia, impaired Ab responses to infection and vaccination, a lack of memory B cells, and Th2-type pathologies in APDS1 (Figs. 1 and 2a, b; Table 1).
Cytotoxic Lymphocytes
The chronic viremia, increased EBV-induced disease, and B cell malignancy in APDS1 patients would predict defects in cytotoxic lymphocytes. PIK3CD GOF patients lack naïve CD8+ T cells and have increased TEM and TEMRA cells [33, 35, 37, 56, 57]. Lucas et al. originally noted that a large proportion of CD8+ T cells in PIK3CD GOF patients expressed CD57, leading to the proposal that hyperactive PI3K signaling promotes T cell senescence, thereby compromising CD8+ T cell function [57]. Subsequent studies found that, in addition to CD57, a substantial proportion of PIK3CD GOF CD8+ T cells co-express elevated levels of receptors associated with senescence/exhaustion, such as 2B4, PD1, and KLRG1 [33, 37, 69]. Functional analysis revealed reduced proliferation and IL-2 production, but increased apoptosis of PIK3CD GOF CD8+ T cells [33, 56, 57, 69]. These defects—accelerated T cell senescence, limited effector function, increased death—likely reflect the altered metabolic state of CD8+ T cells due to constitutive activation of the PI3K pathway, thereby culminating in CD8+ T cell dysfunction [70] (Fig. 2c).
Susceptibility of APDS1 patients to herpes virus infections [32, 35, 56, 57, 60] suggests an inability to generate functional virus-specific CD8+ T cells. However, frequencies of EBV-specific CD8+ T cells were normal/increased in PIK3CD GOF patients compared to healthy donors [33, 37, 57]. Furthermore, most EBV-specific CD8+ T cells in PIK3CD GOF patients exhibited a CD45RA−CCR7− TEM phenotype typical to that of EBV-specific CD8+ T cells in healthy donors [33, 37]. Thus, there is no major defect in generating memory-type EBV-specific cells in these individuals. Despite this, PIK3CD GOF EBV-specific CD8+ T cells acquire a severely senescent/exhausted state [33] and have impaired cytotoxicity against autologous B cells [33, 69]. Collectively, co-expression of high levels of regulatory receptors associated with terminal differentiation and exhaustion, predisposition to apoptosis and impaired cytotoxicity provide cellular mechanisms for the inability of PIK3CD GOF CD8+ T cells to contain EBV infection, resulting in EBV viremia (Figs. 1 and 2c; Table 1).
NK cells also play important roles in anti-viral immunity. NK cells and subsets are generated in normal numbers in PIK3CD GOF patients [33, 71]. However, PIK3CD GOF NK cells also had an altered phenotype and an inability to kill specific target cells [33, 71]. Decreased cytotoxicity of PIK3CD GOF NK cells was attributed to impaired synapse formation between NK and target cells, as well as reduced mobilization of the lytic machinery to the immune synapse in NK cells [71].
Overall, PIK3CD GOF mutations significantly impact the development, differentiation, and/or effector function of all populations of human lymphocytes. These defects operate intrinsically, as well as combinatorially, to manifest as the predominant clinical features of APDS1 patients: impaired humoral immunity (defective B cells, skewed/dysfunctional Tfh cells), allergic disease (aberrant Th2 skewing), and viral susceptibility and B cell lymphoma (impaired CD8 T cell and NK cell cytotoxicity; senescence) (Figs. 1 and 2; Table 1).
Modeling PIK3CD GOF Mutations and Disease in Mice
Findings from Pten-deficient mice have yielded important information regarding the impact of excessive PI3K signaling on immunity [15, 16, 19, 21, 72,73,74]. However, this is not an ideal model for PIK3CD GOF because it is not clear whether PTEN deficiency results in the same temporal and quantitative changes in PI3K signaling. Furthermore, PTEN regulates p110δ, as well as p110α and p110γ [1, 3]. For these reasons, four groups recently and independently generated strains of gene-edited mice that express the common E1021K (E1020 in mice) mutation in one allele of Pik3cd [63, 64, 75, 76]. These studies have complemented analyses of PIK3CD GOF patients and provide an excellent model to elucidate lineage-specific mechanisms of disease pathogenesis in vivo, independent of chronic infection or various therapies that are routinely used to treat the patients.
B Cells
Analysis of BM from Pik3cd GOF mice revealed a decrease in absolute B cell number, a relative increase in pro-B, and significant decrease in mature recirculating B cells. Mutant mice also displayed increases in peritoneal and splenic B1a cells, implicating PI3K in promoting innate B cell expansion. Splenic transitional and MZ B cell populations were also increased, at the expense of follicular B cells [63, 64, 75, 76] (Fig. 2a).
Unimmunized Pik3cd GOF mice displayed an increased number of GC and plasma cells [64, 76]. Interestingly, following immunization with specific Ag, little if any increase in GCs was seen from Pik3cd GOF B cells [63, 64, 76]. However, the structure of the GCs was disordered [64, 76], and Pik3cd GOF murine B cells had substantial defects in CSR in vivo and in vitro [63, 64, 76]. In contrast, affinity maturation was unaffected [63, 64]. Importantly, the in vitro CSR defect could be corrected by the p110δ-specific inhibitor leniolisib [63]. This rescue in CSR directly correlated with increased levels of expression of Aicda by activated murine Pik3cd GOF B cells [63]. These findings provided additional evidence that suppression of CSR by excessive PI3K activity, as previously observed in Pten mutant mice [19, 20], results from repressing induction of Aicda.
CD4+ T Cells
Similar to APDS1 patients, Pik3cd GOF mice have decreased frequencies of naïve CD4+ T cells and a concurrent increase in memory cells, becoming more pronounced over time [31, 64, 75]. In vitro cultures of naïve CD4+ T cells revealed that Pik3cd GOF increases Th1 and Th2 differentiation but inhibited Th17 differentiation [31], suggesting the increased Th2 disease seen in PIK3CD GOF patients is T cell intrinsic.
Unimmunized Pik3cd GOF mice have increased Tfh cells in peripheral lymphoid organs [31, 64, 75], mirroring that observed in the blood of patients [31, 39, 64]. Immunization could increase the numbers of Tfh cells in Pik3cd GOF mice in an ICOS-independent manner [64], inferring that PI3K hyperactivation overcomes the requirement for ICOS-ICOSL interactions needed to generate Tfh cells [7, 24]. However, other studies did not find increased Tfh cells following immunization [31] or infection [77]. These data suggest that the threshold for generating Tfh cells from Pik3cd GOF CD4+ T cells is lower than for wild-type CD4+ T cells; however, once there is sufficient signal for wild-type CD4+ T cells to differentiate, the outcome is comparable to Pik3cd GOF CD4+ T cells [31, 64, 75].
Interestingly, the generation of Tfh cells from wild-type CD4+ T cells was increased in the presence of Pik3cd GOF cells [31]. This suggests that altered activation of non-T cells is an important driver of the increased Tfh cells seen in patients. Despite the generation of Ag-specific Tfh cells from Pik3cd GOF CD4+ T cells, their ability to support B cell responses is significantly impaired [31]. Pik3cd GOF Tfh cells were found to express high levels of FasL and IFNγ, which impaired GC formation and alter class switching, respectively [31].
CD8+ T Cells
These mouse models also supported cell intrinsic effects of hyperactive PI3K signaling on CD8+ T cells with a slight reduction in the CD8+ T cell compartment within the thymus [31, 75] and significantly decreased naïve, and increased memory, CD8+ T cells in the periphery [33, 75]. In vitro cultures of naïve PI3K GOF CD8+ T cells yielded increased expression of IFNγ and TNFα [33]. These changes in cytokine expression occurred independent of infections or immunizations, therefore being exclusively driven by the Pik3cd GOF mutation in CD8+ T cells [33].
Thus, hyperactive PI3K signaling in these mouse models recapitulated the phenotype of APDS1 with (1) defective central and peripheral B cell development, and impaired B cell responses to specific Ags, (2) naïve CD4+ T cell lymphopenia but increased generation of Tfh cells, albeit dysfunctional, and Th2 cells, and (3) aberrant differentiation of CD8+ T cells (Figs. 1 and 2). These mice also developed splenomegaly and autoAbs, further underscoring their utility as models of disease due to PIK3CD GOF mutations in humans.
Related Clinical Phenotypes Resulting from Mutations in the PI3K Pathway
Since the discoveries of recessive PIK3R1 [27, 55] and heterozygous PIK3CD GOF [56, 57, 60] mutations as causes of novel inborn errors of immunity, immune dysregulatory conditions resulting from monoallelic or biallelic mutations in these and other genes encoding key components of the PI3K signaling pathway have been identified.
Heterozygous PIK3R1 LOF Mutations
In 2014, heterozygous germline mutations in PIK3R1 were found to cause a complex immune dysregulatory disorder remarkably similar to PIK3CD GOF patients [44, 45] (Fig. 1; Table 1). To date, > 50 patients with heterozygous PIK3R1 mutations have been identified. Remarkably, all patients have mutations that affect a donor or acceptor splice site resulting in skipping of exon 11 and subsequent in-frame deletion of amino acids 434–475 [44,45,46,47,48,49,50,51,52]. This region of p85 is immediately proximal to the amino acid sequence that binds p110 to regulate its catalytic activity [44, 45, 59]. Consequently, the inability of mutant p85 to regulate p110δ activity directly, or indirectly through a failure to interact with PTEN, results in excessive PI3K p110δ function and signaling [44, 45, 59]. Thus, PIK3R1 LOF splicing mutations functionally mimic PIK3CD GOF mutations, resulting in a common biochemical phenotype despite distinct pathological molecular lesions (Fig. 1; Table 1).
Consistent with the biochemistry, PIK3R1 LOF patients phenocopy PIK3CD GOF patients clinically (recurrent respiratory tract infections, lymphoproliferation, hypogammaglobulinaemia, EBV/CMV viremia, lymphadenopathy, B cell malignancy, autoimmunity) and immunologically (memory B cell deficiency, increased transitional B cells, normal/elevated serum IgM, reduced naïve CD4+ and CD8+ T cells, increased senescent CD8+ T cells; Fig. 1; Table 1) [44,45,46,47,48,49,50,51,52]. This condition has been termed APDS2.
Despite many similarities between APDS1 and APDS2 patients, a key difference is the high frequency of dysmorphic and developmental abnormalities in patients with PIK3R1 mutations [46, 51](Table 1). Interestingly, some PIK3R1 LOF patients were initially diagnosed with SHORT (Short stature, Hyper-extensible joints, Ocular depression, Rieger anomaly, Teething delays) syndrome [47, 49], which is also associated with partial lipodystrophy, insulin resistance, and diabetes. The genetic lesions in SHORT syndrome are heterozygous mutations in distal exons of PIK3R1 [78,79,80]. These disparities between the PIK3R1 LOF and PIK3CD GOF phenotypes could be explained by (1) ubiquitous expression of p85 compared to mainly leukocyte-restricted p110δ, (2) p110δ-independent functions of p85, or (3) involvement of PI3K catalytic isoforms other than p110δ in the disease pathology.
Heterozygous PTEN LOF Mutations
Heterozygous PTEN mutations have long been associated with PTEN harmatoma tumor syndromes, and neurodevelopmental delay [81]. However, PTEN deficiency has recently also been associated with an APDS-like clinical phenotype, including autoimmunity, lymphoid hyperplasia, hypogammaglobulinemia, reduced responses to vaccinations and recurrent infections, CD4+ T cell lymphopenia, transitional B cell accumulation, and reduced memory B cells [39, 53, 54, 82] (Fig. 1; Table 1). The underlying biochemical defect in these patients is likely to be similar to PIK3CD GOF and PIK3R1 LOF individuals, as PTEN mutations also result in enhanced PI3K signaling [39, 53]. However, more detailed analysis of immune defects in individuals with PTEN mutations is required to determine whether the severity and penetrance of their clinical feature are as dramatic as those observed in individuals with PIK3CD GOF or PIK3R1 LOF mutations (Fig. 1; Table 1).
Autosomal Recessive p110δ Deficiency
Three kindreds comprising four individuals have been identified with recessive LOF mutations in PIK3CD that greatly reduce or completely abolish p110δ expression and/or activity [83,84,85]. PIK3CD LOF patients had a history of recurrent sinopulmonary infections, hypogammaglobulinemia, and autoimmune complications (e.g., inflammatory bowel disease). Immune phenotyping revealed dramatically reduced frequencies of peripheral B cells in three patients (normal frequencies in one), with low/normal frequencies of T and NK cells, though the T cells were mostly naïve [83,84,85] (Fig. 1). These findings resemble patients with recessive mutations in PIK3R1 [27, 55] (Fig. 1). Functional analysis revealed intact T cell proliferation but impaired NK cell cytotoxicity [83,84,85]. One patient died of sepsis at age 13 years [84]. Notably, a sibling also died of sepsis at 6 months of age [85]. While this preceded genetic diagnosis, it is likely that this individual was also p110δ-deficient [85].
Interestingly, two siblings with recessive PIK3CD LOF mutations have been reported who also harbor a homozygous mutation in KNSTRN, encoding small kinetochore-associated protein (SKAP), which is involved in regulating microtubule dynamics during mitosis [86]. These patients not only generally shared the immune and clinical features of PIK3CD LOF patients detailed above, but also exhibited dysmorphic features and developmental delay [86]. These latter features are probably due to SKAP-deficiency, while the immune pathology likely results from the PIK3CD lesion but may also be exacerbated by SKAP-deficiency in immune cells. Overall, the discovery of humans with p110δ deficiency, coupled with the striking similarities to p85-deficient humans [27, 55] and p110δ-mutant mice [11,12,13, 25, 26], reinforces the non-redundant role for PI3K catalytic activity in the human immune response.
Conclusions
In the few short years since the seminal finding of GOF mutations in PIK3CD [56, 57], we have witnessed substantial advances in our understanding of the critical and balanced roles that PI3K plays in immune regulation, and how aberrations to this balance can manifest as complex immune diseases. While several key findings had been proposed from studies of gene-targeted mice, analysis of humans and corresponding mice harboring specific pathogenic mutations has provided opportunities to substantially extend these discoveries into novel facets of immunology, including human B cell development and function, Th2 cell differentiation, Tfh effector function, and immune cell senescence. However, many areas remain to be explored, such as regulatory T cells, innate lymphoid cells, and innate immune responses. The generation of E1020K mice has yielded an in vivo model that recapitulates many aspects of the human condition. This will enable the discovery of additional mechanisms of disease pathogenesis without the complication of infection and/or therapeutic intervention, and logistical challenges inherent in studying humans, the impact of PI3K GOF in other immune cell lineages, and the pre-clinical testing of off-label or novel therapies as the next-generation treatment of APDS. Such therapies will potentially have application in immune dysregulatory conditions that phenocopy APDS but have unknown genetic etiology. Thus, while APDS is relatively rare, studies to date again underscore the power of combining well-curated patient cohorts, next-generation sequencing, and in vitro and in vivo cell and molecular biology to make fundamental breakthroughs in basic, clinical, and translational immunology. Ongoing investigation of these and related patients can only accelerate further discoveries that will ultimately improve patient outcomes and human health.
References
Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol. 2013;31:675–704. https://doi.org/10.1146/annurev-immunol-032712-095946.
Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–30. https://doi.org/10.1038/nri1056.
Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605–35. https://doi.org/10.1016/j.cell.2017.07.029.
Jellusova J, Miletic AV, Cato MH, Lin WW, Hu Y, Bishop GA, et al. Context-specific BAFF-R signaling by the NF-kappaB and PI3K pathways. Cell Rep. 2013;5(4):1022–35. https://doi.org/10.1016/j.celrep.2013.10.022.
Limon JJ, Fruman DA. Akt and mTOR in B cell activation and differentiation. Front Immunol. 2012;3:228. https://doi.org/10.3389/fimmu.2012.00228.
Yong PF, Salzer U, Grimbacher B. The role of costimulation in antibody deficiencies: ICOS and common variable immunodeficiency. Immunol Rev. 2009;229(1):101–13. https://doi.org/10.1111/j.1600-065X.2009.00764.x.
Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2009;106(48):20371–6. https://doi.org/10.1073/pnas.0911573106.
Ostiguy V, Allard EL, Marquis M, Leignadier J, Labrecque N. IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. J Leukoc Biol. 2007;82(3):645–56. https://doi.org/10.1189/jlb.0806494.
Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283(5400):393–7.
Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, et al. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 1999;283(5400):390–2.
Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196(6):753–63.
Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297(5583):1031–4. https://doi.org/10.1126/science.1073560.
Jou ST, Carpino N, Takahashi Y, Piekorz R, Chao JR, Carpino N, et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol. 2002;22(24):8580–91.
Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–86. https://doi.org/10.1016/j.cell.2009.08.041.
Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4(3):287–94. https://doi.org/10.1038/ni892.
Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197(5):657–67.
Sander S, Chu VT, Yasuda T, Franklin A, Graf R, Calado DP, et al. PI3 kinase and FOXO1 transcription factor activity differentially control B cells in the germinal center light and dark zones. Immunity. 2015;43(6):1075–86. https://doi.org/10.1016/j.immuni.2015.10.021.
Dominguez-Sola D, Kung J, Holmes AB, Wells VA, Mo T, Basso K, et al. The FOXO1 transcription factor instructs the germinal center dark zone program. Immunity. 2015;43(6):1064–74. https://doi.org/10.1016/j.immuni.2015.10.015.
Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, et al. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25(4):545–57. https://doi.org/10.1016/j.immuni.2006.08.015.
Dengler HS, Baracho GV, Omori SA, Bruckner S, Arden KC, Castrillon DH, et al. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9(12):1388–98. https://doi.org/10.1038/ni.1667.
Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14(5):523–34.
Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177(8):5122–8.
Soond DR, Bjorgo E, Moltu K, Dale VQ, Patton DT, Torgersen KM, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115(11):2203–13. https://doi.org/10.1182/blood-2009-07-232330.
Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LM, et al. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol. 2010;185(7):4042–52. https://doi.org/10.4049/jimmunol.1001730.
Awasthi A, Samarakoon A, Dai X, Wen R, Wang D, Malarkannan S. Deletion of PI3K-p85alpha gene impairs lineage commitment, terminal maturation, cytokine generation and cytotoxicity of NK cells. Genes Immun. 2008;9(6):522–35. https://doi.org/10.1038/gene.2008.45.
Guo H, Samarakoon A, Vanhaesebroeck B, Malarkannan S. The p110 delta of PI3K plays a critical role in NK cell terminal maturation and cytokine/chemokine generation. J Exp Med. 2008;205(10):2419–35. https://doi.org/10.1084/jem.20072327.
Conley ME, Dobbs AK, Quintana AM, Bosompem A, Wang YD, Coustan-Smith E, et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85alpha subunit of PI3K. J Exp Med. 2012;209(3):463–70. https://doi.org/10.1084/jem.20112533.
Crank MC, Grossman JK, Moir S, Pittaluga S, Buckner CM, Kardava L, et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol. 2014;34(3):272–6. https://doi.org/10.1007/s10875-014-0012-9.
Elgizouli M, Lowe DM, Speckmann C, Schubert D, Hulsdunker J, Eskandarian Z, et al. Activating PI3Kdelta mutations in a cohort of 669 patients with primary immunodeficiency. Clin Exp Immunol. 2016;183(2):221–9. https://doi.org/10.1111/cei.12706.
Hartman HN, Niemela J, Hintermeyer MK, Garofalo M, Stoddard J, Verbsky JW, et al. Gain of function mutations of PIK3CD as a cause of primary Sclerosing cholangitis. J Clin Immunol. 2014;35:11–4. https://doi.org/10.1007/s10875-014-0109-1.
Bier J, Rao G, Payne K, Brigden H, French E, Pelham SJ, et al. Activating mutations in PIK3CD disrupt the differentiation and function of human and murine CD4(+) T cells. J Allergy Clin Immunol. 2019. https://doi.org/10.1016/j.jaci.2019.01.033.
Coulter TI, Chandra A, Bacon CM, Babar J, Curtis J, Screaton N, et al. Clinical spectrum and features of activated phosphoinositide 3-kinase delta syndrome: a large patient cohort study. J Allergy Clin Immunol. 2017;139(2):597–606 e4. https://doi.org/10.1016/j.jaci.2016.06.021.
Edwards ESJ, Bier J, Cole TS, Wong M, Hsu P, Berglund LJ, et al. Activating PIK3CD mutations impair human cytotoxic lymphocyte differentiation and function and EBV immunity. J Allergy Clin Immunol. 2019;143(1):276–91 e6. https://doi.org/10.1016/j.jaci.2018.04.030.
Kracker S, Curtis J, Ibrahim MA, Sediva A, Salisbury J, Campr V, et al. Occurrence of B-cell lymphomas in patients with activated phosphoinositide 3-kinase delta syndrome. J Allergy Clin Immunol. 2014;134(1):233–6. https://doi.org/10.1016/j.jaci.2014.02.020.
Goto F, Uchiyama T, Nakazawa Y, Imai K, Kawai T, Onodera M. Persistent impairment of T-cell regeneration in a patient with activated PI3K delta syndrome. J Clin Immunol. 2017;37(4):347–50. https://doi.org/10.1007/s10875-017-0393-7.
Wentink M, Dalm V, Lankester AC, van Schouwenburg PA, Scholvinck L, Kalina T, et al. Genetic defects in PI3Kdelta affect B-cell differentiation and maturation leading to hypogammaglobulineamia and recurrent infections. Clin Immunol. 2017;176:77–86. https://doi.org/10.1016/j.clim.2017.01.004.
Wentink MWJ, Mueller YM, Dalm V, Driessen GJ, van Hagen PM, van Montfrans JM, et al. Exhaustion of the CD8(+) T cell compartment in patients with mutations in phosphoinositide 3-Kinase Delta. Front Immunol. 2018;9:446. https://doi.org/10.3389/fimmu.2018.00446.
Maccari ME, Abolhassani H, Aghamohammadi A, Aiuti A, Aleinikova O, Bangs C, et al. Disease evolution and response to rapamycin in activated phosphoinositide 3-kinase delta syndrome: the European Society for Immunodeficiencies-Activated Phosphoinositide 3-kinase delta syndrome registry. Front Immunol. 2018;9:543. https://doi.org/10.3389/fimmu.2018.00543.
Tsujita Y, Mitsui-Sekinaka K, Imai K, Yeh TW, Mitsuiki N, Asano T, et al. Phosphatase and tensin homolog (PTEN) mutation can cause activated phosphatidylinositol 3-kinase delta syndrome-like immunodeficiency. J Allergy Clin Immunol. 2016;138(6):1672–80 e10. https://doi.org/10.1016/j.jaci.2016.03.055.
Michalovich D, Nejentsev S. Activated PI3 Kinase Delta syndrome: from genetics to therapy. Front Immunol. 2018;9:369. https://doi.org/10.3389/fimmu.2018.00369.
Dulau Florea AE, Braylan RC, Schafernak KT, Williams KW, Daub J, Goyal RK, et al. Abnormal B-cell maturation in the bone marrow of patients with germline mutations in PIK3CD. J Allergy Clin Immunol. 2017;139(3):1032–5 e6. https://doi.org/10.1016/j.jaci.2016.08.028.
Nademi Z, Slatter MA, Dvorak CC, Neven B, Fischer A, Suarez F, et al. Hematopoietic stem cell transplant in patients with activated PI3K delta syndrome. J Allergy Clin Immunol. 2017;139(3):1046–9. https://doi.org/10.1016/j.jaci.2016.09.040.
Okano T, Imai K, Tsujita Y, Mitsuiki N, Yoshida K, Kamae C, et al. Hematopoietic stem cell transplantation for progressive combined immunodeficiency and lymphoproliferation in patients with activated phosphatidylinositol-3-OH kinase delta syndrome type 1. J Allergy Clin Immunol. 2019;143(1):266–75. https://doi.org/10.1016/j.jaci.2018.04.032.
Deau MC, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest. 2014;124(9):3923–8. https://doi.org/10.1172/JCI75746.
Lucas CL, Zhang Y, Venida A, Wang Y, Hughes J, McElwee J, et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med. 2014;211(13):2537–47. https://doi.org/10.1084/jem.20141759.
Elkaim E, Neven B, Bruneau J, Mitsui-Sekinaka K, Stanislas A, Heurtier L, et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase delta syndrome 2: a cohort study. J Allergy Clin Immunol. 2016;138(1):210–8 e9. https://doi.org/10.1016/j.jaci.2016.03.022.
Bravo Garcia-Morato M, Garcia-Minaur S, Molina Garicano J, Santos Simarro F, Del Pino Molina L, Lopez-Granados E, et al. Mutations in PIK3R1 can lead to APDS2, SHORT syndrome or a combination of the two. Clin Immunol. 2017;179:77–80. https://doi.org/10.1016/j.clim.2017.03.004.
Kuhlen M, Honscheid A, Loizou L, Nabhani S, Fischer U, Stepensky P, et al. De novo PIK3R1 gain-of-function with recurrent sinopulmonary infections, long-lasting chronic CMV-lymphadenitis and microcephaly. Clin Immunol. 2016;162:27–30. https://doi.org/10.1016/j.clim.2015.10.008.
Petrovski S, Parrott RE, Roberts JL, Huang H, Yang J, Gorentla B, et al. Dominant splice site mutations in PIK3R1 cause hyper IgM syndrome, lymphadenopathy and short stature. J Clin Immunol. 2016;36(5):462–71. https://doi.org/10.1007/s10875-016-0281-6.
Lougaris V, Faletra F, Lanzi G, Vozzi D, Marcuzzi A, Valencic E, et al. Altered germinal center reaction and abnormal B cell peripheral maturation in PI3KR1-mutated patients presenting with HIGM-like phenotype. Clin Immunol. 2015;159(1):33–6. https://doi.org/10.1016/j.clim.2015.04.014.
Olbrich P, Lorenz M, Cura Daball P, Lucena JM, Rensing-Ehl A, Sanchez B, et al. Activated PI3Kdelta syndrome type 2: two patients, a novel mutation, and review of the literature. Pediatr Allergy Immunol. 2016;27(6):640–4. https://doi.org/10.1111/pai.12585.
Martinez-Saavedra MT, Garcia-Gomez S, Dominguez Acosta A, Mendoza Quintana JJ, Paez JP, Garcia-Reino EJ, et al. Gain-of-function mutation in PIK3R1 in a patient with a narrow clinical phenotype of respiratory infections. Clin Immunol. 2016;173:117–20. https://doi.org/10.1016/j.clim.2016.09.011.
Browning MJ, Chandra A, Carbonaro V, Okkenhaug K, Barwell J. Cowden’s syndrome with immunodeficiency. J Med Genet. 2015;52(12):856–9. https://doi.org/10.1136/jmedgenet-2015-103266.
Driessen GJ, H IJ, Wentink M, Yntema HG, van Hagen PM, van Strien A, et al. Increased PI3K/Akt activity and deregulated humoral immune response in human PTEN deficiency. J Allergy Clin Immunol. 2016;138(6):1744–7 e5. https://doi.org/10.1016/j.jaci.2016.07.010.
Tang P, Upton JEM, Barton-Forbes MA, Salvadori MI, Clynick MP, Price AK, et al. Autosomal recessive Agammaglobulinemia due to a homozygous mutation in PIK3R1. J Clin Immunol. 2017;38:88–95. https://doi.org/10.1007/s10875-017-0462-y.
Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866–71. https://doi.org/10.1126/science.1243292.
Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15(1):88–97. https://doi.org/10.1038/ni.2771.
Takeda AJ, Zhang Y, Dornan GL, Siempelkamp BD, Jenkins ML, Matthews HF, et al. Novel PIK3CD mutations affecting N-terminal residues of p110delta cause activated PI3Kdelta syndrome (APDS) in humans. J Allergy Clin Immunol. 2017;140(4):1152–6 e10. https://doi.org/10.1016/j.jaci.2017.03.026.
Dornan GL, Siempelkamp BD, Jenkins ML, Vadas O, Lucas CL, Burke JE. Conformational disruption of PI3Kdelta regulation by immunodeficiency mutations in PIK3CD and PIK3R1. Proc Natl Acad Sci U S A. 2017;114(8):1982–7. https://doi.org/10.1073/pnas.1617244114.
Jou ST, Chien YH, Yang YH, Wang TC, Shyur SD, Chou CC, et al. Identification of variations in the human phosphoinositide 3-kinase p110delta gene in children with primary B-cell immunodeficiency of unknown aetiology. Int J Immunogenet. 2006;33(5):361–9. https://doi.org/10.1111/j.1744-313X.2006.00627.x.
Rae W, Ramakrishnan KA, Gao Y, Ashton-Key M, Pengelly RJ, Patel SV, et al. Precision treatment with sirolimus in a case of activated phosphoinositide 3-kinase delta syndrome. Clin Immunol. 2016;171:38–40. https://doi.org/10.1016/j.clim.2016.07.017.
Rao VK, Webster S, Dalm V, Sediva A, van Hagen PM, Holland S, et al. Effective 'Activated PI3Kdelta Syndrome'-targeted therapy with the PI3Kdelta inhibitor leniolisib. Blood. 2017;130:2307–16. https://doi.org/10.1182/blood-2017-08-801191.
Avery DT, Kane A, Nguyen T, Lau A, Nguyen A, Lenthall H, et al. Germline-activating mutations in PIK3CD compromise B cell development and function. J Exp Med. 2018;215(8):2073–95. https://doi.org/10.1084/jem.20180010.
Preite S, Cannons JL, Radtke AJ, Vujkovic-Cvijin I, Gomez-Rodriguez J, Volpi S, et al. Hyperactivated PI3Kdelta promotes self and commensal reactivity at the expense of optimal humoral immunity. Nat Immunol. 2018;19(9):986–1000. https://doi.org/10.1038/s41590-018-0182-3.
Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136(4):993–1006 e1. https://doi.org/10.1016/j.jaci.2015.05.036.
Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–21. https://doi.org/10.1016/j.immuni.2010.12.012.
Unger S, Seidl M, van Schouwenburg P, Rakhmanov M, Bulashevska A, Frede N, et al. The TH1 phenotype of follicular helper T cells indicates an IFN-gamma-associated immune dysregulation in patients with CD21low common variable immunodeficiency. J Allergy Clin Immunol. 2018;141(2):730–40. https://doi.org/10.1016/j.jaci.2017.04.041.
Zhang TT, Okkenhaug K, Nashed BF, Puri KD, Knight ZA, Shokat KM, et al. Genetic or pharmaceutical blockade of p110delta phosphoinositide 3-kinase enhances IgE production. J Allergy Clin Immunol. 2008;122(4):811–9 e2. https://doi.org/10.1016/j.jaci.2008.08.008.
Cannons JL, Preite S, Kapnick SM, Uzel G, Schwartzberg PL. Genetic defects in phosphoinositide 3-kinase delta influence CD8(+) T cell survival, differentiation, and function. Front Immunol. 2018;9:1758. https://doi.org/10.3389/fimmu.2018.01758.
Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749–61. https://doi.org/10.1038/nri3307.
Ruiz-Garcia R, Vargas-Hernandez A, Chinn IK, Angelo LS, Cao TN, Coban-Akdemir Z, et al. Mutations in PI3K110delta cause impaired natural killer cell function partially rescued by rapamycin treatment. J Allergy Clin Immunol. 2018;142:605–617.e7. https://doi.org/10.1016/j.jaci.2017.11.042.
Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity. 2009;31(5):749–60. https://doi.org/10.1016/j.immuni.2009.08.026.
Chen Z, Getahun A, Chen X, Dollin Y, Cambier JC, Wang JH. Imbalanced PTEN and PI3K signaling impairs class switch recombination. J Immunol. 2015;195(11):5461–71. https://doi.org/10.4049/jimmunol.1501375.
Miletic AV, Anzelon-Mills AN, Mills DM, Omori SA, Pedersen IM, Shin DM, et al. Coordinate suppression of B cell lymphoma by PTEN and SHIP phosphatases. J Exp Med. 2010;207(11):2407–20. https://doi.org/10.1084/jem.20091962.
Stark AK, Chandra A, Chakraborty K, Alam R, Carbonaro V, Clark J, et al. PI3Kdelta hyper-activation promotes development of B cells that exacerbate Streptococcus pneumoniae infection in an antibody-independent manner. Nat Commun. 2018;9(1):3174. https://doi.org/10.1038/s41467-018-05674-8.
Wray-Dutra MN, Al Qureshah F, Metzler G, Oukka M, James RG, Rawlings DJ. Activated PIK3CD drives innate B cell expansion yet limits B cell-intrinsic immune responses. J Exp Med. 2018;215(10):2485–96. https://doi.org/10.1084/jem.20180617.
Preite S, Huang B, Cannons JL, McGavern DB, Schwartzberg PL. PI3K orchestrates T follicular helper cell differentiation in a context dependent manner: implications for autoimmunity. Front Immunol. 2018;9:3079. https://doi.org/10.3389/fimmu.2018.03079.
Chudasama KK, Winnay J, Johansson S, Claudi T, Konig R, Haldorsen I, et al. SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling. Am J Hum Genet. 2013;93(1):150–7. https://doi.org/10.1016/j.ajhg.2013.05.023.
Dyment DA, Smith AC, Alcantara D, Schwartzentruber JA, Basel-Vanagaite L, Curry CJ, et al. Mutations in PIK3R1 cause SHORT syndrome. Am J Hum Genet. 2013;93(1):158–66. https://doi.org/10.1016/j.ajhg.2013.06.005.
Thauvin-Robinet C, Auclair M, Duplomb L, Caron-Debarle M, Avila M, St-Onge J, et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am J Hum Genet. 2013;93(1):141–9. https://doi.org/10.1016/j.ajhg.2013.05.019.
Marsh DJ, Coulon V, Lunetta KL, Rocca-Serra P, Dahia PL, Zheng Z, et al. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet. 1998;7(3):507–15.
Chen HH, Handel N, Ngeow J, Muller J, Huhn M, Yang HT, et al. Immune dysregulation in patients with PTEN hamartoma tumor syndrome: analysis of FOXP3 regulatory T cells. J Allergy Clin Immunol. 2017;139(2):607–20 e15. https://doi.org/10.1016/j.jaci.2016.03.059.
Zhang K, Husami A, Marsh R, Jordan MB. Identification of a phosphoinositide 3-kinase (PI-3K) p110 delta (PIK3CD) deficent individual. J Clin Immunol. 2013;33:673–4.
Sogkas G, Fedchenko M, Dhingra A, Jablonka A, Schmidt RE, Atschekzei F. Primary immunodeficiency disorder caused by phosphoinositide 3-kinase delta deficiency. J Allergy Clin Immunol. 2018;142(5):1650–3 e2. https://doi.org/10.1016/j.jaci.2018.06.039.
Cohen SB, Bainter W, Johnson JL, Lin TY, Wong JCY, Wallace JG, et al. Human primary immunodeficiency caused by expression of a kinase-dead p110delta mutant. J Allergy Clin Immunol. 2018;143:797–799.e2. https://doi.org/10.1016/j.jaci.2018.10.005.
Sharfe N, Karanxha A, Dadi H, Merico D, Chitayat D, Herbrick JA, et al. Dual loss of p110delta PI3-kinase and SKAP (KNSTRN) expression leads to combined immunodeficiency and multisystem syndromic features. J Allergy Clin Immunol. 2018;142(2):618–29. https://doi.org/10.1016/j.jaci.2017.10.033.
Funding
Research performed in the Tangye and Deenick labs is supported by the National Health and Medical Research Council of Australia, Cancer Council NSW and NIAID, NIH. SGT was a Principal Research Fellow of the NHMRC; JB is supported by the Postgraduate Research Scholarship awarded by Fundação Estudar (Brazil); AL is supported by a Tuition Fee Scholarship from UNSW Sydney; TN is supported by a Research Training Program Scholarship awarded by the Australian Government; GU is supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH; EKD is a Scientia Research Fellow of UNSW Sydney.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tangye, S.G., Bier, J., Lau, A. et al. Immune Dysregulation and Disease Pathogenesis due to Activating Mutations in PIK3CD—the Goldilocks’ Effect. J Clin Immunol 39, 148–158 (2019). https://doi.org/10.1007/s10875-019-00612-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-019-00612-9