Abstract
A change in the intracellular free Ca2+ concentration ([Ca2+]i) functions as a transmitter for signal transduction and shows a broad temporal pattern. Even genetically homogeneous cell types show different Ca2+ response patterns under permanent agonist stimulation. In Ca2+ signaling, the dynamics of the Ca2+ release from the Ca2+ channels during continuous agonist stimulation and the simultaneous effect of the pumps are unclear. In this study, the dynamic interaction of the Ca2+ ATPases in the plasma membrane (PMCA) and the endoplasmic reticulum membrane (SERCA) during continuous ACh stimulation is monitored using Fluo-3 and Fura-2 loaded HEK 293 cells. We characterize Ca2+ release patterns at the sub-maximal and maximal stimulation doses in the absence of extracellular Ca2+. We analyze the responses regarding their types, oscillation frequency and response times. La3+ (PMCA blocker) do not change the frequency and time courses in sub-maximal ACh treatment, while with the maximal stimulation oscillation frequency increase as oscillations superimpose on robust release, and response time of [Ca2+]i is elongated. A similar effect of La3+ is observed in quantal Ca2+ release phenomenon. In the presence of CPA, a SERCA blocker, oscillations are completely abolished, but response time does not change. We also observe that during continuous receptor stimulation, Ca2+ release do not cease. These data may suggest that Ca2+ release continues during agonist stimulation, but SERCA and PMCA form a new steady state and return [Ca2+]i to its physiological concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The generation of Inositol (1,4,5)-Trisphosphate (IP3) in response to agonist stimulation activates its own receptor channel (IP3R) on Endoplasmic Reticulum (ER) membrane. With this activation, IP3R mediates the release of stored Ca2+ from ER into the cytoplasm (Berridge and Irvine 1984; Berridge and Irvine 1989). The increase of free cytosolic Ca2+ concentration ([Ca2+]i) acts as a second messenger that regulates many cellular processes like cell proliferation, muscle contraction and the electrical activities of the cell (Bootman and Berridge 1995).
Increase in [Ca2+]i exhibits many different spatial and temporal dynamics. Spatially, rise of [Ca2+]i may be global (Ca2+ waves) or restricted to a local part of the cell (Ca2+ sparks), and temporally, increase of [Ca2+]i could be observed as a single rise or have an oscillatory pattern (Bootman and Berridge 1995; Shuttleworth and Thompson 1996; Falcke 2004). Cells may use these different Ca2+ signaling dynamics to encode information arriving at the cell surface towards cytoplasmic targets (Falcke 2004). Depending on the cell type and the strength of the agonist stimulation, oscillations may show differences in their amplitudes or frequencies (Marchant et al. 1999; Rooney et al. 1989; Berridge et al. 2003).
Even in a single cell type, different oscillation patterns to the same agonist concentration can be observed. A possible mechanism for this variation may be the immense dependence of IP3R open probability to both [Ca2+]i and [IP3]. Released Ca2+ increase open probability of the adjacent IP3R channels, whereby Ca2+ induces its own release from intracellular stores, known as Ca2+ induced Ca2+ release (CICR) (Salazar et al. 2008; Skupin and Falcke 2007; Berridge 2002). Also, IP3R has three subtypes, and Type 3 IP3R shows inhibition with the high concentrations of Ca2+ (Tu et al. 2005; Hagar et al. 1998).
Two Ca2+-ATPases, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and Plasma Membrane Ca2+-ATPase (PMCA) also have major effects on the [Ca2+]i dynamics (Falcke 2004; Solovyova et al. 2002; Verkhratsky 2005; Petersen et al. 1993). They preserve [Ca2+]i within physiological limits. SERCA uptakes cytosolic Ca2+ to intracellular stores and PMCA extrude Ca2+ across the plasma membrane. Different expression levels of these proteins may also contribute to the diversity (Camacho and Lechleiter 1993).
In the present study, we monitored different temporal Ca2+ release patterns towards equivalent doses of Acetylcholine (Ach) in Fluo-3 and Fura-2 loaded HEK 293 cells expressing endogenous muscarinic receptors. Besides, these [Ca2+] responses are affected variously with the inhibitions of PMCA and SERCA activity by lanthanum and cyclopiazonic acid (CPA), respectively.
We classified Ca2+ release patterns after the sub-maximal and maximal doses of ACh application. We grouped Ca2+ responses by their types (qualitatively), oscillation frequency (number of peaks) and response time (a period of time which start with the increase in the [Ca2+]i after a perturbation and finish when the [Ca2+]i returned to its baseline level).
We analyzed the effects of the perturbations according to these classifications. We demonstrated that in sub-maximal stimulations SERCA is the major component for the generation of the Ca2+ oscillations, shaping the temporal patterns. Besides, with the maximal stimulation PMCA activity dominates the release, suggesting that its activity depends on a high [Ca2+]i threshold. In this study we observed that during agonist stimulation, despite the [Ca2+]i. returns to its base levels, the release from IP3R continues and mainly SERCA and PMCA activities maintain the physiological concentrations of cytoplasm.
Materials and methods
Cell culture
HEK-293 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10 % fetal bovine serum, penicillin (100 IU/ml) and streptomycin (100 μg/ml) in a 5 %CO2 incubator at 37 °C, to about 90 % confluence in tissue culture flasks. They were suspended by gently tapping after incubation with 1 mM EDTA in PBS at 37 °C for 5 min (No trypsin was used). These cells were transferred on glass cover slips in 6-well plates and allowed to attach overnight.
Intracellular calcium measurements
Fluorescent measurements were done on HEK293 cells loaded with Fluo-3 AM or Fura-2 AM (Invitrogen, USA). Cover slips with attached cells were mounted in a Teflon chamber and before Ca2+ measurements cells were washed twice with bathing solution containing 120 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM MgCl2, and 1.5 mM Ca2+ (pH 7.4). All the measurements were done with nominally Ca2+ free bathing solution (Putney 2006; Utz et al. 1999).
Experiments with the Fluo-3 AM loaded cells were carried on confocal microscope (Leica, TCS SP5, Germany) equipped with a 63× water immersion objective. Fluo-3 was excited with Argon laser at 488 nm and fluorescence emission was collected around 530 nm wavelengths. Fluorescence signals from the whole cell field was selected as Region of Interest (ROI) for each separate cell and they were analyzed with software of Leica Imaging System. Approximately 40–50 cells were analyzed per experiment. For data evaluation 4–5 experiments were assessed for each group. To minimize background fluorescence ROIs were assigned from blank and their values were subtracted from the cell fluorescence. Loading differences between cells were excluded by dividing entire record received during the time of the record to beginning record (F/F0).
For the ratiometric fluorescent measurements Fura-2 AM loaded cells were placed on an inverted epifluorescent microscope (Nikon, Japan) plate, and total fluorescence signal from the focal area was collected and analyzed with a fluorescence system and its software (Photon Technology International, USA). Cells were sequentially excited at 340 and 380 nm. Total emission signal from the 40–50 cells in the objective field was collected around 510 nm. The ratio of the emitted fluorescence at 340 and 380 nm (F340/380) was used as an indicator of [Ca2+]i.
Chemicals
Unless specified, the chemicals used were obtained from SIGMA-Aldrich Canada. During the experiments, all the drugs were applied gently into the nominally Ca2+ free bathing solution via micro-pipette. To visualize the effect of the withdrawal of the ACh, experiment solution was perfused without changing other chemical concentrations.
Statistics
Means of all results were given with SEM. Levels of significance of the difference between means were determined using unpaired Student t test with two tailed P value or one way ANOVA as indicated. Significance was defined at the P < 0.05 level.
Results
Release patterns for maximal and Sub-maximal doses of ACh in HEK 293 cells
In HEK 293 cells IP3 induced Ca2+ release is achieved by stimulating the mACh receptor by its own agonist ACh. When cells in whole objective field were treated with 1 μM ACh or 100 μM ACh in nominal Ca2+ free bathing solution, cells showed huge differences in Ca2+ responses as long lasting robust release or transients that vary between single and multiple peaks. In Fig. 1a some selected basic release types were shown for 1 μM ACh (up) and 100 μM ACh (down). In single cell level, diversity of these different temporal patterns becomes much more apparent. Figure 1b shows some response patterns for 100 μM ACh from single cells. One of the aims of this study is to identify these response patterns for sub-maximal and maximal doses of ACh in HEK 293 cells. Therefore we grouped response patterns according to the shape of the peaks as shallow (during robust response) (S) or deep (D). Shallow responses refer to releases with no oscillations or some which do not significantly affect the tendency of monotonic decrease of [Ca2+]i. Deep responses refer to short timed, individual oscillations (approximately 10 s) and the releases that have peaks that disrupt the tendency of monotonic decrease of [Ca2+]i.
Different temporal patterns of [Ca2+]i for the ACh stimulations in HEK 293 cells. a Two different representative responses were shown for 1 μM ACh (up) and 100 μM ACh (down). b Some response patterns for 100 μM ACh recorded from single cells. S represents shallow type responses (response with no oscillations or some which do not significantly affect the tendency of monotonic decrease of [Ca2+]i). D represents deep responses (short timed, individual peaks (approximately 10 s) or the responses that have peaks which disrupt the tendency of monotonic decrease of [Ca2+]i)
Effect of PMCA inhibition on Ca2+ dynamics
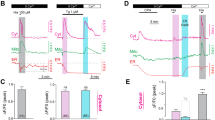
In order to examine the efflux kinetic of [Ca2+]i from plasma membrane, 1 mM (La3+) was used. When used in these concentrations, La3+ blocks the Ca2+ efflux from cells by inhibiting PMCA and cation channels (Van Breemen et al. 1972). La3+ application alone (1 mM) has not created a significant effect (Fig. 2). In Fig. 2, representative data for 1 μM ACh and 100 μM ACh without La3+ and with La3+ is shown. The effect of this perturbation to the agonist induced cytosolic Ca2+ oscillations was examined by comparing their number of peaks, distribution of these oscillations according to their types (D or S type), and release times in Fig. 2b, c and d, respectively. Peaks in each cell were counted and grouped after the perturbations in Fig. 2b. La3+ application had no effect on the distribution of the peak numbers in sub-maximal dose, but with maximal dose of Ach, La3+ increased the number of peaks significantly. However, as shown in Fig. 2a, (right-down) this increase occurred as shallow oscillations, which overlapped on sustained release (an increase to 5 and more peaks).
Effect of PMCA inhibition on [Ca2+]i oscillations and classification of Ca2+ oscillations. a La3+ application alone (1 mM) did not create a significant effect (up). Middle, representative data for 1 μM ACh (left) and 100 μM ACh applications (right), Bottom, successive application of 1 mM La3+ and 1 μM ACh (left) and 1 mM La3+ and 100 μM ACh (right). La3+ with 100 μM ACh significantly elongated the response time and brought an oscillatory pattern to the robust release. b Peaks in each cell were counted and grouped according to the perturbations. c The effect of 1 mM La3+ on the 1 μM ACh and 100 μM ACh induced response types (shallow (S) or deep (D) type responses). *P < 0.05 vs. 1 μM ACh S type responses and # P < 0.05 vs. 1 μM ACh D type responses. (ANOVA) d The effect of 1 mM La3+ on the 1 μM ACh and 100 μM ACh induced Ca2+ response time. *P < 0.05 vs. 1 μM ACh (1 mM La3+) group (ANOVA)
In the presence or absence of 1 mM La3+, 1 μM ACh and 100 μM ACh responses were compared according to the percentage of response types (D or S type) within their groups in Fig. 2c. La3+ had no significant effect on distribution of 1 μM ACh or 100 μM ACh response types. However, 1 μM ACh responses have a slight tendency to shift from D to S.
Effect of 1 mM La3+ application on the 1 μM ACh and 100 μM ACh induced Ca2+ release was investigated in terms of duration in Fig. 2d. La3+ application led to a significant prolongation of Ca2+ release for 100 μM ACh in terms of the release time. However, it did not create a significant change in 1 μM ACh.
Effect of SERCA inhibition on [Ca2+]i oscillations
In order to examine the effect of the SERCA activity on basal [Ca2+]i, SERCA was inhibited by 20 μM CPA. At the indicated time the blocker was added to bath solution, and subsequently [Ca2+]i increased slowly for a while and then gently returned to the baseline (Fig. 3a up).
Effect of SERCA inhibition on [Ca2+]i response patterns. a Up, a representative trace for SERCA inhibition with 20 μM CPA application on basal [Ca2+]i. Middle and down, effect of 20 μM CPA on 1 μM ACh and 100 μM ACh responses, respectively. b Presence of 20 μM CPA did not change the response time of the 1 μM and 100 μM ACh induced Ca2+ increase. However, CPA application alone gradually increased [Ca2+]i and Ca2+ was slowly removed from cytoplasm. *P < 0.05 vs. 20 μM CPA group (ANOVA)
In Fig. 3 middle and down, representative traces show the effect of SERCA blockage to the 1 μM ACh and 100 μM ACh responses, respectively. Before the agonist application, 20 μM CPA was added to bath solution. SERCA blockage caused a total disappearance of the oscillations for both doses of ACh.
The effect of CPA on the agonist induced Ca2+ release was also investigated in terms of duration (Fig. 3b). SERCA blockage did not effect the ACh induced response time. However, CPA application alone increased [Ca2+] gradually and Ca2+ was removed from the cytoplasm in a long term.
The Ca2+ influx continues during the ACh stimulation
Fura 2 AM loaded HEK 293 cells were monitored with a micro-spectrofluorimeter and total fluorescence signal from 40–50 cells in the objective field were analyzed. In Fig. 4a, in order to observe how the SERCA and PMCA affect the Ca2+ dynamics together, 1 mM La3+ and 20 μM CPA were simultaneously applied onto the cells. Without ACh, [Ca2+]i tends to increase slowly. Right after the ACh application, [Ca2+]i increases rapidly and decays slowly.
Co-inhibition of SERCA and PMCA with ACh Stimulation. a Left, SERCA and PMCA co-inhibited with 1 μM La3+ and 20 μM CPA during the indicated time. Right, during the blockage of the both pumps 100 μM ACh was applied at the 60 s. b Left, 20 μM CPA at 30 s and 100 μM ACh at 60 s was applied on the cells. During decrease period of [Ca2+]i 1 mM La was added. Right, at 140 s ACh was removed via perfusion from the bath media while both SERCA and PMCA were inhibited. Measurements were performed by Fura-2 AM with a micro-spectrofluorimeter and fluorescence signals of average 40-50 cells were collected. Dashed lines were drawn for comparison with the base-line
To understand whether the release ceases during the agonist stimulation, we applied La3+ onto the cells right after CPA and ACh application (Fig. 4b left) and observed [Ca2+]i increase after the PMCA blockage.
One point must be specified here is that before the ACh stimulation, [Ca2+]i increase in response to CPA as in Fig. 3a up (confocal microscope data) was not observed in this experiment. Collective data acquisition systems do not enable distinguishing only the responsive cells. A total signal received reflects an average response and the contribution of the unresponsive cell signals will cancel out relatively lower amplitudes as in Ca2+ responses to CPA.
In another experiment (Fig. 4b right) we applied both inhibitors and ACh successively on the cells, and during a relatively stable phase of [Ca2+]i, ACh was removed via perfusion from the bath media. We observed that withdrawal of ACh in the presence of both Ca2+ pump inhibitors decreased [Ca2+]i rapidly. In order to control the effect of perfusion, the group with continuous ACh stimulation, was also perfused without changing the concentration of the chemicals (Fig. 4a right). This experimental result indicates that during the ACh stimulation, Ca2+ release from the stores to the cytoplasm continues, despite the [Ca2+]i reach a new steady state.
PMCA activity in quantal Ca2+ release
Quantal Ca2+ release experiments were performed to explore whether the PMCA activity alters the Ca2+ concentration in ER. As shown in Fig.4b right, decrease of the Ca2+ in the cytoplasm after withdrawal of ACh shows that Ca2+ release from the Ca2+ stores continues during the agonist stimulation. Although efflux is blocked by La3+ and release continues during the ACh stimulation, [Ca2+]i still reach to a new steady state. To explore whether this change in the Ca2+ store capacity of ER affects the PMCA activity, we applied 100 μM ACh after 1 μM ACh pretreatment during 1 mM La3+ application (Fig. 5a). When we compare quantal forms of the 100 μM ACh responses with (w) and without (wo) La3+, the response times were significantly different (Means of the response times: w: 207.9 s ± 3 and wo: 118.6 s ± 4) (Fig. 5b) Although response time was significantly decreased compared to the La3+ application with the 100 μM ACh, still the quantal 100 μM Ach has a greater response time (207.9 s ± 3) to 100 μM ACh alone (148.2 s ± 19) (Fig. 5c).
Effect of Ca2+ store capacity on the PMCA activity. a Effect of La3+ to the maximal dose ACh stimulation in the quantal Ca2+ release phenomenon. b La3+ elongated Ca2+ response times to the maximal agonist stimulation in quantal form also (Student t test) c The all three groups of Ca2+ responses to the 100 μM ACh stimulations were compared regarding their response times (Averages of ≈ 100 cells from the same day experiments). *P < 0.05 vs. 100 μM ACh + La3+ group (ANOVA)
Discussion
In this study, we tried to understand possible reasons of different temporal dynamics of Ca2+ responses after the agonist stimulation from a perspective of the variables as Ca2+ influx, efflux and Ca2+ release. The effects of mutual activities of SERCA and PMCA on Ca2+ dynamics in HEK 293 cells during release were inspected. HEK 293 cells show quite many different Ca2+ release patterns even for the same agonist concentration. Therefore, in order to understand and generalize the properties of Ca2+ release, first we examined the temporal behaviors of Ca2+ responses in cytoplasm for sub-maximal and maximal concentrations of ACh.
Release patterns were grouped according to the oscillation types as shallow responses (S) that refer to releases with no peaks or some which do not significantly affect the tendency of monotonic decrease of [Ca2+]i, or deep responses (D), referring to short timed, individual peaks (approximately 10s) and the releases that have peaks which disrupt the tendency of monotonic decrease of [Ca2+]i.
According to this classification, the distribution of responses to 1 μM and 100 μM ACh were determined. Although it is argued in the literature that in different cell types oscillatory pattern of the Ca2+ release will reduce in maximal stimulus (Berridge 1990; Jacob 1990; Thomas et al. 1996), we clarified the difference: In higher dose, generally during the robust release, shallow oscillations (approximately 2 peaks) were monitored, while in sub-maximal dose, the majority of the cells had individual peaks which showed a wide distribution with an increasing trend towards multiple number of peaks.
According to this classification, the effects of the perturbations on the Ca2+ release were identified. In our experiments, contrary to literature (Bird and Putney 2005), in the presence of 1 mM La3+, sub-maximal dose ACh stimulation did not increase the oscillation frequency. However, in maximal dose ACh stimulation, 1 mM La3+ increased the shallow oscillation frequency on the robust release. The removal time of Ca2+ for 1 μM ACh and 100 μM ACh were measured as nearly equivalent. This equivalence was altered with the PMCA inhibition. During 1 μM ACh stimulation, ceasing Ca2+ efflux with 1 mM La3+ did not change the removal time. However, 100 μM ACh with 1 mM La3+ prolonged the removal of the Ca2+ from the cytoplasm. This prolongation may be related with the differences in the Ca2+ affinity of the two pumps, SERCA and PMCA (Brini and Carafoli 2009; Hove-Madsen and Bers 1993).
Meanwhile, the PMCA blockage without ACh application did not change base level of [Ca2+]i, which indicates that PMCA may not have an active role in resting cell. Approximately ten fold decrease of the pump Kd for Ca2+ during the activation of the pump (from 10 μM to less than 1 μM) (Brini and Carafoli 2009) also supports this hypothesis.
In the resting cell, [Ca2+]i was previously measured in literature to be between 0.03 to 0.1 μM (Mak et al. 1998; Bezprozvanny and Ehrlich 1995; Golovina and Blaustein 1997; Shmigol et al. 1996). If the PMCA activation needs elevated [Ca2+]i, final concentration reached after ACh concentration might have a critical importance. In our experiments we did not observe dramatic effects of “Lanthanide insulation technique (Dupont et al. 2011)” for 1 μM ACh, which may cause sustained oscillations in some non-excitable cells. This difference may be explained with the efficacy differences of the agonists for the threshold [Ca2+]i.
In this study, we also examined the effect of SERCA on Ca2+ oscillations. In line with literature, our experiments demonstrated that basal [Ca2+]i remained stable in the absence of any perturbation, but the application of SERCA inhibitor leads to a gradual increase in [Ca2+]i (Solovyova et al. 2002). This implies a steady state that resulted from the ER Ca2+ leak and SERCA uptake at the basal condition (Verkhratsky 2005). Furthermore, application of SERCA inhibitor with ACh led to higher amplitudes but shorter durations of [Ca2+]i in our experiments. The reason of this rapid decline after the ACh stimulation may arise from PMCA activation, which occurs when [Ca2+]i exceeds the threshold value.
We also detected that in the presence of 20 μM CPA, ACh induced Ca2+ oscillations were completely abolished. These data emphasize that the oscillatory pattern of the Ca2+ release depends on a critical balance between Ca2+ release by IP3R and Ca2+ uptake by SERCA.
To understand the mutual interaction of SERCA and PMCA, they were inhibited by the inhibitors CPA and La3+ before the agonist stimulation during the same experiment. When these two inhibitors were in the bath solution, ACh led to a higher amplitude and longer duration of [Ca2+]i increase. During the perturbations, removal of the ACh from bath solution rapidly ceased the Ca2+ release. These data indicate the continuation of the Ca2+ release from the Ca2+ stores during the agonist stimulation. We also examined the interaction between PMCA and the store capacity. La3+ application with the successive 1 μM ACh and 100 μM ACh stimulations led to a decrease in Ca2+ response time for 100 μM ACh when compared with the effect of only 100 μM ACh with La3+. This alteration could be explained by a change in the activity on PMCA, or less Ca2+ content. It was previously shown that PMCA overexpression caused a reduction in store Ca2+ concentration (Brini et al. 2000). Therefore, there is a significant interaction between PMCA and Ca2+ stores, which could be explained as a reduction in Ca2+ content and therefore less Ca2+. PMCA is observed to be responsible for extrusion of huge amounts of the Calcium from the cytoplasm and has a significant role in Ca2+ homeostasis.
One important question here is about the percentage of the pumps blocked. In the experiments after blocking SERCA and PMCA together with the ACh stimulation, we observed that [Ca2+]i was stable for a certain time. If the pumps were 100 % blocked, this observation may suggest cease of the release from IP3 receptors. However, washing the ACh without changing inhibitor concentrations caused a decrease in [Ca2+]i. These data clearly show that Ca2+ entry to cytoplasm from IP3 receptors and removal from cytoplasm were in a steady state in this condition. It was previously demonstrated in literature that 1 mM La3+ is adequate for inhibiting Ca2+ extrusion at the plasma membrane (Van Breemen et al. 1972). However, 20 μM CPA may not maximally inhibit SERCA, or the other Ca2+ re-uptake mechanisms like mitochondria might be taken into consideration (Halestrap 2009).
In conclusion, the present data show that a PMCA blockage alone or with the low dose ACh stimulation did not make a dramatic effect on temporal [Ca2+]i dynamics in HEK293 cells. The level of [Ca2+]i after the receptor stimulation is critically important for the PMCA activation. During the agonist stimulation Ca2+ release from the stores continues, and in sub-maximal stimulations SERCA may shape the oscillations in this continuous release. With an increased degree of stimulation, mostly PMCA activity shapes the response pattern and preserves the Ca2+ homeostasis.
References
Berridge MJ, Irvine RF (1984) Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nat 312(5992):315–321
Berridge MJ, Irvine RF (1989) Inositol phosphates and cell signalling. Nat 341(6239):197–205. doi:10.1038/341197a0
Bootman MD, Berridge MJ (1995) The elemental principles of calcium signaling. Cell 83(5):675–678
Shuttleworth TJ, Thompson JL (1996) Ca2+ entry modulates oscillation frequency by triggering Ca2+ release. Biochem J 313(Pt 3):815–819
Falcke M (2004) Reading the patterns in living cells - the physics of Ca2+ signaling. Adv Phys 53(3):255–440. doi:10.1080/00018730410001703159
Marchant J, Callamaras N, Parker I (1999) Initiation of IP (3)-mediated Ca (2+) waves in Xenopus oocytes. EMBO J 18(19):5285–5299. doi:10.1093/emboj/18.19.5285
Rooney TA, Sass EJ, Thomas AP (1989) Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem 264(29):17131–17141
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4(7):517–529. doi:10.1038/nrm1155
Salazar C, Politi AZ, Hofer T (2008) Decoding of calcium oscillations by phosphorylation cycles: analytic results. Biophys J 94(4):1203–1215. doi:10.1529/biophysj.107.113084
Skupin A, Falcke M (2007) Statistical properties and information content of calcium oscillations. Genome Inf 18:44–53
Berridge MJ (2002) The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32(5-6):235–249
Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I (2005) Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys J 88(2):1046–1055
Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE (1998) Type III InsP3 receptor channel stays open in the presence of increased calcium. Nat 396(6706):81–84. doi:10.1038/23954
Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A (2002) Ca (2+) dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca (2+)-induced Ca (2+) release triggered by physiological Ca (2+) entry. EMBO J 21(4):622–630
Verkhratsky A (2005) Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85(1):201–279. doi:10.1152/physrev.00004.2004
Petersen CC, Petersen OH, Berridge MJ (1993) The role of endoplasmic reticulum calcium pumps during cytosolic calcium spiking in pancreatic acinar cells. J Biol Chem 268(30):22262–22264
Camacho P, Lechleiter JD (1993) Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science 260(5105):226–229
Putney JW (2006) Calcium signaling (2nd ed., Methods in signal transduction). CRC/Taylor & Francis, Boca Raton
Utz J, Eckert R, Trautwein W (1999) Changes of intracellular calcium concentrations by phenylephrine in renal arterial smooth muscle cells. Pflugers Arch 438(6):725–731
Van Breemen C, Farinas BR, Gerba P, McNaughton ED (1972) Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res 30(1):44–54
Berridge MJ (1990) Calcium oscillations. J Biol Chem 265(17):9583–9586
Jacob R (1990) Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol 421:55–77
Thomas AP, Bird GS, Hajnoczky G, Robb-Gaspers LD, Putney JW Jr (1996) Spatial and temporal aspects of cellular calcium signaling. FASEB J 10(13):1505–1517
Bird GS, Putney JW Jr (2005) Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J Physiol 562(Pt 3):697–706. doi:10.1113/jphysiol.2004.077289
Brini M, Carafoli E (2009) Calcium pumps in health and disease. Physiol Rev 89(4):1341–1378. doi:10.1152/physrev.00032.2008
Hove-Madsen L, Bers DM (1993) Sarcoplasmic reticulum Ca2+ uptake and thapsigargin sensitivity in permeabilized rabbit and rat ventricular myocytes. Circ Res 73(5):820–828
Mak DO, McBride S, Foskett JK (1998) Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol trisphosphate [correction of tris-phosphate] receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci U S A 95(26):15821–15825
Bezprozvanny I, Ehrlich BE (1995) The inositol 1,4,5-trisphosphate (InsP3) receptor. J Membr Biol 145(3):205–216
Golovina VA, Blaustein MP (1997) Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science 275(5306):1643–1648
Shmigol A, Svichar N, Kostyuk P, Verkhratsky A (1996) Gradual caffeine-induced Ca2+ release in mouse dorsal root ganglion neurons is controlled by cytoplasmic and luminal Ca2+. Neuroscience 73(4):1061–1067
Dupont, G., Combettes, L., Bird, G. S., & Putney, J. W. (2011). Calcium oscillations. Cold Spring Harb Perspect Biol, 3(3), doi:10.1101/cshperspect.a004226a004226
Brini M, Bano D, Manni S, Rizzuto R, Carafoli E (2000) Effects of PMCA and SERCA pump overexpression on the kinetics of cell Ca (2+) signalling. EMBO J 19(18):4926–4935. doi:10.1093/emboj/19.18.4926
Halestrap AP (2009) Mitochondrial calcium in health and disease. Biochim Biophys Acta 1787(11):1289–1290. doi:10.1016/j.bbabio.2009.07.011S0005-2728(09)00237-0
Conflicts of interest
No potential conflicts of interest relevant to this article have been reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cicek, F.A., Ozgur, E.O., Ozgur, E. et al. The interplay between plasma membrane and endoplasmic reticulum Ca2+ATPases in agonist-induced temporal Ca2+ dynamics. J Bioenerg Biomembr 46, 503–510 (2014). https://doi.org/10.1007/s10863-014-9587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-014-9587-3