Abstract
Non-small cell lung cancer (NSCLC) is a common cancer with an unfavorable 5-year survival rate. We intended to explore the role of circular RNA_0074027 (circ_0074027) in NSCLC progression. The levels of circ_0074027, messenger RNA (mRNA) of its linear form paired like homeodomain 1 (PITX1), microRNA-2467-3p (miR-2467-3p) and ras homolog family member A (RHOA) mRNA were determined by quantitative real-time polymerase chain reaction (qRT-PCR). Cell Counting Kit-8 (CCK8) assay and plate colony assay were conducted to measure the proliferation ability of NSCLC cells. Transwell assays were used to assess cell migration and invasion abilities. Flow cytometry was utilized to analyze cell apoptosis rate. Dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay were used to test the interaction between miR-2467-3p and circ_0074027 or RHOA. Western blot assay was performed to evaluate the protein level of RHOA in NSCLC cells. Murine xenograft model was built to evaluate the role of circ_0074027 in tumor growth in vivo. Circ_0074027 expression was prominently elevated in NSCLC tissues and cell lines. Circ_0074027 knockdown or miR-2467-3p overexpression suppressed cell proliferation, migration and invasion and facilitated cell apoptosis of NSCLC cells. Circ_0074027 interacted with miR-2467-3p, and RHOA was a target of miR-2467-3p in NSCLC cells. RHOA silencing blocked the malignant potential of NSCLC cells. Circ_0074027 silencing restrained the malignant phenotypes of NSCLC cells largely through up-regulating miR-2467-3p. Circ_0074027 knockdown notably blocked xenograft tumor growth in vivo. In conclusion, circ_0074027 accelerated NSCLC progression by binding to miR-2467-3p to induce RHOA expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) occupies the majority (about 80 %) of all lung cancer cases (Siegel et al. 2018). Although treatment has made great advancement, the prognosis of NSCLC patients remains unfavorable (Proto et al. 2019; Watanabe et al. 2017). Most patients were found to be in an advanced stage when they were diagnosed, leading to low survival rate (Carbone et al. 2017; Chen et al. 2014; Herbst et al. 2018). Therefore, it is of great significance to investigate potential mechanisms underlying NSCLC progression and seek novel therapeutic targets for NSCLC.
Circular RNAs (circRNAs) are newly identified non-coding RNAs (ncRNAs) featured by closed loop structure, and they regulate tumor development via acting as microRNA (miRNA) sponges (Memczak et al. 2013; Meng et al. 2017). Accumulating circRNAs have shown important regulatory roles in NSCLC progression. Wang et al.. claimed that circ_0067934 knockdown restrained the proliferation and motility in NSCLC cells, and high level of circ_0067934 was related to the dismal prognosis of NSCLC patients (Wang and Li 2018). Circ_0076305 enhanced the cisplatin resistance in NSCLC cells through targeting miR-296-5p/STAT3 axis (Dong et al. 2019). Circ_0074027 silencing inhibited cell viability, cell cycle progression, colony formation ability and induced cell apoptosis of NSCLC cells (Yu et al. 2020). As the modulatory mechanisms behind a specific circRNA are complex and crosstalk with each other, we aim to further explore the working mechanism of circ_0074027 in NSCLC progression.
MiR-2467-3p was reported to hamper the progression of diverse cancers, including colorectal cancer (Xiao and Liu, 2020), cervical cancer (Liu and Wen 2020) and NSCLC (Chen et al. 2020). For example, SNHG20 contributed to the progression of NSCLC through positively regulating E2F3 level via sequestering miR-2467-3p (Chen et al. 2020). Nevertheless, the interaction between miR-2467-3p and circ_0074027 remains to be uncovered.
Ras homolog family member A (RHOA) is a member of small GTPase proteins of Ras superfamily, and it is implicated with the remodeling of actin cytoskeleton, thus associating with the motility of cells (Yu et al. 2019a). Accumulating articles have associated the motility of cancer cells with RHOA protein. For instance, long ncRNA (lncRNA) HOTAIR contributed to the proliferation and metastasis abilities of gastric cancer cells through sponging miR-126 to activate CXCR4 and RHOA signaling (Xiao et al. 2019). LncRNA NORAD silencing suppressed the proliferation and motility of lung cancer cells through reducing CXCR4 expression to inhibit RHOA/ROCK signaling (Wu et al. 2020). Disclosing the signal network of RHOA in NSCLC progression is important for finding effective targets of NSCLC treatment.
Circ_0074027 was highly expressed in NSCLC tissues and cell lines. Circ_0074027 silencing suppressed cell proliferation, migration, invasion and promoted cell apoptosis of NSCLC cells. The interacted partners of circ_0074027 were subsequently explored to uncover the working mechanism of circ_0074027 in NSCLC development.
Materials and methods
Clinical samples
Thirty pairs of NSCLC tissues and corresponding non-tumorous tissues were collected from NSCLC patients diagnosed at Shanxi Provincial Cancer Hospital. All subjects had provided written informed consents before surgical resection.
Cell lines
Human bronchial epithelial cell line 16HBE was purchased from Institute of Respiratory Diseases (Guangzhou, China). NSCLC cell lines, including A549, H1299, H358 and H1581, were purchased from BeNa Culture Collection (Beijing, China). All cell lines were maintained with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) plus 10 % (v/v) fetal bovine serum (FBS; Gibco), 100 U/mL penicillin (Sigma, St Louis, MO, USA) and 100 µg/mL streptomycin (Sigma) at the atmosphere of 37℃ and 5 % CO2.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
RNA samples were acquired through disrupting tissues and cells via Trizol reagent (Solarbio, Beijing, China). RNA samples were reversely transcribed into template DNA using iScript™ cDNA Synthesis Kit (for circ_0074027, paired like homeodomain 1 (PITX1) and RHOA; Bio-Rad, Hercules, CA, USA) and miScript reverse transcription kit (for miR-2467-3p; Qiagen, Hilden, Germany). Subsequently, the expression of these molecules was assessed using SYBR™ Green Master Mix (Applied Biosystems, Foster City, CA, USA) and analyzed by the 2−ΔΔCt method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the endogenous control for circ_0074027, PITX1 and RHOA, while nuclear RNA U6 was used as the internal control for miR-2467-3p. The primers were shown in Table 1.
Exonuclease digestion
RNA samples (2 µg) isolated from A549 or H1299 cells were incubated with 6U RNase R (Epicentre Technologies, Madison, WI, USA) for 30 min at room temperature. The levels of circ_0074027 and PITX1 were determined by qRT-PCR.
RNA oligoribonucleotides, plasmids and cell transfection
The small interfering RNA against circ_0074027 (si-circ_0074027) or RHOA (si-RHOA), negative control of siRNA (si-NC), short hairpin RNA of circ_0074027 (sh-circ_0074027), sh-NC, circ_0074027 overexpression plasmid (vector-circ_0074027), pLCDH-cir (vector), miR-2467-3p mimic, NC mimic, miR-2467-3p inhibitor and NC inhibitor were purchased from GenePharma (Shanghai, China). Transfection was conducted using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA), and transfection efficiency was assessed by qRT-PCR to analyze if cells were successfully transfected.
Cell counting Kit-8 (CCK8) assay
Proliferation capacity was analyzed by CCK8 assay through monitoring the number of viable cells after transfection for 0 h, 24 h, 48 h or 72 h. A549 and H1299 cells in 96-well plates were treated with 20 µL CCK8 reagent (Dojindo, Tokyo, Japan) for 4 h at 37℃. The optical density was determined at the wavelength of 450 nm.
Plate colony assay
NSCLC cells were seeded into 6-well plates (200 cells per well) and diluted in culture medium. During 2-week incubation period, complete medium was replenished every 4 d to maintain the culture condition of NSCLC cells. Colonies were stained with 0.5 % crystal violet (Solarbio) for 30 min. The number of colonies containing more than 50 cells was analyzed.
Transwell assays
Transwell chambers were purchased from Shanghai Jrdun Biotechnology (Shanghai, China) and placed in the 24-well plates. Matrigel (Qcbio, Shanghai, China) at the dilution of 1:8 was added to the transwell insert membrane to pre-coat the membrane to conduct transwell invasion assay, and 24-well plates were placed at 37℃ incubator for 30 min for drying. Subsequently, transfected NSCLC cells were digested and diluted in appropriate concentration. Cell suspension (7 × 104 cells in 100 µL serum-free medium) was added to the upper chambers, and 600 µL medium plus 10 % FBS was added to the lower chambers. After 24 h, cells in the upper side of the insert membrane were scraped off with the cotton swab, and invaded cells were fixed using 4 % paraformaldehyde (Leagene Biotechnology, Beijing, China) and stained using 0.5 % crystal violet (Solarbio). Five fields were randomly chosen in each experimental group (100 ×). NSCLC cells passed through the insert membrane were then counted. To assess cell migration ability, transwell migration assay was conducted using un-coated transwell insert membrane, and the other protocols were similar with transwell invasion assay.
Flow cytometry
NSCLC cells were re-suspended in the binding buffer. 10 µL propidium iodide (PI; Beyotime, Shanghai, China) and 5 µL Annexin V-fluorescein isothiocyanate (Annexin V-FITC; Beyotime) were simultaneously added to incubate with NSCLC cells in a dark room for 15 min. Fifteen minutes later, the apoptosis rate was analyzed by the flow cytometer as the percentages of NSCLC cells marked with FITC+ and PI+/−.
Dual‐luciferase reporter assay
We predicted the target partners of circ_0074027 and miR-2467-3p using StarBase software and TargetScan software, respectively.
Dual-luciferase reporter assay was performed to confirm if circ_0074027 and the 3’untranslated region (3’UTR) of RHOA interacted with miR-2467-3p. The fragments of circ_0074027 and the 3’UTR of RHOA were amplified and inserted into the multiple cloning sites in pmirGLO vector (Promega, Madison, WI, USA) which were termed as circ_0074027-wt and RHOA-wt. The miR-2467-3p binding sites in the fragment of circ_0074027 and RHOA were mutated and also cloned into pmirGLO vector (Promega) which termed as circ_0074027-mut and RHOA-mut. NSCLC cells were transfected with NC mimic, miR-2467-3p mimic, NC inhibitor or miR-2467-3p inhibitor and luciferase plasmids for 48 h, and luciferase activities were analyzed by the dual-luciferase assay system kit (Promega).
RNA immunoprecipitation (RIP) assay
EZ-Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) was used in RIP assay. NSCLC cells were disrupted using RIP lysis buffer plus RNase inhibitor (Millipore). A total of 100 µL cell lysate was incubated with RIP buffer containing antibody-precoated magnetic beads. The expression of circ_0074027 and miR-2467-3p which were pulled down was measured by qRT-PCR.
Western blot assay
NSCLC cells were harvested after transfection for 48 h and then Radio-Immunoprecipitation Assay (RIPA) buffer (Solarbio) was utilized for the isolation of protein samples. The BCA kit (Invitrogen) was used to quantify protein samples. Protein samples were mixed with loading buffer and denaturation at 95℃ for 5 min. 25 µg protein samples were added into each well of the 10 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel, and electrophoresis was conducted at the constant voltage of 80V for 2 h. Subsequently, proteins were electro-transferred onto the polyvinylidene fluoride (PVDF) membrane (Millipore) at 110V for 2 h. Blocking was conducted through incubating the PVDF membrane with 5 % non-fat milk. The membrane was then incubated with the primary antibodies at 4℃ overnight, including anti-E-cadherin (ab40772; Abcam, Cambridge, MA, USA), anti-N-cadherin (ab98952; Abcam), anti-Vimentin (ab92547; Abcam), anti-RHOA (ab187027; Abcam) and anti-GAPDH (ab8245; Abcam). After incubating with horseradish peroxidase-conjugated immunoglobulin G (IgG) antibody for 2 h at room temperature, protein bands were measured by the enhanced chemiluminescent visualization (ECL) system kit (Pierce Biotechnology, Rockford, IL, USA).
Murine xenograft model
A549 cell line stably expressing sh-circ_0074027 or sh-NC was established using lentivirus-mediated sh-NC or sh-circ_0074027. BALB/c male nude mice (6-week-old, Vital River Laboratory Animal Technology, Beijing, China) were subcutaneously injected with A549 cells stably expressing sh-circ_0074027 (n = 6) or sh-NC (n = 6) in the dorsal side. The length and width of tumors were measured every week for 5 weeks. Tumor volume was analyzed as width2 × length × 0.5. After inoculation for 5 weeks, nude mice were euthanized, and tumors were resected and weighed. Tumor tissues were stored at -80℃ for further analysis. The expression of circ_0074027, miR-2467-3p and RHOA protein was measured by qRT-PCR or Western blot assay.
Data analysis
GraphPad Prism 7.0 was used to analyze the data from three independent experiments. Statistical data were shown as mean ± standard deviation (SD). The comparisons were analyzed by Student’s t-test and one-way analysis of variance (ANOVA) followed by Tukey’s test as appropriate. The analysis of linear correlation was conducted by Pearson correlation coefficient. P < 0.05 was regarded as statistically significant.
Results
Circ_0074027 interference suppresses the proliferation, migration and invasion while induces the apoptosis of NSCLC cells
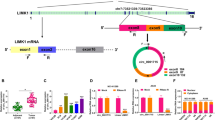
Circ_0074027 was overexpressed in NSCLC tissues relative to that in paired non-tumorous tissues (Fig. 1a). Compared with human bronchial epithelial cell line 16HBE, circ_0074027 expression was observed to be markedly up-regulated in four cancer cell lines (Fig. 1a), especially in A549 and H1299 cell lines. CircRNAs are featured by stable circular structure due to their covalently closed ends (Patop et al. 2019), thus they are resistant to exonuclease. We subsequently assessed the stability of circ_0074027 using RNase R, and its matching linear form PITX1 was used as the control. RNase R treatment remarkably reduced the level of PITX1, while circ_0074027 expression was unchanged with RNase R digestion or not (Fig. 1b), suggesting that circ_0074027 was indeed a circular transcript. Subsequently, we conducted loss-of-function experiments to assess the influences of circ_0074027 silencing on the proliferation, migration, invasion and apoptosis of NSCLC cells. High silencing efficiency of si-circ_0074027 was confirmed by qRT-PCR assay (Fig. 1c). Through analyzing cell proliferation curve in Fig. 1d, we found that circ_0074027 interference significantly suppressed cell proliferation ability. As displayed in Fig. 1e, after incubation for 2 weeks, the number of colonies was prominently reduced with the knockdown of circ_0074027, which further confirmed that circ_0074027 silencing restrained the proliferation of NSCLC cells. Transwell assays were conducted to evaluate cell migration and invasion abilities. Circ_0074027 silencing notably decreased the numbers of migrated and invaded NSCLC cells relative to si-NC group (Fig. 1f). To further confirm the role of circ_0074027 on the motility of NSCLC cells, the expression of three epithelial-mesenchymal transition (EMT)-associated markers (E-cadherin, N-cadherin and Vimentin) was analyzed by Western blot assay. As shown in Supplementary Fig. 1A and 1B, circ_0074027 knockdown significantly down-regulated the expression of N-cadherin and Vimentin whereas up-regulated the level of E-cadherin, suggesting that circ_0074027 silencing restrained the EMT of NSCLC cells. With the silencing of circ_0074027, the apoptosis of NSCLC cells was triggered compared with si-NC transfected group (Fig. 1g), demonstrating that circ_0074027 silencing promoted the apoptosis of NSCLC cells. Taken together, circ_0074027 knockdown hampered the proliferation, migration and invasion and induced the apoptosis of NSCLC cells.
Circ_0074027 interference su and invasion while induces the apoptosis of NSCLC cells. a The level of circ_0074027 was analyzed in a total of 30 pairs of NSCLC tumor (T) tissues and adjacent normal (N) tissues by qRT-PCR. The expression of circ_0074027 in a panel of four NSCLC cell lines and normal human bronchial epithelial cell line (16HBE) was measured by qRT-PCR. b Cyclization validation was conducted to analyze the stability of circ_0074027 and PITX1 with the digestion of RNase R or not. c-g A549 and H1299 cells were transfected with si-NC or si-circ_0074027. c qRT-PCR was implemented to analyze the interference efficiency of si-circ_0074027 in NSCLC cells. d CCK8 assay was conducted to monitor the number of viable NSCLC cells after transfection for 0 h, 24 h, 48 h or 72 h to generate cell proliferation curve. e Plate colony assay was performed to assess the proliferation ability of circ_0074027-silencing NSCLC cells. f Cell migration and invasion abilities were evaluated by transwell assays. g Flow cytometry was carried out to analyze the influence of circ_0074027 silencing on the apoptosis of NSCLC cells. *P < 0.05, **P < 0.01, ***P < 0.001
MiR-2467-3p overexpression restrains the biological phenotypes of NSCLC cells
A prominent decrease in the level of miR-2467-3p was found in NSCLC tumor tissues than that in adjacent non-tumor tissues (Fig. 2a). MiR-2467-3p expression in A549 and H1299 cells was notably reduced compared with 16HBE cells (Fig. 2a). Gain-of-function experiments were conducted to analyze the functions of miR-2467-3p in NSCLC cells. High overexpression efficiency of miR-2467-3p mimic was validated by qRT-PCR (Fig. 2b). Through performing CCK8 assay and plate colony assay, we found that miR-2467-3p overexpression restrained the proliferation of NSCLC cells (Fig. 2c and d). MiR-2467-3p overexpression also prominently suppressed the migration and invasion abilities of NSCLC cells through analyzing the numbers of migrated and invaded NSCLC cells (Fig. 2e and g). Cell apoptosis rate was significantly elevated with the overexpression of miR-2467-3p in NSCLC cells (Fig. 2h). Given the opposite expression tendency of circ_0074027 and miR-2467-3p, we analyzed the linear correlation between circ_0074027 and miR-2467-3p. As displayed in Fig. 2i, circ_0074027 expression was negatively correlated with the expression of miR-2467-3p. Taken together, miR-2467-3p exhibited a tumor suppressor role to inhibit the progression of NSCLC in vitro.
MiR-2467-3p overexpression restrains the biological phenotypes of NSCLC cells. a The level of miR-2467-3p was determined in NSCLC tissues (n = 30), adjacent normal tissues (n = 30), 16HBE cell line and NSCLC cells lines by qRT-PCR. (B-H) A549 and H1299 cells were transfected with NC mimic or miR-2467-3p mimic. b qRT-PCR was conducted to detect the expression of miR-2467-3p in A549 and H1299 cells with the transfection of NC mimic or miR-2467-3p mimic. c Cell proliferation was examined by CCK8 assay. d The number of colonies after incubation for 2 weeks was analyzed by plate colony assay. e-g Transwell assays were performed to analyze the migration and invasion abilities of transfected NSCLC cells. h Cell apoptosis rate was assessed by flow cytometry after transfection for 72 h. i The linear relationship between the expression of miR-2467-3p and circ_0074027 was analyzed by Pearson correlation coefficient. **P < 0.01, ***P < 0.001
Circ_0074027/miR-2467-3p/RHOA axis is identified in NSCLC cells
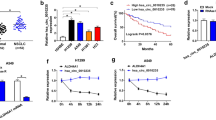
StarBase software predicted that circ_0074027 contained the binding sites with miR-2467-3p (Fig. 3a). Dual-luciferase reporter assay was conducted to investigate if miR-2467-3p interacted with circ_0074027. Prior to conduct dual-luciferase reporter assay, the transfection efficiencies of miR-2467-3p mimic and miR-2467-3p inhibitor were examined by qRT-PCR. As displayed in Fig. 3b, transfection with miR-2467-3p mimic enhanced the expression of miR-2467-3p, while miR-2467-3p expression was reduced after transfecting with miR-2467-3p inhibitor. MiR-2467-3p mimic or miR-2467-3p inhibitor transfection inhibited or promoted the luciferase activity in circ_0074027-wt group compared with their counterparts, respectively (Fig. 3c). Furthermore, luciferase activity was unchanged in circ_0074027-mut group with the co-transfection of miR-2467-3p mimic or miR-2467-3p inhibitor (Fig. 3c), suggesting the target interaction between circ_0074027 and miR-2467-3p in NSCLC cells. As displayed in Fig. 3d and e, both miR-2467-3p and circ_0074027 were enriched in Argonaute-2 (Ago2) group compared with Immunoglobulin G (IgG) group, suggesting that these two molecules were simultaneously existed in RNA-induced silencing complex (RISC) in NSCLC cells. qRT-PCR confirmed that the overexpression efficiency of circ_0074027 plasmid was high in NSCLC cells (Fig. 3f). Circ_0074027 interference up-regulated the expression of miR-2467-3p, while circ_0074027 overexpression notably restrained the expression of miR-2467-3p in NSCLC cells (Fig. 3g), suggesting the negative regulatory relation between circ_0074027 and miR-2467-3p in NSCLC cells. RHOA was predicted to bind to miR-2467-3p via TargetScan software, and the predicted binding sites were shown in Fig. 3h. MiR-2467-3p overexpression suppressed the luciferase activity in RHOA-wt group compared with NC mimic and RHOA-wt group, while miR-2467-3p interference elevated the luciferase activity in RHOA-wt group compared with NC inhibitor and RHOA-wt group (Fig. 3i), suggesting that RHOA was a target of miR-2467-3p in NSCLC cells. The results of RIP assay revealed that miR-2467-3p and RHOA were both pulled down in Ago2 group than that in IgG group, suggesting the interaction between miR-2467-3p and RHOA in NSCLC cells (Fig. 3j and k). The protein level of RHOA was reduced with the overexpression of miR-2467-3p, and miR-2467-3p silencing enhanced the expression of RHOA in NSCLC cells (Fig. 3l). Overall, the target interaction between miR-2467-3p and circ_0074027 or RHOA was identified in NSCLC cells.
Circ_0074027/miR-2467-3p/RHOA axis is identified in NSCLC cells. a The miR-2467-3p-binding sites in circ_0074027 were predicted by StarBase software. b The level of miR-2467-3p in A549 and H1299 cells transfected with NC mimic, miR-2467-3p mimic, NC inhibitor or miR-2467-3p inhibitor was examined via qRT-PCR. c Dual-luciferase reporter assay was conducted to detect the luciferase activities in A549 and H1299 cells transfected with NC mimic, miR-2467-3p mimic, NC inhibitor or miR-2467-3p inhibitor and luciferase plasmids to verify the interaction between circ_0074027 and miR-2467-3p. d and e RIP assay was performed to confirm the target interaction between circ_0074027 and miR-2467-3p. f The level of circ_0074027 in A549 and H1299 cells transfected with vector or vector-circ_0074027 was determined through conducting qRT-PCR. g The evaluation of miR-2467-3p level was performed in NSCLC cells transfected with si-NC, si-circ_0074027, vector or vector-circ_0074027 via qRT-PCR. h TargetScan software was used to predict the downstream interacted partners of miR-2467-3p, and the potential interaction between miR-2467-3p and RHOA was predicted. i Dual-luciferase reporter assay was performed to verify if RHOA interacted with miR-2467-3p via its 3’UTR sequence. j and k RIP assay was implemented for the verification of the target interaction between miR-2467-3p and RHOA. l Western blot assay was conducted to determine the protein level of RHOA in NSCLC cells transfected with NC mimic, miR-2467-3p mimic, NC inhibitor or miR-2467-3p inhibitor. **P < 0.01, ***P < 0.001
RHOA knockdown hampers the malignant properties of NSCLC cells
The protein expression of RHOA was conspicuously enhanced in NSCLC tumor tissues and cell lines in comparison with that in matched non-tumor tissues and 16HBE cell line (Fig. 4a). The interference efficiency of si-RHOA was high in NSCLC cells (Fig. 4b). RHOA interference suppressed the proliferation of NSCLC cells via CCK8 assay and plate colony assay (Fig. 4c and e). The migration and invasion abilities of NSCLC cells were blocked in si-RHOA group than that in si-NC group (Fig. 4f). Finally, cell apoptosis was evaluated by flow cytometry. RHOA silencing promoted cell apoptosis in both NSCLC cells (Fig. 4g). Taken together, RHOA silencing restrained the malignant behaviors of NSCLC cells.
RHOA knockdown hampers the malignant properties of NSCLC cells. a The protein expression of RHOA was examined in NSCLC tumor tissues and corresponding normal tissues by Western blot assay. Western blot assay was also conducted to measure the protein level of RHOA in 16HBE and two NSCLC cell lines (A549 and H1299). b-g A549 and H1299 cells were transfected with si-NC or si-RHOA. b The evaluation of RHOA protein abundance was carried out via Western blot assay. (C and D) CCK8 assay was conducted to obtain cell proliferation curve to analyze the proliferation ability in RHOA silencing-NSCLC cells and si-NC-transfected cells. e Plate colony assay was used to allow NSCLC cells transfected with si-NC or si-RHOA to form single-cell colonies to assess cell colony formation ability. f Transwell assays were conducted to analyze the abilities of migration and invasion in transfected NSCLC cells. g The apoptosis rate of transfected NSCLC cells was analyzed via flow cytometry. **P < 0.01, ***P < 0.001
Circ_0074027 interference suppresses cell proliferation, migration and invasion and promotes cell apoptosis through enhancing miR-2467-3p expression in NSCLC cells
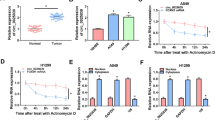
We introduced miR-2467-3p inhibitor into A549 and H1299 cells to rescue the expression of RHOA in circ_0074027-silenced NSCLC cells. As displayed in Fig. 5a, circ_0074027-silencing mediated down-regulation in RHOA expression was overturned by the addition of miR-2467-3p inhibitor in NSCLC cells. Si-circ_0074027-induced suppressive effect in cell proliferation was attenuated by the addition of miR-2467-3p inhibitor via CCK8 assay (Fig. 5b). With the interference of circ_0074027, the number of colonies was decreased, and cell colony formation ability was recovered in si-circ_0074027 and miR-2467-3p inhibitor co-transfected group (Fig. 5c). Cell migration ability and invasion ability were restrained by the silencing of circ_0074027, which were rescued with the addition of miR-2467-3p inhibitor (Fig. 5d). Circ_0074027 knockdown-mediated cell apoptosis was suppressed by the addition of miR-2467-3p inhibitor (Fig. 5e). These results suggested that circ_0074027 silencing-mediated effects were based on the up-regulation of miR-2467-3p in NSCLC cells.
Circ_0074027 interference suppresses cell proliferation, migration and invasion and promotes cell apoptosis through enhancing miR-2467-3p expression in NSCLC cells. a-e A549 and H1299 cells were transfected with si-NC, si-circ_0074027, si-circ_0074027 + NC inhibitor or si-circ_0074027 + miR-2467-3p inhibitor. a The relative level of RHOA was determined by Western blot assay. b Cell proliferation ability was assessed by CCK8 assay. c Cell colony formation ability was evaluated via plate colony assay. d The abilities of migration and invasion were assessed by transwell assays. e Flow cytometry was performed to assess cell apoptosis rate. *P < 0.05, **P < 0.01
Circ_0074027 knockdown suppresses NSCLC progression in vivo
To further confirm the role of circ_0074027 in NSCLC progression in vivo, we built xenograft tumor model using A549 cells stably expressing sh-circ_0074027 or sh-NC. Tumor volume and weight were reduced with the interference of circ_0074027 compared with that in sh-NC group (Fig. 6a and b). Circ_0074027 expression was significantly reduced in tumor tissues generated from A549 cells stably expressing sh-circ_0074027 (Fig. 6c). Besides, the expression of RHOA was prominently down-regulated in sh-circ_0074027 group than that in sh-NC group (Fig. 6e), which exhibited a similar tendency to circ_0074027. The level of miR-2467-3p was notably elevated with the knockdown of circ_0074027 compared with sh-NC group (Fig. 6d). Taken together, circ_0074027 silencing suppressed the growth of xenograft tumors in vivo.
Circ_0074027 knockdown suppresses NSCLC progression in vivo. a Tumor volume was recorded as width2 × length × 0.5 every week to generate tumor growth curve. b Tumors were weighed after 5-week inoculation. c and d The expression of circ_0074027 and miR-2467-3p was determined by qRT-PCR. e Western blot assay was implemented to measure the protein level of RHOA in two groups. **P < 0.01
Discussion
CircRNAs exert important roles in the initiation and development of various neoplasms, including NSCLC (Meng et al. 2017; Zhang et al. 2018). We found that circ_0074027 expression was higher in NSCLC tissues and NSCLC cell lines than that in adjacent non-tumor tissues and 16HBE cell line. The results of knockdown experiments revealed that circ_0074027 was found to play an oncogenic role in NSCLC to accelerate cell proliferation, migration, invasion and inhibit cell apoptosis. Through using bioinformatic softwares, miR-2467-3p/RHOA signal axis was found to locate in the downstream of circ_0074027 to regulate NSCLC progression.
CircRNAs were found to modulate the progression of many malignancies. For instance, circ_0078767 restrained NSCLC progression through elevating RASSF1A level via sequestering miR-330-3p (Chen et al. 2019). Circ_0091570 restrained the development of hepatocellular cancer through acting as miR-1307 sponge (Wang et al. 2019). Circ_0074027 was found to accelerate the proliferation and invasion of glioblastoma cells through miR-518a-5p/IL17RD axis (Qian et al. 2019). Furthermore, circ_0074027 was also identified to play a pro-tumor role in NSCLC by former articles (Gao et al. 2020; Yu et al. 2020). Gao et al.. demonstrated that circ_0074027 was overexpressed in NSCLC tissue samples and cell lines, and circ_0074027 accelerated the proliferation and invasion and hampered the apoptosis of NSCLC cells through miR-185-3p mediated BRD4/MADD activation (Gao et al. 2020). Yu et al.. claimed that circ_0074027 was highly expressed in NSCLC tissues and cell lines, and circ_0074027 accelerated NSCLC development via miR-335-5p/CUL4B axis (Yu et al. 2020). In line with former works, circ_0074027 expression was found to be significantly enhanced in NSCLC, and circ_0074027 interference suppressed the proliferation, migration and invasion and triggered the apoptosis of NSCLC cells.
Subsequently, we aimed to investigate how circ_0074027 exhibited an oncogenic role in NSCLC development. MiR-2467-3p was predicted to be a candidate target of circ_0074027 by StarBase database, and the interaction between circ_0074027 and miR-2467-3p was then confirmed via dual-luciferase reporter assay and RIP assay in NSCLC cells. Previous works have identified miR-2467-3p as a tumor suppressor in many malignancies, including colorectal cancer (Xiao and Liu, 2020), cervical cancer (Liu and Wen 2020) and NSCLC (Chen et al. 2020). For instance, circ_0053277 accelerated colorectal cancer development through sponging miR-2467-3p to enhance MMP14 expression (Xiao and Liu, 2020). Chen et al.. claimed that SNHG20 interference restrained NSCLC progression through miR-2467-3p/E2F3 signaling (Chen et al. 2020), which suggested that miR-2467-3p suppressed NSCLC development. We found that miR-2467-3p overexpression suppressed the proliferation and motility and induced the apoptosis of NSCLC cells, and the tumor suppressor role of miR-2467-3p was consistent with previous articles.
In addition, RHOA was confirmed as a downstream target of miR-2467-3p in NSCLC cells. RHOA has been verified to be targeted by many miRNAs (Mizoguchi et al. 2013; Yu et al. 2019b). For example, LINC00974 accelerated the migration and invasion of oral squamous cell carcinoma cells through enhancing RHOA expression via sponging miR-122 (Tian et al. 2021). Lu et al.. claimed that miR-133b facilitated neurite outgrowth through targeting RHOA in PC12 cells (Lu et al. 2015). RHOA interference hampered the proliferation, migration and invasion and promoted cell apoptosis in NSCLC cells.
Through transfecting si-circ_0074027 alone or together with miR-2467-3p inhibitor into NSCLC cells, we found that circ_0074027 knockdown down-regulated the expression of RHOA through up-regulating miR-2467-3p in NSCLC cells. This study is the first study to confirm the interaction between miR-2467-3p and circ_0074027 or RHOA in modulating NSCLC development. The results of rescue experiments uncovered that circ_0074027 silencing restrained the malignant phenotypes of NSCLC cells through up-regulating miR-2467-3p expression.
Given the pro-tumor role of circ_0074027 in NSCLC progression in vitro, A549 cell line stably expressing sh-circ_0074027 was built to investigate its role in vivo. Tumors generated from A549 cells stably expressing sh-circ_0074027 grew more slowly than that in sh-NC group, suggesting that circ_0074027 promoted NSCLC xenograft tumor growth in vivo.
In summary, circ_0074027 aggravated NSCLC progression through sponging miR-2467-3p to enhance the expression of RHOA. The identification of circ_0074027/miR-2467-3p/RHOA axis may provide novel underlying therapeutic target for NSCLC.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M et al (2017) First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 376(25):2415–2426
Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK (2014) Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14(8):535–546
Chen T, Yang Z, Liu C, Wang L, Yang J, Chen L et al (2019) Circ_0078767 suppresses non-small-cell lung cancer by protecting RASSF1A expression via sponging miR-330-3p. Cell Prolif 52(2):e12548
Chen H, Tan X, Ding Y (2020) Knockdown SNHG20 suppresses nonsmall cell lung cancer development by repressing proliferation, migration and invasion, and inducing apoptosis by regulating miR-2467-3p/E2F3. Cancer Biother Radiopharm. https://doi.org/10.1089/cbr.2019.3430
Dong Y, Xu T, Zhong S, Wang B, Zhang H, Wang X et al (2019) Circ_0076305 regulates cisplatin resistance of non-small cell lung cancer via positively modulating STAT3 by sponging miR-296-5p. Life Sci 239:116984
Gao P, Wang Z, Hu Z, Jiao X, Yao Y (2020) Circular RNA circ_0074027 indicates a poor prognosis for NSCLC patients and modulates cell proliferation, apoptosis, and invasion via miR-185-3p mediated BRD4/MADD activation. J Cell Biochem 121(3):2632–2642
Herbst RS, Morgensztern D, Boshoff C (2018) The biology and management of non-small cell lung cancer. Nature 553(7689):446–454
Liu F, Wen C (2020) LINC01410 knockdown suppresses cervical cancer growth and invasion via targeting miR-2467-3p/VOPP1 axis. Cancer Manag Res 12:855–861
Lu XC, Zheng JY, Tang LJ, Huang BS, Li K, Tao Y et al (2015) MiR-133b Promotes neurite outgrowth by targeting RhoA expression. Cell Physiol Biochem 35(1):246–258
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495(7441):333–338
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P et al (2017) CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer 16(1):94
Mizoguchi F, Murakami Y, Saito T, Miyasaka N, Kohsaka H (2013) miR-31 controls osteoclast formation and bone resorption by targeting RhoA. Arthritis Res Ther 15(5):R102
Patop IL, Wüst S, Kadener S (2019) Past, present, and future of circRNAs. EMBO J 38(16):e100836
Proto C, Ferrara R, Signorelli D, Lo Russo G, Galli G, Imbimbo M et al (2019) Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev 75:39–51
Qian L, Guan J, Wu Y, Wang Q (2019) Upregulated circular RNA circ_0074027 promotes glioblastoma cell growth and invasion by regulating miR-518a-5p/IL17RD signaling pathway. Biochem Biophys Res Commun 510(4):515–519
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Tian Y, Zhong L, Gao S, Yu Y, Sun D, Liu X et al (2021) LncRNA LINC00974 downregulates miR-122 to upregulate RhoA in oral squamous cell carcinoma. Cancer Biother Radiopharm 36(1):18–22
Wang J, Li H (2018) CircRNA circ_0067934 silencing inhibits the proliferation, migration and invasion of NSCLC cells and correlates with unfavorable prognosis in NSCLC. Eur Rev Med Pharmacol Sci 22(10):3053–3060
Wang YG, Wang T, Ding M, Xiang SH, Shi M, Zhai B (2019) hsa_circ_0091570 acts as a ceRNA to suppress hepatocellular cancer progression by sponging hsa-miR-1307. Cancer Lett 460:128–138
Watanabe SI, Nakagawa K, Suzuki K, Takamochi K, Ito H, Okami J et al (2017) Neoadjuvant and adjuvant therapy for Stage III non-small cell lung cancer. Jpn J Clin Oncol 47(12):1112–1118
Wu Y, Shen QW, Niu YX, Chen XY, Liu HW, Shen XY (2020) LncNORAD interference inhibits tumor growth and lung cancer cell proliferation, invasion and migration by down-regulating CXCR4 to suppress RhoA/ROCK signaling pathway. Eur Rev Med Pharmacol Sci 24(10):5446–5455
Xiao H, Liu M (2020) Circular RNA hsa_circ_0053277 promotes the development of colorectal cancer by upregulating matrix metallopeptidase 14 via miR-2467-3p sequestration. J Cell Physiol 235(3):2881–2890
Xiao J, Lai H, Wei SH, Ye ZS, Gong FS, Chen LC (2019) lncRNA HOTAIR promotes gastric cancer proliferation and metastasis via targeting miR-126 to active CXCR4 and RhoA signaling pathway. Cancer Med 8(15):6768–6779
Yu G, Wang Z, Zeng S, Liu S, Zhu C, Xu R et al (2019a) Paeoniflorin inhibits Hepatocyte Growth Factor- (HGF-) induced migration and invasion and actin rearrangement via suppression of c-met-mediated RhoA/ROCK signaling in glioblastoma. Biomed Res Int 2019:9053295
Yu X, Wang D, Wang X, Sun S, Zhang Y, Wang S et al (2019b) CXCL12/CXCR4 promotes inflammation-driven colorectal cancer progression through activation of RhoA signaling by sponging miR-133a-3p. J Exp Clin Cancer Res 38(1):32
Yu C, Ying J, Yu K, Shen W, Jiang M (2020) Circ_0074027 contributes to nonsmall cell lung cancer progression by upregulating CUL4B expression through miR-335-5p. Cancer Biother Radiopharm. https://doi.org/10.1089/cbr.2020.3579
Zhang HD, Jiang LH, Sun DW, Hou JC, Ji ZL (2018) CircRNA: a novel type of biomarker for cancer. Breast Cancer 25(1):1–7
Author information
Authors and Affiliations
Contributions
Ying Liu designed and supervised the study, Zhihui Duan conducted the experiments and drafted the manuscript. Shuqing Wei involved in methodology development and analyzed the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Clinical study was approved by the Ethics Committee of Shanxi Provincial Cancer Hospital and was carried out according to the guidelines of Declaration of Helsinki. Animal experiment was permitted by the Animal Care and Use Committee of Shanxi Provincial Cancer Hospital and performed in accordance with the guidelines of the National Animal Care and Ethics Institution.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Fig. 1
Circ_0074027 knockdown suppresses the EMT of NSCLC cells. (A and B) Western blot assay was employed to determine the expression of three EMT-associated markers (E-cadherin, N-cadherin and Vimentin) in A549 and H1299 cells transfected with si-NC or si-circ_0074027. **P < 0.01
Rights and permissions
About this article
Cite this article
Duan, Z., Wei, S. & Liu, Y. Circ_0074027 contributes to non‐small cell lung cancer progression through positively modulating RHOA via sequestering miR-2467-3p. J Bioenerg Biomembr 53, 223–233 (2021). https://doi.org/10.1007/s10863-021-09876-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-021-09876-6