Abstract
A number of studies have shown that self-rated health reliably predicts mortality. This study assessed the impact of perseveration on self-rated health, physical functioning, and physical symptoms (pain, fatigue, breast cancer symptoms) among breast cancer patients. We hypothesized that cancer-related distress would serve as an intervening variable between both worry and rumination and self-rated health, physical functioning, and physical symptoms. Women (N = 124) who were approximately 7 weeks post-surgery but pre adjuvant treatment completed the Impact of Events Scale, the Penn State Worry Questionnaire, and the Rumination Scale. They also rated their pain, fatigue, physical functioning, and self-rated health using the RAND-36 and breast cancer symptoms with the Breast Cancer Prevention Trial Symptom Checklist (BCPT). Covariates included body mass index, age, cancer stage, menopause status, and physical comorbidities. Worry was associated with higher cancer-related distress, which in turn predicted greater pain and breast cancer symptoms, poorer physical functioning, and lower self-rated health. Rumination also predicted greater cancer-related distress, which ultimately contributed to greater pain along with poorer physical functioning and self-rated health. Models with fatigue as an outcome were not significant. These findings suggest that perseveration can heighten cancer-related distress and subsequent perceptions of physical symptoms and health among breast cancer patients prior to adjuvant treatment. Perseveration early in the cancer trajectory can adversely increase the impact of a cancer diagnosis and treatment on functioning and quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poor self-rated health, a risk factor for cardiovascular disease, diabetes, lung disease, arthritis, and stroke, consistently predicts mortality (Latham & Peek, 2013; Schnittker & Bacak, 2014). People who rate their health as poor have a two-fold increase in mortality compared to those who rate it as excellent (DeSalvo et al., 2006). Further, individuals reporting poorer self-rated health are less likely to engage in preventative health behaviors, thus increasing risk for morbidity and mortality (Idler & Benyamini, 1997). These effects hold after accounting for objective health status, behavioral risk factors, and sociodemographic characteristics.
Breast cancer patients experience many troubling symptoms during and after cancer treatment. Nearly 30% of breast cancer survivors suffer from chronic pain 5 years after treatment (Sheridan et al., 2012). Advanced cancer patients also often endure pain and fatigue simultaneously (Laird et al., 2011). Fatigue may also persist for years after cancer treatment (Bower et al., 2006) and can worsen self-reported physical symptoms above and beyond oncological treatment (Hagen et al., 2016). These unpleasant physical symptoms increase depression and/or anxiety (Bower et al., 2000; Vahdaninia et al., 2010) and thus reduce participation in everyday pleasant activities and negatively impact quality of life (Curt et al., 2000).
Alongside these physical symptoms and increased distress, cancer treatment and survivorship present a number of uncertainties: fears of the disease progression, recurrence, side effects, changes in physical functioning, and early mortality. Each of these uncertainties can contribute to anxiety and depression. A recent meta-analysis found higher rates of anxiety and depression among breast cancer survivors compared to women with no cancer history (Carreira et al., 2018). Worry (thinking about things that may or may not happen in the future) and rumination (thinking back over past losses and failures) may boost cancer-related distress (Borkovec et al., 1983; Nolen-Hoeksema, 2000). These processes, collectively termed perseveration, also contribute to poorer self-rated health in physically healthy adults (Verkuil et al., 2010). Consistent with the perseverative cognition hypothesis (PCH), worry and rumination heighten emotional intensity and create distress by prolonging both relatively innocuous and stressful situations. Further, this heightened emotional intensity resulting from perseveration prolongs physiological reactivity to stress. Perseveration also provokes anxiety and depression, respectively, among both healthy individuals and cancer patients (Brosschot et al., 2006; Deimling et al., 2006). Consequently, perseveration serves as an important cognitive risk factor for mental health difficulties throughout diagnosis and treatment.
The Self-Regulatory Model of Illness Behavior highlights the importance of illness perceptions and emotional responses when managing a health threat such as cancer (Leventhal et al., 1980). Within this model, poor self-regulation, including heightened perseveration, leads to increased distress and maladaptive coping behaviors. The heightened cancer-related distress among breast cancer patients who are more anxious and have more health problems may be augmented by worry and rumination (Bleiker et al., 2000). A history of anxiety or depression increases cancer-related distress (O’Connor et al., 2011). This distress can remain high up to 2 years following treatment (Bleiker et al., 2000). Cancer-related distress thus influences breast cancer patients’ perceptions of both psychological and physical symptoms (Jim et al., 2007).
This study drew upon the PCH and the Self-Regulatory Model of Illness Behavior, which emphasize how self-regulatory failures such as worry and rumination lead to heightened distress and physical dysregulation. The primary goal was address the impact of perseveration on cancer related-distress, physical symptoms (pain, fatigue, and breast cancer symptoms), physical functioning, and self-rated health among breast cancer patients. We assessed cancer-related distress and its links to these perseverative processes to physical symptoms, physical functioning, and self-rated health. The hypothesized model (Fig. 1) shows how greater worry and rumination could heighten cancer-related distress and contribute to low physical functioning and self-rated health along with high pain, fatigue, and breast cancer symptoms. We also expected that worry and rumination would indirectly relate to these outcomes through cancer-related distress; specifically, worry and rumination would be associated with higher cancer-related distress, which in turn would promote poorer health perceptions.
The proposed direct and indirect pathways of worry and rumination linking to self-rated health and physical symptoms among breast cancer patients. Perseveration was measured via worry and rumination. Self-rated symptom and health measures include the following outcomes: pain, fatigue, breast cancer symptoms, physical functioning, and self-rated health
Methods
Participants
Women (N = 124) were recruited as part of a larger ongoing parent study examining cardiovascular function among breast cancer patients. Breast cancer patients were stage I-IIIA, 18–80 years old, and were 52.07 days post-surgery (SD = 24.83) but pre-adjuvant treatment. Exclusion criteria included a prior history of any malignancy except for basal or squamous cell skin cancers, recent strokes, diabetes mellitus, current heart disease or uncontrolled hypertension, peripheral vascular disease, liver disease, autoimmune and/or inflammatory diseases including rheumatoid arthritis and ulcerative colitis, alcohol or drug abuse, or other medical conditions that would limit participation in the study (e.g., implantable defibrillator, pacemaker or life-threating heart conditions such as congestive heart failure, pulmonary disease, orthopedic problems, major psychiatric illness, cognitive dysfunction, or an acute medical problem).
Measures
Predictor variables
The 16-item Penn State Worry Questionnaire (PSWQ) (Meyer et al., 1990) assesses pathological worry. The respondent rates items such as “I worry all the time” on five-point scales with 1 = not at all typical of me and 5 = very typical of me. The Rumination Scale (Armey et al., 2009) is a 10-item measure of conscious, repetitive, and aversive thoughts (e.g. when I have a problem, I tend to think of it a lot of the time”).
Intervening variables
The 22-item Impact of Events Scale (IES) (Horowitz et al., 1979) asked women to respond based on their recent cancer diagnosis. The instructions were modified to ask the women to respond based on their recent cancer diagnosis. The current study utilized the IES total score as a global measure of cancer-related distress.
Outcome variables
The revised Breast Cancer Prevention Trial symptom checklist (BCPT) (Stanton et al., 2005) provided information about symptoms related to the breast cancer diagnosis and treatment. This study used the BCPT mean score. The RAND-36 (Hays & Morales, 2001) provides a non-disease specific measure of functioning and well-being. It indexes eight health concepts, five of which were used for the current study as they directly related to physical wellbeing: physical functioning, bodily pain, role limitations due to physical health problems, energy/fatigue, and general health perceptions.
Covariates
All analyses controlled for age, cancer stage, menopause status, body mass index (BMI), and medical comorbidities. The Charlson comorbidity index, originally developed with breast cancer patients (Charlson et al., 1994), assessed physical comorbidities. The Charlson assigns weights to 19 medical conditions with greater scores equal to a greater comorbidity burden.
Procedures
The Ohio State University biomedical institutional review board approved this study (#2014C0012). Every participant provided written informed consent. Participants completed questionnaires as part of a full-day study visit at the Ohio State Clinical Research Center where they completed several other tasks relevant to the parent study (ate a standardized meal, had their blood taken to assess meal responses, and underwent metabolic measurements).
Data analysis plan
All analyses were conducted using SPSS version 26. Bivariate correlations tested associations between the predictor and dependent variables with the intervening variable. The hypotheses illustrated in Fig. 1 were tested using Hayes’ SPSS PROCESS macro (Hayes, 2012). We first assessed whether worry and rumination were associated with cancer-related distress as well as pain, fatigue, physical functioning, general health, and breast cancer symptoms. Then we examined whether worry and rumination were indirectly linked to these same variables through cancer-related distress. Indirect effects were tested with 95% bias-corrected confidence intervals and 10,000 bootstrapped samples. Separate models were run with worry and rumination as predictors with each outcome. Body mass index, physical comorbidities, cancer stage, menopause status, and age were included as covariates in all models.
Results
Participant demographics and covariates
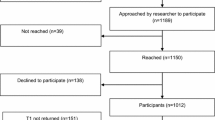
Initially, 437 women were approached for the current study and chose not to participate for various reasons. Of the 212 women who expressed initial interest in participating, 150 completed the informed consent process; 26 women were excluded from our analyses due to missing data. The women’s mean age was 52.45 years (SD = 10.7), and they were predominantly white (86.0%) and married (70.0%). Most participants were highly educated (60.0% had at least a college education) and were working part- or full-time (76.0%).
Women had a mean BMI of 28.68 (SD = 5.8) and a low number of physical comorbidities (M = 0.20, SD = 0.5). A total of 49.3% of participants were diagnosed with Stage I breast cancer while 47.3% were diagnosed with Stage II and 3.3% with Stage IIIA.
Worry, cancer-related distress, and health
We first tested the model with worry as the predictor. BMI had a direct effect on fatigue (b = 0.11, SE = 0.18, p = 0.02) and self-rated health (b = −0.81, SE = 0.22, p < 0.001) while no other covariates had a direct effect on cancer-related distress or the outcome variables. As expected, worry was associated with greater cancer-related distress (b = 0.36, SE = 0.08, p < 0.001) and higher fatigue (b = −0.34, SE = 0.15, p = 0.02). Worry was not directly related to pain (b = −0.14, SE = 0.17, p = 0.43), self-rated health (b = −0.18, SE = 0.10, p < 0.001), physical functioning (b = 0.14, SE = 0.11, p = 0.24), or breast cancer symptoms (b = 0.004, SE = 0.003, p = 0.23) (Table 1).
When cancer-related distress was added to the models, worry was no longer significant, and only cancer-related distress was associated with pain (b = −0.61, SE = 0.18, p = 0.001), physical function (b = −0.29, SE = 0.13, p = 0.02), breast cancer symptoms (b = 0.01, SE = 0.00, p = 0.02), and self-rated health (b = −0.25, SE = 0.11, p = 0.02). Cancer-related distress was not associated with fatigue (b = −0.07, SE = 0.16, p = 0.66). Worry had indirect effects on pain, breast cancer symptoms, physical functioning, and self-rated health (see Table 2 for coefficients). Thus, the more breast cancer patients worried, the greater their cancer-related distress, which in turn contributed to greater pain and breast cancer symptoms, poorer physical functioning and poorer self-rated health.
Rumination, cancer-related distress, and health
We tested rumination as a predictor in separate models. BMI had a direct effect on fatigue (b = −0.65, SE = 0.31, p = 0.04) and self-rated health (b = −0.76, SE = 0.22, p < 0.001) while no other covariates had a direct effect on cancer-related distress or the outcome variables. As expected, rumination was associated with greater cancer-related distress (b = 1.24, SE = 0.25, p < 0.001), as well as higher fatigue (b = −1.29, SE = 0.49, p < 0.01) and breast cancer symptoms (b = 0.02, SE = 0.01, p = 0.05). Rumination was not directly related to self-rated health (b = −0.60, SE = 0.34, p = 0.08), physical functioning (b = 0.35, SE = 0.38, p = 0.37), or pain (b = −0.14, SE = 0.57, p = 0.80).
When cancer-related distress was added to the models, rumination was not significant, and only cancer-related distress was related to pain (b = −0.66, SE = 0.19, p < 0.001), self-rated health (b = −0.26, SE = 0.11, p = 0.02), and physical function (b = −0.29, SE = 0.13, p = 0.03). Similar to the effects of worry, rumination also had indirect effects on pain, physical functioning, and self-rated health (see Table 2 for coefficients). Thus, the more women ruminated, the greater their cancer-related distress, which in turn contributed to worse pain, poorer physical functioning and poorer self-rated health. The direct effect of cancer-related distress on breast cancer symptoms was marginally significant (b = 0.01, SE = 0.00, p = 0.06). Cancer-related distress was not associated with fatigue (b = −0.04, SE = 0.16, p = 0.75).
Alternative models
It is plausible that cancer-related distress influences worry and rumination, resulting in increased physical symptoms and poorer self-rated health. We therefore tested alternative models using cancer-related distress as the predictor variable and worry or rumination as intervening variables. When cancer-related distress served as the predictor, worry had indirect effects on fatigue (b = −0.15, SE = 0.07, 95% CI = −0.30, −0.03). In contrast, there were no indirect effects of worry on pain (b = −0.09, SE = 0.08, 95% CI = −0.25, −0.06), self-rated health (b = −0.07, SE = 0.05, 95% CI = −0.16, 0.02), physical function (b = 0.06, SE = 0.05, 95% CI = −0.04, 0.17), or breast cancer symptoms (b = −0.002, SE = 0.001, 95% CI = −0.009, 0.004). Rumination had indirect effects on breast cancer symptoms (b = 0.003, SE = 0.002, 95% CI = 0.001, 0.006) and fatigue (b = −0.18, SE = 0.08, 95% CI = −0.35, −0.04). There were no indirect effects of rumination on pain (b = −0.06, SE = 0.08, 95% CI = −0.23, 0.11), self-rated health (b = −0.05, SE = 0.04, 95% CI = −0.13, 0.05), or physical functioning (b = −0.05, SE = 0.06, 95% CI = −0.05, 0.17).
Discussion
Findings from this study suggest that perseveration plays an important role in heightening cancer-related distress and subsequent perceptions of physical health among breast cancer patients. The breast cancer patients in this study had undergone surgery but had not yet begun adjuvant treatment (e.g., radiation, chemotherapy, or immune therapy). This period is particularly important as psychological health prior to adjuvant treatment can set the stage for future psychological and physical distress throughout treatment and survivorship (Gil et al., 2012; Iwamitsu et al., 2005; Jim et al., 2007; Oh & Cho, 2020). Even after controlling for important covariates including cancer stage, there was a direct link between worry and rumination and cancer-related distress, pain, fatigue, physical functioning, and self-rated health, indicating that perseveration primes the focus on physical symptoms and health. Consistent with the perseverative cognition hypothesis, processes such as worry and rumination can prolong emotional and physical stress responses (Brosschot et al., 2006).
Perseveration and its ties to cancer-related distress influenced patients’ physical functioning, self-rated health, and physical symptoms. These findings are consistent with the Self-Regulatory Model of Illness Behavior, where poor regulation, characterized in this study as high rates of perseveration, increase distress. Anxiety and depression can provoke worry and rumination, respectively (Brosschot et al., 2006) and greater cancer-related distress heightens depression and anxiety before and during diagnosis and treatment (Siglen et al., 2007). Cancer-related distress thus serves as a downstream consequence of perseverative processes and their associated mental health correlates. When coupled with cancer-related distress, the burden of worry and rumination can promote poor emotional and physical health.
These data have several important implications. The early detection and treatment advances in breast cancer have increased survival time (Kaplan et al., 2015). As a consequence, the quality of life and mental and physical health adjustments of breast cancer survivors is an important consideration (Alfano & Rowland, 2006). By identifying those women who have heightened cancer-related distress, clinicians may be able to better intervene on these symptoms and subsequently reduce both the psychological and physical burden associated with cancer treatment and survivorship.
This study has several notable strengths. This sample included breast cancer patients who had undergone surgery but not begun adjuvant treatment. The assessment of women at this stage in cancer treatment provides novel insights into their psychological and physical health that can predict functioning throughout treatment and survivorship. These findings extend existing self-rated health research by identifying cancer-related distress as an intervening variable linking perseveration to self-rated health. The links among perseveration, cancer-related distress, physical functioning and pain among breast cancer patients expand our understanding of the importance of assessing multiple domains of physical health. Further, given previous research linking perseveration to physical functioning (Ottaviani et al., 2016), assessing how perseveration may influence self-rated health and physical symptoms among breast cancer patients provides information about the processes that contribute to problematic psychological and physical functioning. However, the cross-sectional nature of these data limits causal claims regarding the impact of perseveration and cancer-related distress on self-rated health and physical symptoms.
Early in the cancer trajectory, perseveration can prime psychological distress, which in turn can exacerbate physical symptoms during treatment and survivorship. Future work should continue to explore ways to assess for and intervene on worry and rumination among breast cancer patients with the goal of lessening the psychological and physical burden of cancer diagnosis and treatment.
References
Alfano, C. M., & Rowland, J. H. (2006). Recovery issues in cancer survivorship: A new challenge for supportive care. The Cancer Journal, 12, 432–443.
Armey, M. F., Fresco, D. M., Moore, M. T., Mennin, D. S., Turk, C. L., Heimberg, R. G., et al. (2009). Brooding and pondering: Isolating the active ingredients of depressive rumination with exploratory factor analysis and structural equation modeling. Assessment, 16, 315–327.
Bleiker, E. M., Pouwer, F., Van Der Ploeg, H. M., Leer, J.-W. H., & Ader, H. J. (2000). Psychological distress two years after diagnosis of breast cancer: Frequency and prediction. Patient Education and Counseling, 40, 209–217.
Borkovec, T. D., Robinson, E., Pruzinsky, T., & DePree, J. A. (1983). Preliminary exploration of worry: Some characteristics and processes. Behaviour Research and Therapy, 21, 9–16.
Bower, J. E., Ganz, P. A., Desmond, K. A., Bernaards, C., Rowland, J. H., Meyerowitz, B. E., et al. (2006). Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer, 106, 751–758.
Bower, J. E., Ganz, P. A., Desmond, K. A., Rowland, J. H., Meyerowitz, B. E., & Belin, T. R. (2000). Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. Journal of Clinical Oncology, 18, 743–743.
Brosschot, J. F., Gerin, W., & Thayer, J. F. (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60, 113–124.
Carreira, H., Williams, R., Müller, M., Harewood, R., Stanway, S., & Bhaskaran, K. (2018). Associations between breast cancer survivorship and adverse mental health outcomes: A systematic review. JNCI: Journal of the National Cancer Institute, 110, 1311–1327.
Charlson, M., Szatrowski, T. P., Peterson, J., & Gold, J. (1994). Validation of a combined comorbidity index. Journal of Clinical Epidemiology, 47, 1245–1251.
Curt, G. A., Breitbart, W., Cella, D., Groopman, J. E., Horning, S. J., Itri, L. M., et al. (2000). Impact of cancer-related fatigue on the lives of patients: New findings from the fatigue coalition. The Oncologist, 5, 353–360.
Deimling, G. T., Bowman, K. F., Sterns, S., Wagner, L. J., & Kahana, B. (2006). Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology, 15, 306–320.
DeSalvo, K. B., Bloser, N., Reynolds, K., He, J., & Muntner, P. (2006). Mortality prediction with a single general self-rated health question: A meta-analysis. Journal of General Internal Medicine, 21, 267–275.
Gil, F., Costa, G., Hilker, I., & Benito, L. (2012). First anxiety, afterwards depression: Psychological distress in cancer patients at diagnosis and after medical treatment. Stress & Health, 28, 362–367.
Hagen, K. B., Aas, T., Kvaløy, J. T., Eriksen, H. R., Søiland, H., & Lind, R. (2016). Fatigue, anxiety and depression overrule the role of oncological treatment in predicting self-reported health complaints in women with breast cancer compared to healthy controls. The Breast, 28, 100–106.
Hayes, A. F. (2012). PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. Lawrence, KS: University of Kansas.
Hays, R. D., & Morales, L. S. (2001). The RAND-36 measure of health-related quality of life. Annals of Medicine, 33, 350–357.
Horowitz, M., Wilner, N., & Alvarez, W. (1979). Impact of event scale: A measure of subjective stress. Psychosomatic Medicine, 41, 209–218.
Idler, E. L., & Benyamini, Y. (1997). Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior, 38, 21–37.
Iwamitsu, Y., Shimoda, K., Abe, H., & Okawa, M. (2005). Anxiety, emotional suppression, and psychological distress before and after breast cancer diagnosis. Psychosomatics, 46, 19–24.
Jim, H. S., Andrykowski, M. A., Munster, P. N., & Jacobsen, P. B. (2007). Physical symptoms/side effects during breast cancer treatment predict posttreatment distress. Annals of Behavioral Medicine, 34, 200–208.
Kaplan, H. G., Malmgren, J. A., Atwood, M. K., & Calip, G. S. (2015). Effect of treatment and mammography detection on breast cancer survival over time: 1990-2007. Cancer, 121, 2553–2561.
Laird, B. J., Scott, A. C., Colvin, L. A., McKeon, A.-L., Murray, G. D., Fearon, K. C., et al. (2011). Pain, depression, and fatigue as a symptom cluster in advanced cancer. Journal of Pain and Symptom Management, 42, 1–11.
Latham, K., & Peek, C. W. (2013). Self-rated health and morbidity onset among late midlife US adults. Journals of Gerentology Series B: Psychological Sciences and Social Sciences, 68, 107–116.
Leventhal, H., Meyer, D., & Nerenz, D. (1980). The common sense representation of illness danger. Contributions to Medical Psychology, 2, 7–30.
Meyer, T. J., Miller, M. L., Metzger, R. L., & Borkovec, T. D. (1990). Development and validation of the penn state worry questionnaire. Behaviour Research, 28, 487–495.
Nolen-Hoeksema, S. (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology, 109, 504–511.
O’Connor, M., Christensen, S., Jensen, A. B., Møller, S., & Zachariae, R. (2011). How traumatic is breast cancer? Post-traumatic stress symptoms (PTSS) and risk factors for severe PTSS at 3 and 15 months after surgery in a nationwide cohort of Danish women treated for primary breast cancer. British Journal of Cancer, 104, 419.
Oh, P.-J., & Cho, J.-R. (2020). Changes in fatigue, psychological distress, and quality of life after chemotherapy in women with breast cancer: A prospective study. Cancer Nursing, 43, E54–E60.
Ottaviani, C., Thayer, J. F., Verkuil, B., Lonigro, A., Medea, B., Couyoumdjian, A., et al. (2016). Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin, 142, 231.
Schnittker, J., & Bacak, V. (2014). The increasing predictive validity of self-rated health. PliS One, 9, e84933.
Sheridan, D., Foo, I., O’Shea, H., Gillanders, D., Williams, L., Fallon, M., et al. (2012). Long-term follow-up of pain and emotional characteristics of women after surgery for breast cancer. Journal of Pain and Symptom Management, 44, 608–614.
Siglen, E., Bjorvatn, C., Engebretsen, L. F., Berglund, G., & Natvig, G. K. (2007). The influence of cancer-related distress and sense of coherence on anxiety and depression in patients with hereditary cancer. Journal of Genetic Counseling, 16, 607–615.
Stanton, A. L., Bernaards, C. A., & Ganz, P. A. (2005). The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. Journal of the National Cancer Institute, 97, 448–456.
Vahdaninia, M., Omidvari, S., & Montazeri, A. (2010). What do predict anxiety and depression in breast cancer patients? A follow-up study. Social Psychiatry and Psychiatric Epidemiology, 45, 355–361.
Verkuil, B., Brosschot, J. F., Gebhardt, W. A., & Thayer, J. F. (2010). When worries make you sick: A review of perseverative cognition, the default stress response and somatic health. Journal of Experimental Psychopathology, 1, 87–118.
Funding
This study was funded by NIH grant number R01CA186720 which was awarded to Dr. Kiecolt-Glaser. Dr. Renna is also supported by T32CA229114-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Renna, M.E., Rosie Shrout, M., Madison, A.A. et al. Worry and rumination in breast cancer patients: perseveration worsens self-rated health. J Behav Med 44, 253–259 (2021). https://doi.org/10.1007/s10865-020-00192-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10865-020-00192-9