Abstract
Single crystals of Ba3Na(OH)3(CO3)2 were grown using the hydroflux method and characterized by single crystal X-ray diffraction. Ba3Na(OH)3(CO3)2 crystallizes in the hexagonal, non-centrosymmetric space group P63cm with a = 9.31360(10) Å and c = 6.2218(2) Å. The material exhibits a three-dimensional crystal structure consisting of chains of face-sharing Na(OH)6 octahedra that are hydrogen bonded to carbonate anions; the charge balance is maintained by barium atoms connected to the trigonal planar carbonate groups and surrounding the Na(OH)6 chains.
Graphical Abstract
The synthesis and crystal structure of the non-centrosymmetric hydroxycarbonate, Ba3Na(OH)3(CO3)2, is reported.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The exploratory crystal growth from high temperature solutions [1] has been exceedingly effective in yielding high quality crystals with new compositions and structures. Among the high temperature solutions, hydroxide fluxes have turned out to be especially effective and, by adjusting the water content of the melt, often the specific product that forms can be controlled [2]. Recently, several papers have appeared that describe a hydroflux, a water rich hydroxide melt that exists as a solid below 100 °C but that melts at very low temperatures, less than 250 °C [3,4,5]. This hydroflux has proven to be very effective at crystallizing transition metal hydroxometallates, such as A2B(OH)2, where A is an alkali or alkaline earth metal and B is a transition metal. In this paper we discuss an expansion of this work by reporting on a new hydroxycarbonate, Ba3Na(OH)3(CO3)2, that formed under hydroflux growth conditions.
Hydroxycarbonates have been used extensively as precursors for the preparation of binary catalysts, such as copper–zinc oxide catalysts [6], and many of the hydroxycarbonates exist as minerals in nature, for example aurichalcite and rosasite [7]. Typically, co-precipitation was used to prepare the hydroxycarbonates [8]. Other well-known hydroxycarbonate minerals are malachite, Cu2(OH)2CO3, azurite Cu3(OH)2(CO3)2 and hydrozincite Zn5(OH)6(CO3)2 [9, 10]. Many other complex hydroxycarbonates are known, including Ln2Cd(OH)6(CO3), [11], NaAl(OH)2(CO3), [12],Ce(OH)(CO3) [13, 14], and NaPb(OH)(CO3)2 [15].
The growth of crystals of hydroxycarbonates has been achieved via hydrothermal routes and herein, we describe an alternative method to crystallizing hydroxycarbonates using a hydroflux.
Experimental
Materials and Methods
Single crystals of Ba3Na(OH)3(CO3)2 were grown out of a sodium hydroxide hydroflux. The reaction was carried out in a 23 mL polytetrafluoroethylene (PTFE) lined stainless steel autoclave. A mixture of 4.3 mmol (1.3497 g) of Ba(OH)2·8H2O (Alfa Aesar, 98%), 2.85 mmol (0.3021 g) of Na2CO3 (Fisher, 100.0%), 9 g (9.0089 g) of NaOH (Macron, ACS grade), and 7 g (7.0013 g) of deionized H2O were added to the autoclave, and the autoclave was sealed. The autoclave was heated to 180° at 5 °C/min, then heated to 230° at 0.3 °C/minute, held for 24 h, and then cooled slowly to 80 °C at a rate of 0.3 °C/minute, at which point the oven was shut off. After cooling, the liquid in the autoclave was poured out, and the crystals inside were scraped off the sides and rinsed out of the autoclave with methanol.
Energy-Dispersive Spectroscopy (EDS)
Elemental analysis was performed on the crystals using an FEI Quanta 200 scanning electron microscope (SEM) with EDS capabilities. The crystals were mounted on carbon tape and analyzed using a 30 kV accelerating voltage. The elemental crystal compositions obtained by EDS matched the ratio obtained from the single crystal structure. The EDS measurements indicated the presence of Ba and Na, and the absence of other extraneous elements.
Crystallographic Study
X-ray intensity data from a hexagonal needle crystal were collected at 296(2) K using a Bruker SMART APEX diffractometer (Mo Kα radiation, λ = 0.71073 Å) [15]. The data collection covered 100% of reciprocal space to 2θmax = 75.6°, with an average reflection redundancy of 14.2 and Rint = 0.032 after absorption correction. The raw area detector data frames were reduced and corrected for absorption effects with the SAINT+ and SADABS programs. [16] Final unit cell parameters were determined by least-squares refinement of 8638 reflections from the data set. An initial structural model was obtained with direct methods [17]. Subsequent difference Fourier calculations and full-matrix least-squares refinement against F2 were performed with SHELXL-20142 using the ShelXle interface [18].
The compound crystallizes in the hexagonal system. Systematic absences in the intensity data were consistent with the space groups P3c1, P-3c1, P63cm, P-6c2, and P63/mcm. The non-centrosymmetric space group P63cm (No. 185) was eventually confirmed by structure solution. The absolution structure (Flack) parameter was − 0.009(19) after the final refinement cycle, indicating the correct absolute structure and the absence of inversion twinning. There are six atomic positions in the asymmetric unit. Barium Ba(1) and the hydroxyl group atoms O(2) and H(2) are located on mirror planes (site 6c, site symmetry..m), sodium Na(1) is located on site 2a with site symmetry 3.m, oxygen O(1) is located on a general position (site 12d), and carbon atom C(1) is located on the threefold axis (site 4b, site symmetry 3..). All atoms were refined with anisotropic displacement parameters. The hydroxyl hydrogen was located in a difference map and refined with an isotropic displacement parameter subject to a d(O–H) = 0.84(2) Å distance restraint. Free refinement led to a short O–H distance of 0.68 Å. No deviation from full occupancy was observed for either of the metal atoms. The largest residual electron density peak and hole in the final difference map are + 1.33 and − 0.68 e−/Å3, located 0.64 and 1.25 Å from Ba(1) and C(1), respectively. Details about the single-crystal refinement are given in Table 1, atomic positions are given in Table 2, select bond lengths in Table 3 and hydrogen bonding information in Table 4. The CCSD number for Ba3Na(OH)3(CO3)2 is 1814353.
Results and Discussion
Synthetic Considerations
Clear colorless needles of Ba3Na(OH)3(CO3)2 were grown out of a hydroflux and were isolated in good yield. This is the first example of a hydroflux yielding a hydroxycarbonate product. The presence of the carbonate in the product crystals is due to the presence of carbonate impurities in commercially available hydroxides. All hydroxides, by the time they reach the chemistry laboratory, contain carbonate impurities. Normally this is not an issue, however, in the hydroflux method the relative ratio of hydroxides to reagents is very large, since a significant excess of hydroxide is used. Under such conditions even a small impurity will be present in significant amounts. During hydroflux syntheses, simple carbonates, such as BaCO3, often crystallize simultaneously with the reaction product, however, in this case, the carbonate was incorporated into the product itself. This suggests that this method could be used to crystallize new hydroxycarbonate structures by adding sodium or potassium carbonate to the hydroflux itself. It is possible that this represents a significant new approach to prepare hydroxycarbonates.
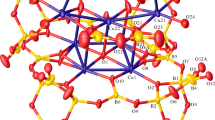
The structure, solved by single crystal X-ray diffraction, contains infinite chains of Na(OH)6 face sharing octahedra, Fig. 1, consisting of slightly distorted octahedra with Na–O bonds of 3 × 2.321(3) and 3 × 2.488(4) Å. Each Na(OH)6 octahedra is surrounded by six barium atoms that are located just outside the faces of the octahedron at a Na–Ba distance of 3.3483(13) Å (Fig. 2). The barium atoms are in a 9-coordinate environment, surrounded by 6 oxygens and 3 hydroxide groups, resulting in a local environment of BaO6(OH)3 with Ba–O bonds ranging from 2.752(3) to 2.911(2) Å. Figure 3. The Ba6Na(OH)6 units, in turn, are surrounded by planar CO32− groups (Fig. 4). Each CO32− group has C–O bond lengths of 1.286(2) Å and each oxygen of the carbonate group is connected to one carbon and three barium atoms, placing the oxygens into a distorted tetrahedral bonding environment (Fig. 5). This results in a 3D structure consisting of a barium hydroxide carbonate channel structure containing chains of face sharing Na(OH)6 octahedra (Figs. 6).
The structure of Ba3Na(OH)3(CO3)2 crystallizes in the non-centrosymmetric space group P63cm. Non-centrosymmetric crystal structures are often found to exhibit second harmonic generation (SHG) effects, however, testing using Powder SHG measurements performed on a modified Kurtz nonlinear-optical (NLO) system using a pulsed Nd:YAG laser with a wavelength of 1064 nm indicated that Ba3Na(OH)3(CO3)2 does not exhibit significant SHG effects.
Conclusion
A new hydroxycarbonate, Ba3Na(OH)3(CO3)2, was crystallized out of a hydroflux and its non-centrosymmetric structure was described. The hydroflux approach may be a new avenue for growing single crystals of complex hydroxycarbonates.
References
Bugaris DE, zur Loye H-C (2012) Angew Chem Int Ed 51:3780–3811
Mugavero III SJ, Gemmill WR, Roof IP, zur Loye H-C (2009) J Solid State Chem 182:1950–1963
Chance WM, Bugaris DE, Sefat AS, zur Loye H-C (2013) Inorg Chem 52:11723–11733
Bugaris DE, Smith MD, zur Loye H-C (2013) Inorg Chem 52:3836–3844
Latshaw AM, Chance WM, Trenor N, Morrison G, Smith MD, Yeon J, zur Loye H-C (2015) CrystEngComm 17:4691–4698
Stoilova D, Koleva V, Vassileva V (2002) Spectrochim Acta A 58:2015
Alwan AK, Thomas JH, Williams PA (1980) Trans Metal Chem 76:51–53
Del Arco M, Trujillano R, Rives V (1998) J Mater Chem 8:761–767
Stoilova D, Koleva V, Vassileva V (2002) Spectrochim Acta A 58:2051
Kang J, Lee C, Kremer RK, Whangbo M-H (2009) J Phys Cond Mater 21:392201
Song J-L, Cui J-Q, Wu C, Yang G, Zhang C (2016) Inorg Chim Acta 444:217–220
Frost RL, López A, Scholz R, Sampaio NP, De Oliveira FAN (2015) Spectrochim Acta A 136:918–923
Guo Z, Du F, Li G, Cui Z (2006) Inorg Chem 45:4167–4169
Sorbello C, Jobbágy M (2017) Cryst Growth Des 17:5660–5666
Belokoneva EL, Al’-Ama AG, Dimitrova OV, Kurazhkovskaya VS, Stefanovich SY (2002) Crystallog Rep 47:217–222
SMART Version 5.631, SAINT + Version 6.45 and SADABS Version 2.10. Bruker Analytical X-ray Systems, Inc., Madison, Wisconsin (2003)
Sheldrick GM (2008) Acta Cryst A64:112–122
Hübschle CB, Sheldrick GM, Bittrich B (2011) J Appl Cryst 44:1281–1284
Acknowledgements
Research supported by the U.S. National Science Foundation through Grant DMR-1301757.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
zur Loye, K.D., Latshaw, A.M., Smith, M.D. et al. Ba3Na(OH)3(CO3)2: A Non-centrosymmetric Hydroxycarbonate Crystallized Using the Hydroflux Method. J Chem Crystallogr 48, 103–108 (2018). https://doi.org/10.1007/s10870-018-0716-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-018-0716-4