Abstract
Single crystals of Na3GaF6 were prepared via a mild hydrothermal method and the crystal structure was characterized by single crystal X-ray diffraction. Na3GaF6 crystallizes in the monoclinic space group P21/n with a = 5.4724(3) Å, b = 5.6742(3) Å, c = 7.8866(4) Å, γ = 90.361(1)˚, V = 244.89(2) Å3, and Z = 2. The compound exhibits a cryolite-type crystal structure consisting of corner-shared GaF6 and Na(1)O6 polyhedra. The Ga and Na(1) atoms are found in almost regular octahedra, whereas the Na(2) atom is observed in a highly distorted square antiprismatic coordination environment.
Graphical Abstract
The synthesis and crystal structure of the hexafluorogallate, Na3GaF6 is reported.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Materials belonging to a cryolite-type structure, A3BF6 (A = monovalent and B = trivalent cations), have been continuously investigated due to not only their important function as host materials for luminescence ions, but also interesting structural phase transitions [1,2,3]. While many fluoride compounds have been traditionally prepared by high temperature reactions including solid-state method or flux growth [4,5,6,7,8], hydrothermal process can also provide an effective synthetic route for the synthesis of the fluoride materials at a relatively mild reaction condition [9,10,11]. In the course of our research for uranium fluorides, Na3GaF6 was crystallized through hydrothermal reactions. Although there is a report on the preparation of Na3GaF6 powder by mechanochemical method that utilize a ball milling process [12], the crystal structure has not been analyzed yet. In this paper, we report on the hydrothermal synthesis and crystal structure of Na3GaF6.
Experimental
Materials and Method
Single crystals of Na3GaF6 were grown by a mild hydrothermal route during reactions to prepare Na3GaU6F30 which is published elsewhere [13]. For the synthesis, 1 mmol of UO2(CH3CO2)2·2H2O, 2 mmol of Ga2O3, and 1 mmol of NaF were combined with 1 mL of H2O and 1 mL of HF. The reaction mixture was placed into 23 mL Teflon–lined autoclaves. The autoclaves were closed, heated to 200 °C at a rate of 5 °C m−1, held for 1 day, and cooled to room temperature at a rate of 6 °C h−1. The mother liquor was decanted from the single crystal products, which were isolated by filtration and washed with distilled water and acetone. Colorless crystals of Na3GaF6 were found from the reaction as a minor phase.
Crystallographic Study
X-ray intensity data from a colorless polyhedral crystal (approximate dimensions 0.10 × 0.05 × 0.04 mm3) were measured at 294(1) K on a Bruker SMART APEX CCD diffractometer (Mo Kα radiation, λ = 0.71073 Å) [14]. The raw area detector data frames were reduced with SAINT+ [14]. Data were corrected for absorption effects using the multi-scan technique implemented in SADABS [14]. The reported unit cell parameters were determined by least-squares refinement of large sets of strong reflections taken from each data set. Full-matrix least-squares refinement against F2 of the structural models and difference Fourier calculations were performed with SHELXTL [15].
The most important crystallographic data and selected interatomic distances from the single crystal structure refinements for Na3GaF6 are found in Tables 1 and 2, respectively.
Results and Discussion
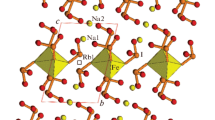
Na3GaF6 crystallizes in the monoclinic space group P21/n, and is isostructural with Na3AlF6 [16] that belongs to the cryolite type structure with a general formulae A3BF6 (A = Li, Na, K, Rb, Tl, NH4 and M = Al, Sc, V, Cr, Fe, etc.) (Figs. 1, 2). It is also closely related to a double perovskite structure A2BB’O6, where the A site includes 8- to 12-coordinated cations and the B and B’ sites contain octahedrally coordinated metal cations. In such a way, for the title compound if it is written as Na2NaGaF6, the Na(2) atom takes on the A site, and the Na(1) and Ga atoms occupy the B and B’ sites. Na3GaF6 exhibits a three-dimensional framework consisting of corner-shared alternating Na(1)F6 and GaF6 polyhedra. The Ga atom is located at a nearly regular octahedron with Ga–F distances of 1.879(2)–1.889(2) Å. The Na(1) atom is also observed in an almost regular octahedron with Na-F distances of between 2.235(2) and 2.293(2) Å, whereas the Na(2) atom is coordinated to nine F atoms creating a highly distorted square antiprism with Na(2)–F lengths ranging from 2.281(2) to 2.876(2) Å. The average angle of Na(1)–Ga–Na(1) and Ga–Na(1)–Ga is approximately 140° that is deviated from 180° for the ideal double perovskite structure, which is likely due to the presence of the small Na(2) cation between the framework, enforcing the GaF6 and Na(1)F6 polyhedra tilted. Bond valence sum calculation [17, 18] resulted in values of 3.01, 0.97–1.21 for Ga3+ and Na+ ions, respectively, consistent with the expected oxidation states.
Polyhedral representation of Na3GaF6 along the (top) a- and (bottom) c-axis. The corner-shared GaF6 and Na(1)F6 polyhedra build up a three-dimensional framework, wherein the Na(2) cations reside. The blue, green, orange, and red polyhedra/spheres represent the Ga, Na(1), Na(2) and F atoms, respectively. (Color figure online)
Illustration of the local coordination environments of the metal atoms. The Ga and Na(1) atoms are located at nearly regular octahedra, whereas the Na(2) is found in a highly distorted antisquare prism. The blue, green, orange, and red polyhedra/spheres represent the Ga, Na(1), Na(2) and F atoms, respectively. (Color figure online)
Supplementary Material
Further details of the crystal structure investigation can be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany; Fax: +49-7247-808-666; E-mail address: crystdata@fizkarlsruhe.de on quoting the depository number CSD-429,839 for Na3GaF6.
References
Ahrens M, Schuschke K, Redmer S, Kemnitz E (2007) Solid State Sci 9:833
Zhou Q, Kennedy BJ (2004) J Solid State Chem 177:654
Flerov IN, Gorev MV, Grannec J, Tressaud A (2002) J Fluor Chem 116:9
Yue Y, Hu Z, Chen C (2008) J Cryst Growth 310:1264
Birol Y (2007) J Alloys Compd 443:94
Slater PR, Gover RKB (2001) J Mater Chem 11:2035
Choy J-H, Kim J-Y, Kim S-J, Sohn J-S, Han OH (2001) Chem Mater 13:906
Caramanian A, Souron J-P, Gredin P, de Kozak A (2001) J Solid State Chem 159:234
Parhi P, Kramer J, Manivannan V (2008) J Mater Sci 43:5540
Wang X, Zhuang J, Peng Q, Li Y (2006) Inorg Chem 45:6661
Hua RN, Jia ZH, Xie DM, Shi CS (2002) Chin Chem Lett 13:1021
Lu J, Zhang Q, Wang J, Saito F (2004) J Am Ceram Soc 87:1814
Yeon J, Smith MD, Morrison G, zur Loye H-C (2015) Inorg Chem 54:2058
SMART Version 5.625, SAINT + Version 6.45 and SADABS Version 2.05 (2001) Bruker Analytical X-ray Systems, Inc., Madison
SHELXTL Version 6.14 (2000) Bruker analytical X-ray Systems, Inc., Madison
Hawthorne FC, Ferguson RB (1975) Can Miner 13:(Pt 4):377
Brese NE, O’Keeffe M (1991) Acta Crystallogr B 47:192
Brown ID, Altermatt D (1985) Acta Crystallogr B 41:244
Acknowledgements
Research supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award DE-SC0008664.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeon, J., zur Loye, HC. Hydrothermal Synthesis and Crystal Structure of Hexafluorogallate, Na3GaF6 . J Chem Crystallogr 47, 129–132 (2017). https://doi.org/10.1007/s10870-017-0687-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-017-0687-x