Abstract
Archaea represent an important component of marine picoplankton. Marine archaeal communities are generally dominated by Marine Group I (MGI) and Marine Group II (MGII), which are affiliated to the phyla Thaumarchaeota and Euryarchaeota. We used pyrosequencing-based analysis of archaeal 16S rRNA genes to investigate archaeal community composition throughout the water column (0–5000 m) at the subarctic (station K2) and subtropical (station S1) stations in western North Pacific. Sampling was conducted on four occasions between 2010 and 2011 to examine seasonal variability in community composition. Four phylogenetic groups (MGI-α, MGI-γ, MGII-α, and MGII-β) belonging to Thaumarchaeota and Euryarchaeota were found throughout the water column, but the relative abundance of each group varied depending on depth. In the surface water, archaeal communities displayed marked seasonal variability at both stations, whereas in deeper waters (300–5000 m), temporal variability in community composition was less evident. Sequences similar to those of the uncultured Marine Benthic Group A, to date found only in marine sediments, were detected throughout the water column at station S1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Archaea represent a widespread and diverse component of marine picoplankton (DeLong 1992; Fuhrman et al. 1992). Marine archaeal communities are generally dominated by two major groups, Marine Group I (MGI) and Marine Group II (MGII), which are affiliated to the phyla Thaumarchaeota (previously classified as Crenarchaeota) and Euryarchaeota, respectively (DeLong 1992; Fuhrman and Davis 1997; Galand et al. 2009). Typically, MGI is the major archaeal group in meso-, bathy-, and abyssopelagic layers (e.g., Karner et al. 2001; Massana et al. 1997; Nunoura et al. 2015), where they appear to oxidize ammonia and fix carbon (Könneke et al. 2005; Santoro et al. 2015), but there is also evidence for heterotrophy or mixotrophy by this group (Alonso-Sáez et al. 2012; Connelly et al. 2014; Hansman et al. 2009; Qin et al. 2014). In contrast, MGII is more prevalent in surface waters, especially in temperate oceans (DeLong et al. 2006; Herndl et al. 2005; Massana et al. 2000). However, the functional role of MGII, which could be either heterotrophic, chemoautotrophic, or both (Baker et al. 2013; Herndl et al. 2005; Teira et al. 2006), has yet to be fully determined.
In surface ocean waters, the seasonal dynamics of MGI and MGII are distinct from each other, displaying annually recurring succession in archaeal community compositions (Galand et al. 2010; Herfort et al. 2007; Hugoni et al. 2013). However, there are limited data on seasonal variability of archaeal community composition in meso- and bathypelagic layers. The currently available data on seasonal dynamics of deep oceanic archaeal communities only provide information at a broad phylogenetic level (Church et al. 2003; Karner et al. 2001; Winter et al. 2009). It is yet to be determined whether archaeal community composition in deep oceanic waters shows seasonal variation at finer levels of phylogenetic resolution.
As a part of the K2S1 project (Honda et al. 2015), during which repeated surveys were conducted at fixed stations deployed in the subarctic gyre (station K2) and subtropical region (station S1) of the western North Pacific, we collected data on seasonal changes in archaeal community composition throughout the water column (0–5000 m). Sampling was conducted on four occasions between 2010 and 2011. We used massively parallel 16S rRNA gene pyrosequencing (e.g., Sogin et al. 2006) to gain a detailed picture of variability in archaeal community composition.
2 Materials and methods
2.1 Water sampling
The sampling sites used in this study are established time series observation stations in the subarctic gyre (station K2; 47°N–160°E, depth 5300 m) and the subtropical region (station S1; 30°N–145°E, depth 5800 m) of the western North Pacific (Honda et al. 2015). Forty seawater samples for DNA analysis were collected from five depths (0, 300, 1000, 2000, and 5000 m) during four cruises of R/V Mirai (19 January–24 February 2010, 18 October–16 November 2010, 14 April–5 May 2011, and 27 June–4 August 2011). Two or 4 L of seawater from each depth were pre-filtered through a 3.0-μm pore size Nuclepore polycarbonate membrane filter (Whatman, Maidstone, UK), and microbial cells were collected onto a 0.22-μm Millipore Sterivex filter unit (EMD Millipore, Darmstadt, Germany). Samples were frozen immediately and stored at −80 °C for later analysis in the laboratory.
2.2 DNA extraction
Genomic DNA was extracted according to the instructions provided with the ChargeSwitch Forensic DNA purification kit (Invitrogen, Carlsbad, CA, USA) with the following modifications. First, we cut open the cartridge of the 0.22-μm Sterivex filter using a sterilized pipe cutter. Next, we used a sterile razor blade to cut the filter, which was placed into a sterilized 2.0-mL screw cap tube with zirconium beads (ZircoPrep Mini; Nippon Genetics Co. Ltd., Tokyo, Japan) containing ChargeSwitch Lysis Buffer (L13). Lysis of microbial cells was accomplished via proteinase K incubation followed by bead beating at 5000 rpm for 30 s using a bead-beater (Micro Smash MS-100R; Tomy Seiko Co., Ltd., Tokyo, Japan). After cell wall lysis, 1.0 mL of the supernatant was recovered after spin down at 2000×g for 1 min, and the crude DNA in the supernatant was purified according to the manufacturer’s instructions. The cell lysis and DNA purification steps were repeated twice to extract genomic DNA from each sample. The extracted DNA samples were kept at −20 °C until further analysis.
2.3 Polymerase chain reaction amplification and 454 sequencing
Polymerase chain reaction (PCR) amplification of the V1–V3 hypervariable regions of archaeal 16S rRNA gene was performed with the archaea-specific primer 21F (5′-TCCGGTTGATCCYGCCG-3′) and the universal primer 519R (5′-GWATTACCGCGGCKGCTG-3′). The forward primer contained the sequence of 454 adapter A (5′-CCATCTCATCCCTGCGTGTCTCCGACTCAG-3′) and the multiplex identifiers (MIDs), and the reverse primer contained the sequence of 454 adapter B (5′-CCTATCCCCTGTGTGCCTTGGCAGTCTCAG-3′). Amplifications were carried out using Ex Taq HS DNA Polymerase (Takara Bio., Shiga, Japan) and the following sequence: denaturation at 94 °C for 3 min followed by 27 cycles at 98 °C for 10 s, primer annealing at 57 °C for 30 s and at 72 °C for 50 s, followed by a final extension at 72 °C for 7 min. To minimize the potential effect of PCR biases in single reactions (Polz and Cavanaugh 1998), eight independent PCR products were pooled. The pooled PCR product was purified using an Agencourt AMPure XP kit (Beckman Coulter, Brea, CA, USA) in accordance with the manufacturer’s instructions. The purity and concentration of PCR products were checked using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and equal amounts of the PCR amplicons from different samples were mixed. Pyrosequencing of the amplicon mixture was performed using a Roche emPCR Lib-L kit (Roche Diagnostics, Branford, CT, USA) and was carried out using 454 GS-FLX Titanium chemistry (Roche Diagnostics).
2.4 Quality filtering and processing of 454 pyrosequencing sequences
The raw 454 sequences were processed to remove low-quality reads using the bioinformatics software mothur v.1.33.3 (Schloss et al. 2009) as described below. Initially, the raw 454 sequences containing more than two mismatches in the primer sequence or more than one mismatch in the MID sequence were removed, and quality-based filtering was performed using the AmpliconNoise algorithm (Quince et al. 2011) implemented in mothur (Schloss et al. 2011). Next, quality trimming was carried out by removing sequences with read length <400 or >600 base pairs, containing one or more ambiguous bases (Ns), or with a homopolymer longer than eight bases. The sequences were aligned against the SILVA alignment database provided on the website for the mothur project, and then the sequencing noise was further reduced using single linkage pre-clustering (Huse et al. 2007). Putative chimeric sequences were identified using UCHIME v4.2.40 (Edgar et al. 2011) and removed. A distance matrix of the high-quality sequences was constructed, and the sequences were clustered into operational taxonomic units (OTUs) at a 98 % similarity level (Harris et al. 2015) with average neighbor clustering.
2.5 Phylogenetic analysis
After quality filtering, the sequences were classified using a k-mers nearest neighbor searching method (Wang and Qian 2009) implemented in mothur using the SILVA 119 reference database (Yilmaz et al. 2014) at an 80 % threshold. Sequences affiliated with eukaryotes or bacteria and unknown sequences were eliminated. Phylogenetic trees based on maximum likelihood algorithms were constructed in MEGA6 (Tamura et al. 2013) after alignment using MUSCLE (Edgar 2004). Phylogenetic groups belonging to MGI Thaumarchaeota and MGII Euryarchaeota were further classified into sub-clusters using a protocol proposed by Massana et al. (2000). This protocol has been used in a number of previous studies (e.g., Bano et al. 2004; Galand et al. 2010; Hu et al. 2011).
2.6 Accession numbers and data availability
The pyrosequencing data have been submitted to the Sequence Read Archive of the DNA Data Bank of Japan (DDBJ) under accession number DRP002844.
2.7 Data analyses
Archaeal richness and diversity were assessed using the abundance-based coverage estimator (ACE; Chao and Lee 1992) and Simpson’s reciprocal index (Haegeman et al. 2014; Hill 1973). Good’s coverage was calculated as G = 1−n/N, where n represents the number of OTUs observed at least once and N represents the total number of reads. To compare these indices among all archaeal communities in this study, we normalized the sequence number of each sample to 465 reads (the lowest among the 40 samples). Rarefaction curves for each sample were generated using a program implemented in mothur. Community classification of archaeal assemblages from different samples was evaluated by hierarchical clustering with the Bray–Curtis dissimilarity index (Bray and Curtis 1957). Weighted UniFrac analyses were performed to test whether the archaeal communities differed significantly based on the permutation test (Lozupone and Knight 2005). All the analyses above were calculated based on the number of taxa at OTU-level using programs implemented in mothur. The relationship between the relative abundance of major archaeal phylogenetic groups and environmental variables was analyzed by the distance-based redundancy analysis (dbRDA) (Legendre and Anderson 1999) using R software (R Core Team 2014) and vegan package (Oksanen et al. 2013). The set of environmental variables that best explained the archaeal relative abundance variability was identified using the model selection based on the Akaike information criteria (AIC).
3 Results
3.1 Environmental parameters
The physicochemical parameters of seawater samples, which were retrieved from the database for time series stations K2 and S1 (http://ebcrpa.jamstec.go.jp/k2s1/en/index.html), are shown in Table S1. In the upper water column (0 and 300 m), temperature and salinity were lower at station K2 than at station S1, whereas concentrations of nutrients (nitrate and phosphate) and chlorophyll-a were higher at station K2 than at station S1. The dissolved oxygen concentration at 300 m differed greatly between station K2 (14.86–20.30 μmol kg−1) and station S1 (192.13–200.14 μmol kg−1). In deeper waters (>1000 m), temperature and salinity differed little between the two stations, with an overall range of 1.50–3.96 and 34.26–34.69 °C, respectively.
3.2 Overview of pyrosequencing data
A total of 376,330 raw sequences were obtained from 40 PCR amplicon libraries, and a total of 285,103 sequences of archaeal 16S rRNA genes with a mean ± standard deviation (SD) of 7127 ± 2324 sequences per sample remained after quality checks (Table S2). These high quality sequences were categorized into 593 OTUs based on 98 % similarity threshold. The observed OTUs were not saturated as shown in rarefaction curves (Fig. S1), but the Good’s coverage value for each sample indicated that 99.5–99.9 % of the archaeal species at the two sampling sites could be represented by the pyrosequencing. The representative sequences from 593 OTUs were classified into different taxa using the SILVA database. Phylogenetic placement of 39 major OTUs (98.3 % of total reads, >1 % relative abundance in each sample) are shown in the maximum likelihood trees (Fig. 1a, b).
Phylogenetic tree of Thaumarchaeota (a) and Euryarchaeota (b) based on 250 base pairs of archaeal 16S rRNA gene sequences in samples from the water column in the western North Pacific. These phylogenetic trees were constructed using the maximum-likelihood method in MEGA6. Bootstrap values at branching nodes indicate the level of bootstrap support (>50 %) based on 1000 resamplings. Numbers in parentheses indicate accession numbers of GenBank/DDBJ/EMBL. Operational taxonomic units (OTUs) that were observed at all depths (0, 300, 1000, 2000, and 5000 m) at both stations are indicated with asterisks
3.3 Phylogenetic assignment of 16S rRNA gene sequences
Most OTUs were affiliated with MGI Thaumarchaeota and MGII Euryarchaeota, representing 83.7 and 15.6 % of total reads, respectively. These two phylogenetic groups were further grouped into four sub-clusters, namely MGI-α, MGI-γ, MGII-α, and MGII-β (Fig. 1a, b). At >98 % sequence similarity, OTUs within MGI-α (16.2 % of total reads; Fig. 1a) were related to the ammonia-oxidizing archaeon Nitrosopumilus maritimus SCM1, 67.3 % of total reads were related to uncultured MGI-γ (Fig. 1a), 1.5 % were closely related to the MGII-α sub-cluster (Fig. 1b), and 13.9 % were affiliated with MGII-β (Fig. 1b). Marine Benthic Group A (MBG-A; 0.31 % of total reads; Fig. 1a) was commonly found in samples from station S1. The remaining OTUs with relative abundance <1 % (not shown in Fig. 1) included those belonging to the uncultured member of MGI-β, Marine Group III, the Marine Hydrothermal Vent Group, pSL12, AK31, Candidatus Halobonum, and unclassified sequences from Thaumarchaeota and Euryarchaeota. These minor groups were rare at both stations.
3.4 Archaeal community structure and distribution
At stations K2 and S1, the archaeal community was dominated by MGI and MGII (Fig. 2). These two groups collectively accounted for >93.5 % of total archaeal abundance throughout the water column (0–5000 m), regardless of season (Fig. 2). In addition to these groups, MBG-A affiliated with Thaumarchaeota (Fig. 1a) was always present at low levels (0.22–3.5 % of total archaeal abundance) in deeper waters (300–5000 m) at station S1, with the highest abundance being found at 300 m in July 2011 (Fig. 2). MBG-A was not detected in the water column at station K2 (Fig. 2).
Phylogenetic trees containing four distinct archaeal sub-clusters (MGI-α, MGI-γ, MGII-α, and MGII-β) with high bootstrap values were constructed (Fig. 1a, b). At station K2, the relative abundance of MGI-α was consistently high (range 29–47 %) at depths of 300 and 5000 m and low (<2.7 %) at depths of 1000 and 2000 m, whereas the corresponding abundance was highly variable (range 0.4–78 %) in surface waters (Fig. 2). At station S1, MGI-α abundance was relatively high at 5000 m (range 17–23 %), low at 300, 1000, and 2000 m (<3.5 %), and variable over the seasons in surface waters (range 0–17 %). At both stations, the relative abundance of MGI-γ was low (range 0–1.9 %) in surface waters, whereas it was high (range 43–94 %) in deeper waters (300–5000 m). MGII-β was found throughout the water column at both stations and its relative abundance varied in a relatively small range of 4.63–30.5 % among all the samples examined. Seasonal and geographic variability in MGII-β abundance was evident in surface waters, and was characterized by high relative abundance (range 68.1–90.8 %) in February 2010, October 2010, and April 2011 at station S1 and in October 2010 at station K2. MGII-α also displayed a large seasonal and geographic variation in surface waters, with the highest relative abundance (66.8–88.7 %) in July at both stations. Unlike MGII-β, MGII-α abundance was generally low (<1.7 %) in deeper waters (300–5000 m), except that this group was a significant member of the archaeal community (relative abundance, 5 %) at 1000 m in February 2010 at station K2.
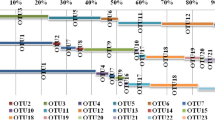
At a finer phylogenetic level, seven OTUs from MGI and two OTUs from MGII (78.5 and 6.98 % of total reads, respectively; asterisked OTUs in Fig. 1a, b) were found at almost all depths at both stations. Different OTUs displayed different vertical distribution patterns (Fig. 3). The relative abundance of OTU1 (MGI-γ) generally increased with increasing depth. OTU2 (MGI-γ), OTU4 (MGI-γ), and OTU5 (MGII-β) tended to be relatively abundant between 300 and 2000 m, whereas their relative abundances were low in the surface waters and at 5000 m. In contrast, the relative abundance of OTU3 (MGI-α) was higher in surface waters and at 5000 m relative to 1000 and 2000 m, although the relative abundance of this group at 300 m was high and low at station K2 and station S1, respectively.
Spatiotemporal distribution pattern of the nine operational taxonomic units (OTUs; marked with asterisks in Fig. 1) observed at all depths at both stations K2 and S1
3.5 Similarity among the archaeal communities
Results of hierarchical clustering analysis using the Bray–Curtis dissimilarity index revealed significant seasonal and site-dependent differences (p < 0.001) in the archaeal community in surface waters with 10–35 % dissimilarity (Fig. 4). Both seasonal and site-dependent dissimilarity of the archaeal community was largely attenuated (<10 % dissimilarity in most cases) in deeper waters (300–5000 m). In these deep waters, archaeal communities were separated into four clusters; cluster A (300 m, station S1), cluster B (300 m, station K2), cluster C (1000 and 2000 m, stations K2 and S1), and cluster D (5000 m, stations K2 and S1).
3.6 Diversity of the planktonic archaeal communities
The species richness (ACE) of archaea varied in the range of 12–216 at station K2 and 55–229 at station S1, displaying large seasonal variability depending on the depth and station (Fig. 5a). Depth-dependent patterns in the ACE index averaged over seasons (Fig. 5c) showed that the archaeal species richness were high at 300 m depth at both stations and the species richness tend to be lower at the surface relative to deeper waters. At station K2, Simpson’s diversity index varied in the range of 1.39–4.95 (Fig. 5b). This diversity index averaged over seasons (Fig. 5d) displayed a systematic vertical trend, characterized by low values at the surface and at 5000 m relative to other depths. Similarly, at station S1, Simpson’s diversity index (range 1.92–12.38) systematically varied with depth. This index averaged over seasons displayed a prominent peak at a depth of 300 m, whereas values were low at 5000 m (Fig. 5d).
Diversity metrics of archaeal communities from the western North Pacific. a Temporal dynamics of archaeal species richness based on the abundance-based coverage estimator (ACE) index, b temporal dynamics of archaeal diversity based on Simpson’s reciprocal index, c depth profile of the ACE index (mean ± SD), d depth profile of Simpson’s index (mean ± SD)
3.7 Relationship between the relative abundance of major archaeal group and environmental variables
The model selection based on AIC indicated that DOC, temperature, pH, concentrations of dissolved oxygen, chlorophyll a, phosphate, ammonia and nitrate gave the closest fit to the changes in relative abundances of archaeal groups. Based on the dbRDA results (Fig. S2), it was suggested that water temperature influenced the relative abundance of MGII-α (p = 0.032), while the relative abundance of MGII-β was related to DOC concentrations (p = 0.002) and pH levels (p = 0.001). None of the environmental variables examined by this study was selected as a significant predictor variable accounting for the variability in the relative abundances of MGI-α, MGI-γ and MBG-A (p > 0.05).
4 Discussion
Previous studies have used fluorescence in situ hybridization (FISH) to evaluate seasonal variability in archaeal communities at depth based on the abundance of specific groups of archaea (MGI and MGII) relative to total picoplankton abundance. These studies have found large seasonal variability in MGI and MGII abundances in both surface and deep oceanic waters (Church et al. 2003; Winter et al. 2009). In contrast, our data on archaeal community composition are based on the number of retrieved 16S rRNA gene sequences of specific groups normalized to the total number of retrieved archaeal 16S rRNA gene sequences for each sample. It is important to note that our data cannot be directly compared with the FISH data reported in the literature, given the difference in the quantification procedures and principles. The archaeal abundance data reported in the present paper must be regarded as semi-quantitative. Despite these limitations, it is notable that the archaeal community composition (relative abundance of different sub-clusters) in deeper waters (300–5000 m) was remarkably constant among four sampling occasions, as indicated by visual inspection of Fig. 2, as well as by the low Bray–Curtis distances (<0.1 in most cases) between the data collected at a given depth from 300 to 5000 m (Fig. 4). In contrast, our data revealed high seasonal variability in archaeal community composition in surface waters. Thus, our data demonstrate that seasonal variability in archaeal community composition in deeper waters of the western North Pacific was much less pronounced than the corresponding variability in surface waters.
Archaeal community composition in deep waters (300–5000 m) was characterized by the dominance of MGI, especially sub-cluster MGI-γ. These data agree with previous results obtained in other oceanic regions (e.g., Anderson et al. 2013; Galand et al. 2009; Massana et al. 2000). Another sub-cluster, MGI-α, which harbors the ammonia-oxidizing archaeon N. maritimus, was occasionally abundant in surface waters at station K2, but was less abundant at intermediate depths (300–2000 m), except for 300 m at station K2. Some previous studies have also reported similar vertical distribution patterns of these MGI sub-clusters in other oceanic regions (Beman et al. 2008; Massana et al. 1997; Massana et al. 2000; Mincer et al. 2007), suggesting the presence of niche separation among these sub-clusters (e.g., Christman et al. 2011; De Corte et al. 2009; Kalanetra et al. 2009; Prosser and Nicol 2008). Our dbRDA results (Fig. S2) showed that the factors influencing the distribution of MGI-α and MGI-γ abundances were not significantly related to the environmental factors examined by the present study. Previous cultivation experiments (Merbt et al. 2012) showed that the inhibition by light is one of the potential factors to determine the distribution patterns of ammonia-oxidizing archaea and bacteria in aquatic environments. The higher relative abundance of MGI-α than MGI-γ in surface waters was presumably caused by the higher sensitivity of MGI-γ to photoinhibition than the sensitivity of MGI-α. Furthermore, some other investigations (Hu et al. 2011; Mincer et al. 2007) have suggested that the gradient of light intensity across the water column is a plausible factor for the niche partitioning of MGI sub-groups with depth. Resent study based of single-cell genomics (Luo et al. 2014) found the key genes that may be related to mechanisms to reduce light-induced damage from shallow-water Thaumarchaeota clade.
MGI-α was commonly found at a depth of 5000 m at both stations, but with higher abundances at station K2 (Fig. 2). Similarly, the increasing of the relative abundance of MGI-α in deep part of ocean was reported in the water column of the Challenger Deep (Nunoura et al. 2015). Although the 16S rRNA gene sequences of MGI-α from 0, 300, and 5000 m showed high similarity (>98 %), given the large differences in environmental conditions between surface and deep waters, each of these sequences might represent ecotypes originating from different habitat-specific members of MGI-α (Muller et al. 2010; Park et al. 2014). Representatives of MGI-α have been found in benthic archaeal communities (Durbin and Teske 2010; Gillan and Danis 2007); therefore, benthic MGI-α could have been introduced into the water column through the resuspension of sediment caused by bottom current-induced shear stress on the seafloor (Eittreim et al. 1976; Kolla et al. 1976; McCave 1986). Sediment resuspension from the seafloor to the water column has been reported to reach up to several hundred meters (e.g., Hunkins et al. 1969; McCave 1986). The flux of suspended organic matter (particulate organic matter or dissolved organic matter) from the resuspended sediment possibly changed the activities (Boetius et al. 2000) and abundances (Wells and Deming 2003; Wells et al. 2006) of archaea at 5000 m at both stations. Alternatively, the DNA of MGI-α recovered from waters at 5000 m could be remnant DNA of surface archaeal communities delivered to deeper waters by attachment to settling particles (Dell’Anno et al. 1999).
The relative abundances of MGII-α and MGII-β in the surface waters were constantly high at station S1, but were highly variable at station K2 throughout the seasons. There are no cultivated representatives of MGII Euryarchaeota; therefore, their metabolic requirements and the mechanisms underlying their distribution pattern remain unknown. Results from dbRDA (Fig. S2) showed that water temperature, DOC and pH might be factors influencing the distribution pattern of MGII sub-groups. Another possible factor that might influence the distribution of MGII is light intensity. Recent metagenomic studies have found that some members of MGII possessed the proteorhodopsin gene, indicating the capability of using light to gain metabolic energy (Frigaard et al. 2006; Iverson et al. 2012). Furthermore, the distribution pattern and dynamics of MGII in epipelagic ocean waters were presumably related to spatiotemporal differences in sunlight intensity (Galand et al. 2010; Herfort et al. 2007). On the contrary, Deschamps et al. (2014) reported that genes encoding proteorhosopsin homologs were not found in MGII metagenome data from deep-Mediterranean water, and they also noted the deep sea MGII have abundant genes that are typical of heterotrophic prokaryotes.
Uncultured MBG-A Thaumarchaeota was first found in deep-sea sediments of the northwestern Atlantic (Vetriani et al. 1999). Since that first report, MBG-A has only been found in marine sediments (Dang et al. 2010; Inagaki et al. 2006; Teske 2006). Our finding that MBG-A was commonly distributed in waters from 300 to 5000 m at station S1 (range 0.22–3.5 %; Fig. 2) indicates that this group of archaea may distribute in oceanic environments not only in sediments but also in the water column.
Nine OTUs belonging to MGI-α, MGI-γ, and MGII-β were found at all depths at both stations and represented a large fraction of total reads (85.5 %; Fig. 3). These phylotypes displayed distinct vertical distribution patterns regardless of season. These results suggest that these groups represent the dominant group of archaea in the western North Pacific and might be important in the mediation of carbon and/or nutrient cycles in the water column of this area.
Cluster analysis showed strong vertical stratification of archaeal communities (Fig. 4). Species richness and the diversity indices fluctuated according to depths and season (Fig. 5). Archaeal communities at the depths of 0 and 300 m differed between the stations, whereas those at the depths of 1000–5000 m differed little between the stations (Fig. 4). Therefore, both the subarctic station (K2) and the subtropical station (S1) appear to share similar archaeal communities in deep waters.
Our data revealed that the archaeal community composition and diversity at 300 m differed between station K2 and station S1. Currently, none of physicochemical parameters obtained in this study were sufficient to explain these differences. During our study period, subtropical mode water (Suga and Hanawa 1990), an outcrop from the oceanic regions further north was identified at station S1 at 100–400 m, depending on the season. Therefore, one of the possible hypotheses to account for the discrepancy of archaeal communities in 300 m between station S1 and station K2 might be the advection of different archaeal communities from adjacent waters. This may contribute to the development of the distinct archaeal community at that location, although this hypothesis requires further verification.
5 Conclusion
In summary, we have shown distribution patterns of four cosmopolitan archaeal groups within MGI Thaumarchaeota (MGI-α and MGI-γ) and MGII Euryarchaeota (MGII-α and MGII-β) throughout the water columns from the surface to the bottom of 5000 m depth at the subarctic and the subtropical stations in the western North Pacific Ocean. Archaeal community structures in >300 m deep waters remained relatively stable over time and similar to each other between stations, although those in surface waters displayed marked spatiotemporal changes. Our results imply that four major lineages of marine archaea may differently adapt to their own niches and also the lineages predominantly found in deep waters are scarcely affected by spatiotemporal fluctuation of biotic and abiotic factors in the surface waters. To date, marine planktonic archaea have yet been isolated, hampering our better understanding of their metabolic capabilities and ecological functions in the ocean. In future studies, archaeal genome information directly obtained from their inhabiting environments using metagenomics and single-cell genomics are highly valuable for an in-depth understanding of these ubiquitous archaea.
References
Alonso-Sáez L, Waller AS, Mende DR et al (2012) Role for urea in nitrification by polar marine archaea. Proc Natl Acad Sci USA 109(44):17989–17994
Anderson RE, Beltran MT, Hallam SJ, Baross JA (2013) Microbial community structure across fluid gradients in the Juan de Fuca Ridge hydrothermal system. FEMS Microbiol Ecol 83(2):324–339
Baker BJ, Sheik CS, Taylor CA et al (2013) Community transcriptomic assembly reveals microbes that contribute to deep-sea carbon and nitrogen cycling. ISME J 7(10):1962–1973
Bano N, Ruffin S, Ransom B, Hollibaugh JT (2004) Phylogenetic composition of Arctic Ocean archaeal assemblages and comparison with Antarctic assemblages. Appl Environ Microbiol 70(2):781–789
Beman JM, Popp BN, Francis CA (2008) Molecular and biogeochemical evidence for ammonia oxidation by marine Crenarchaeota in the Gulf of California. ISME J 2(4):429–441
Boetius A, Springer B, Petry C (2000) Microbial activity and particulate matter in the benthic nepheloid layer (BNL) of the deep Arabian Sea. Deep Sea Res II 47(14):2687–2706
Bray JR, Curtis JT (1957) An ordination of upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Chao A, Lee SM (1992) Estimating the number of classes via sample coverage. J Am Stat Assoc 87(417):210–217
Christman GD, Cottrell MT, Popp BN, Gier E, Kirchman DL (2011) Abundance, diversity, and activity of ammonia-oxidizing prokaryotes in the coastal Arctic ocean in summer and winter. Appl Environ Microbiol 77(6):2026–2034
Church MJ, DeLong EF, Ducklow HW, Karner MB, Preston CM, Karl DM (2003) Abundance and distribution of planktonic archaea and bacteria in the waters west of the Antarctic Peninsula. Limnol Oceanogr 48(5):1893–1902
Connelly TL, Baer SE, Cooper JT, Bronk DA, Wawrik B (2014) Urea uptake and carbon fixation by marine pelagic bacteria and archaea during the Arctic summer and winter seasons. Appl Environ Microbiol 80(19):6013–6022
Dang HY, Luan XW, Chen RP, Zhang XX, Guo LZ, Klotz MG (2010) Diversity, abundance and distribution of amoA-encoding archaea in deep-sea methane seep sediments of the Okhotsk Sea. FEMS Microbiol Ecol 72(3):370–385
De Corte D, Yokokawa T, Varela MM, Agogué H, Herndl G (2009) Spatial distribution of Bacteria and Archaea and amoA gene copy numbers throughout the water column of the Eastern Mediterranean Sea. ISME J 3(2):147–158
Dell’Anno A, Fabiano M, Mei ML, Danovaro R (1999) Pelagic-benthic coupling of nucleic acids in an abyssal location of the Northeastern Atlantic Ocean. Appl Environ Microbiol 65:4451–4457
DeLong EF (1992) Archaea in coastal marine environments. Proc Natl Acad Sci USA 89(12):5685–5689
DeLong EF et al (2006) Community genomics among stratified microbial assemblages in the ocean’s interior. Science 311(5760):496–503
Deschamps P, Zivanovic Y, Moreira D, Rodriguez-Valera F, Lopez-Garcia P (2014) Pangenome evidence for extensive inter-domain horizontal transfer affecting lineage-core and shell genes in uncultured planktonic Thaumarchaeota and Euryarchaeota. Genome Biol Evol 6(7):1549–1563
Durbin AM, Teske A (2010) Sediment-associated microdiversity within the Marine Group I Crenarchaeota. Environ Microbiol Rep 2(5):693–703
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200
Eittreim S, Thorndike EM, Sullivan L (1976) Turbidity distribution in the Atlantic Ocean. Deep Sea Res 23:1115–1137
Frigaard NU, Martinez A, Mincer TJ, DeLong EF (2006) Proteorhodopsin lateral gene transfer between marine planktonic bacteria and archaea. Nature 439(7078):847–850
Fuhrman JA, Davis AA (1997) Widespread archaea and novel bacteria from the deep sea as shown by 16S rRNA gene sequences. Mar Ecol Prog Ser 150:275–285
Fuhrman JA, McCallum K, Davis AA (1992) Novel major archaebacterial group from marine plankton. Nature 356(6365):148–149
Galand PE, Casamayor EO, Kirchman DL, Potvin M, Lovejoy C (2009) Unique archaeal assemblages in the Arctic Ocean unveiled by massively parallel tag sequencing. ISME J 3(7):860–869
Galand PE, Gutiérrez-Provecho C, Massana R, Gasol JM, Casamayor EO (2010) Inter-annual recurrence of archaeal assemblages in the coastal NW Mediterranean Sea (Blanes Bay Microbial Observatory). Limnol Oceanogr 55(5):2117–2125
Gillan DC, Danis B (2007) The archaebacterial communities in Antarctic bathypelagic sediments. Deep Sea Res II 54(16–17):1682–1690
Haegeman B, Sen B, Godon JJ, Hamelin J (2014) Only Simpson diversity can be estimated accurately from microbial community fingerprints. Microb Ecol 68:169–172
Hansman RL, Griffin S, Watson JT et al (2009) The radiocarbon signature of microorganisms in the mesopelagic ocean. Proc Natl Acad Sci USA 106(16):6513–6518
Harris S, Croft J, O’Flynn C et al (2015) A pyrosequencing investigation of differences in the feline subgingival microbiota in health, gingivitis and mild periodontitis. PLoS One 10(11):e0136986
Herfort L, Schouten S, Abbas B et al (2007) Variations in spatial and temporal distribution of Archaea in the North Sea in relation to environmental variables. FEMS Microbiol Ecol 62(3):242–257
Herndl GJ, Reinthaler T, Teira E, van Aken H, Veth C, Pernthaler A, Pernthaler J (2005) Contribution of archaea to total prokaryotic production in the deep Atlantic Ocean. Appl Environ Microbiol 71(5):2303–2309
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54(2):427–432
Honda MC, Kawakami H, Matsumoto K et al (2015) Comparison of sinking particles in the upper 200 m between subarctic station K2 and subtropical station S1 based on drifting sediment trap experiments. J Oceanogr. doi:10.1007/s10872-015-0280-x
Hu A, Jiao N, Zhang CL (2011) Community structure and function of planktonic Crenarchaeota: changes with depth in the South China Sea. Microb Ecol 62(3):549–563
Hugoni M, Taib N, Debroas D et al (2013) Structure of the rare archaeal biosphere and seasonal dynamics of active ecotypes in surface coastal waters. Proc Natl Acad Sci USA 110(15):6004–6009
Hunkins K, Thorndike EM, Mathieu G (1969) Nepheloid layers and bottom currents in the Arctic Ocean. J Geophys Res 74(28):6995–7008
Huse S, Huber J, Morrison H, Sogin M, Welch MD (2007) Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol 8(7):R143
Inagaki F, Nunoura T, Nakagawa S et al (2006) Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean margin. Proc Natl Acad Sci USA 103(8):2815–2820
Iverson V, Morris RM, Frazar CD, Berthiaume CT, Morales RL, Armbrust EV (2012) Untangling genomes from metagenomes: revealing an uncultured class of marine euryarchaeota. Science 335(6068):587–590
Kalanetra KM, Bano N, Hollibaugh JT (2009) Ammonia-oxidizing archaea in the Arctic Ocean and Antarctic coastal waters. Environ Microbiol 11(9):2434–2445
Karner MB, DeLong EF, Karl DM (2001) Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409(6819):507–510
Kolla V, Sullivan L, Streeter SS, Langseth MG (1976) Spreading of Antarctic bottom water and its effects on the floor of the Indian Ocean inferred from bottom-water potential temperature, turbidity, and sea-floor photography. Mar Geol 21(3):171–189
Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437(7058):543–546
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69:1–24
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71(12):8228–8235
Luo H, Tolar BB, Swan BK, Zhang CL, Stepanauskas R, Moran AM, Hollibaugh JT (2014) Single-cell genomics shedding light on marine Thaumarchaeota diversification. ISME J 8(3):732–736
Massana R, Murray AE, Preston CM, DeLong EF (1997) Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara Channel. Appl Environ Microbiol 63(1):50–56
Massana R, DeLong EF, Pedrós-Alió C (2000) A few cosmopolitan phylotypes dominate planktonic archaeal assemblages in widely different oceanic provinces. Appl Environ Microbiol 66(5):1777–1787
McCave IN (1986) Local and global aspects of the bottom nepheloid layers in the world ocean. Neth J Sea Res 20(2–3):167–181
Merbt SN, Stahl DA, Casamayor EO, Martí E, Nicol GW, Prosser JI (2012) Differential photoinhibition of bacterial and archaeal ammonia oxidation. FEMS Microbiol Lett 327:41–46
Mincer TJ, Church MJ, Taylor LT, Preston CM, Karl DM, DeLong EF (2007) Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ Microbiol 9(5):1162–1175
Muller F, Brissac T, Le Bris N, Felbeck H, Gros O (2010) First description of giant Archaea (Thaumarchaeota) associated with putative bacterial ectosymbionts in a sulfidic marine habitat. Environ Microbiol 12(8):2371–2383
Nunoura T, Takaki Y, Hirai M et al (2015) Hadal biosphere: insight into the microbial ecosystem in the deepest ocean on Earth. Proc Natl Acad Sci USA 112(11):E1230–E1236
Oksanen J, Blanchet FG, Kindt R, et al (2013) Vegan: community ecology package. R package version 2.0-10. Available at http://CRAN.R-project.org/package=vegan. Accessed 3 Oct 2015
Park SJ, Ghai R, Martín-Cuadrado AB, Rodríguez-Valera F et al (2014) Genomes of two new ammonia-oxidizing archaea enriched from deep marine sediments. PLoS One 9(5):e96449
Polz FM, Cavanaugh MC (1998) Bias in template-to-product ratios in multitemplate PCR. Appl Environ Microbiol 64(10):3724–3730
Prosser JI, Nicol GW (2008) Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 10(11):2931–2941
Qin W, Amin SA, Martens-Habbena W et al (2014) Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation. Proc Natl Acad Sci USA 111:12504–12509
Quince C, Lanzen A, Davenport RJ, Turnbaugh PJ (2011) Removing noise from pyrosequenced amplicons. BMC Bioinform 12:38
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/. Accessed 10 Sep 2015
Santoro AE, Dupont CL, Richter RA et al (2015) Genomic and proteomic characterization of “Candidatus Nitrosopelagicus brevis”: an ammonia-oxidizing archaeon from the open ocean. Proc Natl Acad Sci USA 112(4):1173–1178
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Schloss PD, Gevers D, Westcott SL (2011) Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One 6(12):e27310
Sogin ML, Morrison HG, Huber JA et al (2006) Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc Natl Acad Sci USA 103(32):12115–12120
Suga T, Hanawa K (1990) The mixed layer climatology in the northwestern part of the North Pacific subtropical gyre and the formation area of subtropical mode water. J Mar Res 48(3):543–566
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mole Biol Evol 30(12):2725–2729
Teira E, Aken HMV, Veth C, Herndl GJ (2006) Archaeal uptake of enantiomeric amino acids in the meso-and bathypelagic waters of the North Atlantic. Limnol Oceanogr 51(1):60–69
Teske A (2006) Microbial communities of deep marine subsurface sediments: molecular and cultivation surveys. Geomicrobiol J 23(6):357–368
Vetriani C, Jannasch HW, MacGregor BJ, Stahl DA, Reysenbach AL (1999) Population structure and phylogenetic characterization of marine benthic archaea in deep-sea sediments. Appl Environ Microbiol 65(10):4375–4384
Wang Y, Qian PY (2009) Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One 4(10):e7401
Wells LE, Deming JW (2003) Abundance of bacteria, the Cytophaga-Flavobacterium cluster and archaea in cold oligotrophic waters and nepheloid layers of the Northwest Passage, Canadian Archipelago. Aquat Microb Ecol 31(1):19–31
Wells LE, Cordray M, Bowerman S, Miller LA, Vincent WF, Deming JW (2006) Archaea in particle-rich waters of the Beaufort Shelf and Franklin Bay, Canadian Arctic: clues to an allochthonous origin? Limnol Oceanogr 5(1):47–59
Winter C, Kerros M-E, Weinbauer MG (2009) Seasonal changes of bacterial and archaeal communities in the dark ocean: evidence from the Mediterranean Sea. Limnol Oceanogr 54(1):160–170
Yilmaz P, Wegener Parfrey L, Yarza P et al (2014) The SILVA and ‘All-species Living Tree Project (LTP)’ taxonomic frameworks. Nucl Acids Res 42:D643–D648
Acknowledgments
We thank T. Saino from Japan Agency for Marine-Earth Science and Technology (JAMSTEC) for his invaluable contribution in initiating the K2S1 project. This work was supported by KAKENHI (Grant-in-Aid for Scientific Research on Innovative Areas) Grant Number 24241002. We are Grateful to T. Fujiki, H. Kawakami, K. Kimoto, M. Kitamura, K. Matsumoto, K. Sasaoka, and M. Wakita for their support in sample collection and physicochemical analyses, and to MC. Honda, H. Fukuda, and M. Uchimiya for their helpful comments. We acknowledge the help of the captain, officers, and crew of the R/V Mirai during cruises, and the marine technicians of Global Ocean Development Inc. and Marine Works Japan Ltd. for their on-board work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaneko, R., Nagata, T., Suzuki, S. et al. Depth-dependent and seasonal variability in archaeal community structure in the subarctic and subtropical western North Pacific. J Oceanogr 72, 427–438 (2016). https://doi.org/10.1007/s10872-016-0372-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-016-0372-2