Abstract
It has recently been demonstrated that accurate near surface electrostatic potentials can be calculated for proteins from solvent paramagnetic relaxation enhancements (PREs) of amide protons measured using spin labels of similar structures but different charges (Yu et al. in Proc Natl Acad Sci 118(25):e2104020118, 2021). Here we develop methodology for extending such measurements to intrinsically disordered proteins at neutral pH where amide spectra are of very poor quality. Under these conditions it is shown that accurate PRE values can be measured using the haCONHA experiment that has been modified for recording 1Hα transverse relaxation rates. The optimal pulse scheme includes a spin-lock relaxation element for suppression of homonuclear scalar coupled evolution for all 1Hα protons, except those derived from Ser and Thr residues, and minimizes the radiation damping field from water magnetization that would otherwise increase measured relaxation rates. The robustness of the experiment is verified by developing a second approach using a band selective adiabatic decoupling scheme for suppression of scalar coupling modulations during 1Hα relaxation and showing that the measured PRE values from the two methods are in excellent agreement. The near surface electrostatic potential of a 103-residue construct comprising the C-terminal intrinsically disordered region of the RNA-binding protein CAPRIN1 is obtained at pH 5.5 using both 1HN and 1Hα-based relaxation rates, and at pH 7.4 where only 1Hα rates can be quantified, with very good agreement between potentials obtained under all experimental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
NMR spectroscopy is an extremely powerful technique for quantifying site-specific molecular dynamics (Mittermaier and Kay 2006; Palmer 2014; Anthis and Clore 2015). Most frequently this is accomplished through the measurement of heteronuclear (15N, 13C, 2H, 31P, 19F) spin relaxation rates that can then be recast in terms of motional parameters in the context of a preferred model of dynamics (Lipari and Szabo 1982a, b). In this regard, the use of heteronuclear spins as probes of motion, as opposed to measurements involving 1H spins, offers several advantages. Importantly, it is often the case that the relaxation of heteronuclei can be quantitatively analyzed in terms of a small number of well-defined interactions, greatly simplifying data analysis. An example is the popular series of 15N R1, R2, and heteronuclear NOE experiments where a relatively simple two-spin 15N–1HN spin system is sufficient to describe the experiments (Kay et al. 1989). The situation is more complex for 13C, as coupled relaxation between spin interactions, such as 13C–1H dipolar pairs in methylene and methyl groups (Vold and Vold 1976; Werbelow and Grant 1977), complicates the analysis (Kay and Torchia 1991), as does scalar coupling and relaxation between proximal 13C spins in uniformly 13C labeled samples (Yamazaki et al. 1994). The development of labeling schemes involving the placement of isolated 13C spins in the system of interest (Goto et al. 1999; Kainosho et al. 2006; Teilum et al. 2006; Lundström et al. 2007; Kasinath et al. 2013), in concert with the substitution of protons with deuterons (Ishima et al. 1999; Tugarinov and Kay 2005), can simplify the spin system so that it is well approximated as a two-spin 13C–1H pair. In some cases experiments are relatively benign to the effects of scalar couplings that manifest in uniformly 13C labeled molecules, such as carbon-13 CEST (Vallurupalli et al. 2013), for example, while special pulse schemes have been developed for mitigating the effects of the homonuclear 13C couplings in 13C relaxation measurements (Yamazaki et al. 1994). Other applications exploit the inherent complexity that is introduced by the multiplicity of interactions within methylene and methyl groups to obtain additional insights into dynamics using experiments that rely on cross-correlated relaxation (Sun et al. 2011; Tugarinov and Clore 2021). Measuring 2H spin relaxation (Muhandiram et al. 1995) is advantageous in that the decay is dominated by the quadrupolar interaction, and the obtained rates can be cross-validated by recording as many as five independent decay times for each deuteron (Millet et al. 2002). A limitation is that the experiments are less sensitive than those quantifying 15N and, often, 13C relaxation, so that applications are largely focused on methyl groups (Kay et al. 1998).
Far fewer biomolecular applications involving 1H relaxation have appeared in the literature, reflecting the fact that 1H spins are most often both dipolar and scalar coupled to neighboring protons in fully protonated molecules, leading to the facile transfer of magnetization between protons and, therefore, contaminating measured relaxation rates. Labeling strategies involving partial deuteration are helpful in this regard, producing isolated 1H spins at significant numbers of backbone and sidechain positions (Lundström et al. 2009; Hansen et al. 2012). However, it remains of interest to establish robust methods for measurement of relaxation rates in fully protonated proteins, in particular focusing on backbone 1Hα spins, which is the subject matter of this report. Our interest in the measurement of 1Hα relaxation rates concerns quantification of solvent paramagnetic relaxation enhancements (PREs) in an attempt to map near surface electrostatic potentials (Yu et al. 2021) in intrinsically disordered proteins (IDPs). As PRE effects scale with the square of the gyromagnetic ratios of the probe spins (Abragam 1961), there are clear advantages to proton-based experiments. The obvious choice is to record 15N–1HN HSQC spectra that measure 1HN rates, as these can be faithfully obtained via simple spin-echo schemes in which evolution from 1HN–1H scalar couplings is refocused by the application of an 1HN-selective pulse in the center of a relaxation period (Donaldson et al. 2001). Alternatively, the effects of 1HN–1H J-modulation can be “removed” during analysis of spectra recorded with non-selective 1HN chemical shift refocusing pulses by using identical relaxation times for both paramagnetic and diamagnetic samples and fitting paramagnetic relaxation rates directly from intensity ratios of corresponding peaks in the resulting pairs of spectra (Iwahara et al. 2004, 2007). Yet in some applications, especially those involving IDPs or intrinsically disordered regions (IDRs) in otherwise folded molecules that must be performed at neutral pH, amide spectra are severely compromised due to rapid hydrogen exchange. In these cases an approach that circumvents both the recording of 1HN chemical shifts and measurement of 1HN relaxation rates, that are likely to be contaminated by exchange with water, complicating extraction of robust exchange rates, would be preferred. Herein we develop a pseudo-4D experiment for measurement of 1Hα relaxation rates based on the haCONHA pulse scheme that records (13COi,15Ni+1,1Hαi) correlations, where the chemical shifts of 13CO and 1Hα spins of residue i are correlated with the 15N spin of the subsequent residue, i + 1 (Mäntylahti et al. 2011; Wong et al. 2020a). Important considerations for the design and optimization of the pulse scheme are described, along with applications to the C-terminal region of the RNA binding protein CAPRIN1 (Kedersha et al. 2016; Nakayama et al. 2017), so as to establish the robustness of the approach and its utility in studies of IDPs at neutral pH values and higher.
Material and methods
Sample preparation
The C-terminal region of CAPRIN1 (residues 607–709, Uniprot: Q14444) was expressed and purified as described previously (Kim et al. 2019; Wong et al. 2020a). As reported in our previous study (Wong et al. 2020a), residues N623-G624 and N630-G631 slowly form isoaspartate (IsoAsp)-Gly peptide linkages over time. As the formation of IsoAsp can alter the charge distribution of the CAPRIN1 molecule, N623T and N630T double mutations were introduced; the double Thr mutant was used in all of the experiments (and is referred to as CAPRIN1 in the discussion which follows). These mutations were introduced by using Quikchange site-directed mutagenesis (Agilent). Uniformly 13C, 15N-labeled CAPRIN1 was produced by bacterial growth, with expression using minimal media supplemented with [U-13C]-glucose and 15NH4Cl as the sole carbon and nitrogen sources, respectively. The NMR samples were comprised of 280–300 μM U-13C,15N CAPRIN1, 25 mM MES-NaOH (pH 5.5) or 25 mM HEPES–NaOH (pH 7.4), and 3% D2O. For solvent PRE measurements, 3-carboxy-PROXYL (Sigma-Aldrich) or 3-carbamoyl-PROXYL (Sigma-Aldrich) was added to a final concentration of 5 mM from a ~ 100 mM stock solution. The concentration of the paramagnetic co-solutes in the stock solution was measured by 1H 1D NMR after reducing the spin-label, using a procedure established by Iwahara and co-workers (Yu et al. 2021).
NMR measurements
All NMR measurements were performed at 23.5 Tesla (1 GHz 1H frequency) on a Bruker Avance Neo spectrometer or at 14.0 Tesla (600 MHz 1H frequency) on a Bruker Avance III HD spectrometer, equipped with cryogenically cooled x, y, z pulsed-field gradient triple-resonance probes. All spectra were processed and analyzed using the NMRPipe suite of programs (Delaglio et al. 1995) and visualized using the Python package nmrglue (Helmus and Jaroniec 2013). Peak intensities were extracted either by using the Peakipy software package (https://github.com/j-brady/peakipy) for 2D datasets, or by analyzing the time-domains of pseudo-4D datasets (haCONHA of Fig. 1A.1 or A.3), as described previously (Long et al. 2015; Wong et al. 2020b). In time-doming fitting, the reference 3D spectrum recorded with the first relaxation delay was reconstructed using SMILE (Ying et al. 2017), and the peak list required for the time-domain fitting was obtained by analyzing the processed data.
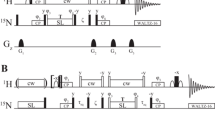
The haCONHA pulse sequence for measuring 1Hα R2 rates by observing (13COi,15Ni+1,1Hαi) correlations. Many of the details of the pulse scheme are as described previously (Wong et al. 2020a); however, for completeness they are repeated here. All 90° (180°) rectangular pulses are denoted by narrow (wide) bars and applied along the x-axis unless otherwise indicated. The 1H carrier is on resonance with the water line (~ 4.7 ppm), the 13C carrier is at 176 ppm between points b and c and otherwise at 58 ppm, and the 15N carrier is at 119 ppm (but see below for specific details about each of the schemes in panel A). 1H WALTZ-16 decoupling is applied with a field of ~ 6.25 kHz. 13Cα and 13CO 90° and 180° rectangular pulses are applied with fields of ΔΩ/√15 and ΔΩ/√3, respectively, where ΔΩ = 118 ppm, ensuring minimal excitation of 13CO spins when 13Cα pulses are applied and vice versa (Kay et al. 1990). 13Cα WALTZ-16 decoupling during t3 acquisition uses a field of ~ 2 kHz (600 MHz spectrometer). Inset A shows three approaches for measuring 1Hα R2 rates, including (i) application of a 1H spin lock (A.1), (ii) application of 1Hβ, 1HN adiabatic decoupling (A.2), and a spin-echo scheme (A.3). In scheme A.1 the 1H and 13C carriers are placed at 4.35 ppm and 50 ppm, respectively, with the placement of the 13C carrier so as to reduce off-resonance effects for 13Cα of Gly. The 1H spin-lock is achieved with a 1 kHz CW field along x, at the center of which a high power 180y pulse is applied. Prior to the spin-lock, 1H spins are aligned along their effective fields via a pulse/delay scheme, described previously (Hansen and Kay 2007), where χ and ζ are set to 1/ωSL − (4/π)pw and (2/π)pw, respectively, where ωSL is the RF field strength for the 1H spin-lock and pw is the 1H high power 90° pulse width. The relaxation delay, Trelax, was varied from 0 to 30 ms. In scheme A.2, selective 1H decoupling is achieved using a constant adiabaticity WURST decoupling element (Kupce and Wagner 1996) swept from 1.1 to 3.3 ppm (8 ms WURST (Kupce and Freeman 1995) pulse width) centered at 2.2 pm, a typical 1Hβ shift value, along with a second field swept from 7.4 to 8.6 ppm centered on the amide 1HN protons. Trelax was varied from 0 to 40 ms in 8 ms spacing intervals so as to be synchronous with the 1H decoupling sequence. The delays are: τ1 = 1.7 ms, τ3 = 4.5 ms, τ4 = 15 ms, with τe sufficiently long to accommodate gradient g13. The delay τ2 is set to 2.3 ms, a compromise so as to obtain cross peaks from all residues including Gly. 13Cβ decoupling is achieved using a constant adiabaticity WURST decoupling element swept from 41 to 15 ppm (5 ms WURST pulse width), along with a second field swept from 68 to 72 ppm. 13CO chemical shift evolution during t1 is acquired in a semi-constant time mode (Grzesiek et al. 1993; Logan et al. 1993) as depicted in B. The phase cycle used is: φ1 = 2(x),2(− x); φ2 = y + 48.5° (600 MHz); φ3 = x, − x; and φrec = x,2(− x),x. The phase change applied to φ2 corrects for the Bloch-Siegert shift caused by application of the uncompensated 13Cα pulse during the t1 period. Quadrature detection in t1 and t2 is achieved by STATES-TPPI (Marion et al. 1989) of φ1 and φ3, respectively. Gradients are applied with the following durations (ms) and strengths (in % maximum): g1: (0.5, 24%), g2: (1.0, 24%), g3: (0.256, 15%), g4: (0.5, 52.8%), g5: (1.0, 40%), g6: (1.25, 80%), g7: (1.5, 80%), g8: (0.9, 50%), g9: (1.0, 15%), g10: (0.512, 90%), g11: (0.4, 40%), g12: (0.3, 15%), g13: (0.256, 90.3%)
1Hα R2 or 1Hα–13Cα longitudinal order relaxation measurements were recorded with pulse schemes that are based on the haCONHA experiment (Wong et al. 2020a) (see Fig. 1), and were performed in a pseudo-4D manner where the indirect 13CO and 15N dimensions were non-uniformly sampled using a Poisson-gap sampling schedule (Hyberts et al. 2010) (1Hα R2, measured using the schemes of Fig. 1A.1 or A.3) or by measuring 2D 13CO–1Hα or 15N–1Hα planes (1Hα R2, measured by the adiabatic scheme of Fig. 1A.2; \(2{I}_{z}^{\alpha }{C}_{z}^{\alpha }\) longitudinal order relaxation using the scheme shown schematically in Fig. 3B that replaces A in Fig. 1). Measurements were performed at 600 MHz and 25 °C with relaxation delays set to 0, 4, 8, 12, 16, 20, 25, and 30 ms for the scheme of Fig. 1A.1 and A.3, or 0–40 ms, in 8 ms steps, for the scheme of Fig. 1A.2. Longitudinal order decay rates were quantified with delays of 0, 4, 8, 12, 16, 20, 25, and 30 ms.
1HN R2 relaxation measurements (pH 5.5 sample) were performed using a transverse relaxation-optimized spectroscopy (TROSY) scheme (Pervushin et al. 1997), with a 1H spin-echo variable delay interval inserted immediately prior to direct detection. A selective REBURP pulse (Geen and Freeman 1991) (length of 1800 μs and centered at 7.7 ppm, 1 GHz) during the 1H spin-echo period refocuses homonuclear J-evolution of 1HN spins. The measurements were performed at 1 GHz and 25 °C, with relaxation delays of 2, 4, 6, 8, 12, 16, 22, and 30 ms.
Fitting of 1Hα and 1HN relaxation rates
1Hα PREs were quantified by fitting intensity ratios of corresponding peaks in the “paramagnetic” (with 5 mM 3-carboxy-PROXYL or 5 mM 3-carbamoyl-PROXYL, denoted by − or N, respectively) and “diamagnetic” (no PRE co-solute molecules) experiments to the single exponential decay function,
where \({I}^{para.,i}({T}_{relax})\) and \({I}^{dia.}\left({T}_{relax}\right)\) are signal intensities at time Trelax for peaks in the paramagnetic and diamagnetic samples, and Γ2,i is the PRE contribution to the 1Hα R2 rate (i \(\in\) {− , N}). As described by Iwahara, Clore and co-workers (Iwahara et al. 2004, 2007; Yu et al. 2022), by taking the ratio of intensities it is possible to divide out contributions from 1H–1H J-modulations that would otherwise contaminate the relaxation rates. Nevertheless, scalar-coupled evolution does attenuate the signals so that it is highly desirable to suppress the modulations in the first place, and this is possible for 1Hα spins from all residues with the exception of Ser and Thr, as described in detail below. We have also observed that for a number of non-Ser/Thr residues there is a slight deviation from single exponential decay, presumably because of modulation from couplings that are not completely suppressed by the 1 kHz spin-lock field of Fig. 1A.1 that selectively locks 1Hα magnetization. Use of Eq. (1) is beneficial for these cases as well.
1HN PREs are quantified by fitting the decay of signals to a single exponential function to obtain \({R}_{2}^{para.,i}\) and \({R}_{2}^{dia.}\) rates, from which the PRE contribution is calculated as \({\Gamma }_{2,i}={R}_{2}^{para.,i}-{R}_{2}^{dia.}\). Fits made use of in-house written programs (Python 3.7), exploiting the Levenberg–Marquardt algorithm of the Lmfit python software package (https://lmfit.github.io/lmfit-py/).
Calculations of near-surface electrostatic potentials
Near-surface electrostatic potentials were calculated from the PRE rates obtained with 3-carboxy-PROXYL (\({\Gamma }_{2,-}\)) and 3-carbamoyl-PROXYL (\({\Gamma }_{2,N}\)) derivatives using the following equation, as described previously (Yu et al. 2021),
where kB is Boltzmann’s constant (8.62 × 10–5 eV/K), T is temperature (298.15 K), and e is the charge of an electron. Note that the denominator was set to 1e as the difference in charge between 3-carboxy-PROXYL and 3-carbamoyl-PROXYL is 1. In the calculations, residues with \({\Gamma }_{2,-}\) or \({\Gamma }_{2,N}\) larger than 0.5 s−1 were used.
Simulations of 1Hα scalar coupled evolution with and without a spin-locking field
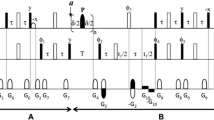
The scalar-coupled evolution of 1Hα magnetization was simulated by calculating the time-evolution of the density matrix (Sørensen et al. 1984). The operative Hamiltonian (\({\widehat{\mathcal{H}}}_{0}\)) is composed of chemical shift (\({\widehat{\mathcal{H}}}_{CS}\)), scalar coupling (\({\widehat{\mathcal{H}}}_{J}\)), and spin-lock field (\({\widehat{\mathcal{H}}}_{SL}\)) terms, as follows,
where Ωi is the offset frequency (rad/sec) of proton spin i from the radio frequency carrier, Ix and Iz denote the x and z components of 1H spin angular momentum, respectively, Jij is the homonuclear scalar coupling constant between spins i j, ω1 is the 1H spin-lock field strength in rad/sec (1000 × 2π rad/sec was used in experiments), and it is understood that only one of the terms of the form 2πJαβ1Iα·Iβ1 or 2πJβ1αIβ1·Iα is included, for example. The number of spins, and the chemical shifts and J coupling constants used in each simulation are indicated in the schematics of Fig. 2. In the calculations, only 2-bond or 3-bond homonuclear J couplings were considered and heteronuclear couplings were not included. In the case of a “typical amino-acid”, such as shown in Fig. 2A (top left), for example, a set of 4 spins i,j \(\in\) {α, β1, β2, HN} was considered. Relaxation was not included in the simulations. The time evolution of the density matrix, evolving with the scheme of Fig. 2A (top right), was calculated by numerically solving the Liouville von-Neumann equation,
Simulating the evolution of 1Hα magnetization due to 1Hα-1H scalar couplings. A Evolution of 1Hα magnetization in a spin system typical for amino acids, where 1HN and two 1Hβ protons are scalar-coupled to 1Hα. (top left) The chemical shifts of each spin and the J-coupling constants used are shown. (top right) Pulse scheme elements, similar to those used in experiments of Fig. 1A.1 (orange; 1Hα magnetization, \(2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\), is locked along its effective field at the start of the spin-lock, as is done experimentally) and in 1A.3 (navy, starting from \({I}_{x}^{\alpha }\)). (bottom) Plots of the trajectories of 1Hα x-magnetization calculated with different 1Hβ chemical shifts (left: 2 ppm, center: 3 ppm, and right: 3.5 ppm), in the presence (orange) and absence (navy) of a 1 kHz spin-lock field centered at 4.35 ppm. B Evolution of 1Hα magnetization in methionine (left), serine (center), and threonine (right) spin systems. (top) The chemical shifts of each spin and the J-coupling constants are shown. (bottom) Trajectories of calculated 1Hα x-magnetization with (orange) and without (navy) a 1 kHz spin-lock field centered at 4.35 ppm. The chemical shifts of each 1H spin were taken from a tabulation of random coil values (Wishart et al. 1995) and the J-coupling constants were set to those measured in unfolded proteins (Hähnke et al. 2010) or those typically observed in folded proteins
with σ(0) = \({I}_{x}^{\alpha }\) (in-phase 1Hα magnetization). Terms from other spins were set to zero initially. Note that, experimentally, the proton magnetization of interest during the relaxation period is antiphase with respect to the attached 13C spin (\(2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\), see Fig. 1) and other transverse magnetization components coupled to 13Cα (such as \(2{I}_{x}^{\beta }{C}_{z}^{\alpha }\)) are not present at the beginning of this period. Thus, when considering a homonuclear spin system exclusively, the analogous situation is one where initial magnetization components with the exception of \({I}_{x}^{\alpha }\) are set to 0. The expectation value of the x-transverse magnetization at time Trelax (M(Trelax)) was calculated by taking the trace of the product of σ(Trelax) and \({I}_{x}^{\alpha }\). M(Trelax) profiles for 0 ≤ Trelax ≤ 30 ms, with a time step of 1 ms, were calculated from
and plotted in Fig. 2A, B.
Simulating the effects of cross-relaxation
As described in the text, a spin-lock field has been used to minimize evolution of magnetization due to homonuclear scalar couplings. We wondered whether dipolar cross-relaxation between neighboring spins would become an issue (ROE effect) under these conditions, leading to non-exponential decay of 1Hα magnetization and to mixing of PREs from proximal 1H spins. We have, therefore, simulated an I-C-M three spin system, where I, C, and M are 1Hα, 13Cα, and 1Hβ spins, respectively, considering relaxation and scalar coupled evolution. In principle, a complete description of this spin system requires a basis of 64 elements. However, as Cz is the only 13C operator considered (13C pulses are not applied), a reduced basis set suffices, composed of 30 elements (excluding the identity operator). The normalized basis set can be expressed by using a column vector with 30 Cartesian product operators (Ernst et al. 1987; Allard et al. 1998),
where + denotes the transpose operation.
The operative Hamiltonian in this case is,
where ΩI and ΩM are the offsets of 1H spins I and M from the 1H carrier, JIM and JIC are I-M and I-C homo- and hetero-nuclear scalar coupling constants, respectively, and ω1 (1000 × 2π rad/sec) is the strength of an applied field on spins I and M. In all simulations, JIM = 7 Hz, JIC = 140 Hz and a static magnetic field of 14.0 Tesla (600 MHz 1H resonance frequency) was used.
The evolution of the density matrix, σ, during the scheme of Fig. 2A (top right) was calculated by numerically solving
In Eq. (8) \(\widehat{\widehat{\mathcal{L}}}\) is a 30 × 30 Liouvillian matrix that includes J and chemical shift evolution, \(\widehat{\widehat{\mathcal{R}}}\) is a relaxation matrix, including auto- and cross-relaxation terms, and \({\widehat{\widehat{U}}}^{y,\pi }\) is a rotation matrix that “applies” a 1H 180° pulse along y in the center of the spin lock element. Each component of \(\widehat{\widehat{\mathcal{L}}}\) and \({\widehat{\widehat{U}}}^{y,\pi }\) was calculated as
where |r > and |s > are density elements listed in Eq. (6).
The relaxation matrix, \(\widehat{\widehat{\mathcal{R}}}\), includes auto-relaxation terms for each operator and cross-relaxation terms coupling Ix ↔ Mx, Iy ↔ My, Iz ↔ Mz, 2IxMz ↔ 2IzMx, 2IyMz ↔ 2IzMy, 2IxMy ↔ 2IyMx, 2IxCz ↔ 2MxCz, 2IyCz ↔ 2MyCz, 2IzCz ↔ 2MzCz, 4IxMzCz ↔ 4IzMxCz, 4IyMzCz ↔ 4IzMyCz, and 4IxMyCz ↔ 4IyMxCz. Auto-relaxation was calculated by including 1H-1H dipolar interactions between each of spins I and M and a pair of external proton spins (one unique external 1H for each of I and M; rHH,ext = 2.1 Å for both I and M spins), 1H-1H dipolar interactions between spins I and M (rHH,IM = 1.9 Å), and 1H-13C dipolar interactions between spins I and M and their directly bonded 13C nuclei (rCH = 1.1 Å). Only terms proportional to the spectral density evaluated at zero frequency are included in our analysis. Although a separate carbon spin one-bond coupled to spin M is not explicitly included in the spin system under consideration (Eq. (6)), we have, nevertheless, included a C-M dipolar interaction that would normally be present in the U-13C,15N proteins that are studied experimentally. The relaxation matrix can, thus, be defined as follows (Allard et al. 1997),

The auto-relaxation rates (R11–R1515), anti-phase and multiple-quantum cross-relaxation terms (R79, R97, R810, R108, R1213 and R1312) are given by Desvaux et al. (1994) and Allard et al. (1997)
and the transverse and longitudinal cross-relaxation rates, σROE and σNOE, are defined as
where μ0 denotes the vacuum permeability, γH and γC are the gyromagnetic ratios of 1H and 13C spins, respectively, ℏ is Planck’s constant devided by 2π, and τC is the correlation time (Abragam 1961; Cavanagh et al. 2007). Relaxation of longitudinal magnetization to its thermal equilibrium is not included in the calculation, as the evolution of anti-phase magnetization is considered (see below).
In our experimental scheme (Fig. 1A.1) antiphase 1Hα magnetization (\(2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\)) is spin-locked along its effective field using a previously described alignment element (Hansen and Kay 2007). Thus, in our simulations the initial value of the density matrix is given by
where \({I}_{z}^{^{\prime}}\) is the aligned magnetization in the tilted frame and θI is the angle between the z-axis of the tilted frame and the axis parallel to the static magnetic field (tanθ = ω1/ΩI). The expectation value of the spin-locked magnetization at time Trelax (M(Trelax)) was obtained by solving Eq. (8) and then extracting the \(2{I}_{z}^{^{\prime}}{C}_{z}\) element as,
In our simulations a value of τC = 2 × 10–9 s was assumed, consistent with previous relaxation measurements (Kim et al. 2021), so that the corresponding cross-relaxation rates are σROE = 4.84 s−1 and σNOE = − 2.42 s−1. In all simulations, the chemical shift of spin I and the carrier position were fixed to 4.4 and 4.35 ppm, respectively (ΩI = (4.4 − 4.35) × 600 × 2π rad/sec), and the chemical shift of spin M was varied from 2 to 4 ppm (ΩM = (2 to 4 − 4.35) × 600 × 2π rad/sec). The trajectory of \(2{I}_{z}^{^{\prime}}{C}_{z}\) was calculated from 0 to 120 ms (fourfold longer than experimental relaxation times, Fig. 3A) with a time step of 1 ms. Similar simulations were performed in the absence of cross-relaxation by setting σROE and σNOE to 0 in the relaxation matrix of Eq. (11).
Cross-relaxation has a minimal effect on the evolution of \(2{I{^{\prime}}}_{z}^{\alpha }{C}_{z}^{\alpha }\) magnetization. A (top) Schematic of the 3-spin {I, C, M}-spin system used in the simulations; a pair of external protons are included, in addition, contributing only to the auto-relaxation of proton spins I and M. (bottom) The evolution of anti-phase 1Hα magnetization (\(2{I{^{\prime}}}_{z}^{\alpha }{C}_{z}^{\alpha }\)) spin-locked along its effective field (Eq. (14)) in the presence (navy, line) and absence (pink, dotted line) of cross-relaxation. The chemical shift of spin M (1Hβ mimic) was set to 2 (left), 3 (center), and 4 (right) ppm, respectively. A 1 kHz spin-lock field is applied, centered at 4.35 ppm. Note that the distinctly non-exponential profile for the case where the 1Hβ resonance frequency is 4 ppm is due to homonuclear J-evolution that is not suppressed using the 1H spin-lock field. B Relaxation of longitudinal order (\(2{I}_{z}^{\alpha }{C}_{z}^{\alpha }\)) was quantified using the scheme (left) that replaces A in Fig. 1. The delay τ1 and gradient strengths of g3 and g5 are indicated in the Fig. 1 legend. Water magnetization is either initially in the transverse plane (Scheme 1) or dephased at the start of the relaxation interval (Scheme 2) during Trelax. The relaxation rates of \(2{I}_{z}^{\alpha }{C}_{z}^{\alpha }\) two-spin order (CAPRIN1, 25 °C, 600 MHz) are shown as a function of 1Hα chemical shift (right)
Results and discussion
Description of pulse scheme for the measurement of 1Hα transverse relaxation rates in IDPs
The original experiments to quantify near surface electrostatic potentials of proteins, developed by Iwahara and co-workers (Yu et al. 2021), based in part on work from the Clore group (Okuno et al. 2020), focused on the measurement of amide 1HN transverse relaxation rates in the presence and absence of variously charged spin labels. The measurement of 1HN as opposed to other proton relaxation rates has the obvious advantage in that there is only a single homonuclear scalar coupling to consider, involving 1Hα spins, and evolution from the 3JHN-Hα coupling can be refocused by the application of a 1HN-selective 180° pulse in the center of the 1H evolution period that is required to quantify transverse relaxation (Donaldson et al. 2001). Unfortunately, however, studies of IDPs at physiological pH values cannot be performed using amide correlation spectra as the rapid exchange of amide protons with water deteriorates the quality of the resulting spectra. Moreover, in such cases the relaxation of 1HN spins is contaminated by exchange with water, with effective rates that are often non-exponential. These rates, further, can vary significantly depending on where in the pulse scheme they are interrogated (Ishima et al. 1998; Yuwen et al. 2016). By contrast, relaxation rates of 1Hα protons are not sensitive to pH (exchange with water) and 1Hα-detect experiments remain of high quality even when amide protons exchange rapidly with solvent. As the resolution of 1Hα-13Cα correlations in 2D heteronuclear spectra of IDPs is poor, we prefer to measure relaxation data using 3D haCONHA-type experiments in which correlations of the form (ωCO, ωN, ωHα) are recorded, exploiting the resolution in 13CO and 15N dimensions (Mäntylahti et al. 2011; Wong et al. 2020a). A pseudo-4D dataset is, thus, obtained, in which each 3D spectrum corresponds to a single time point that is used to quantify transverse relaxation.
Figure 1 highlights the pulse scheme that we prefer (Scheme A.1), along with a sequence that is used to cross-validate the results (Scheme A.2). For completeness, we also show a simple spin-echo variant, similar to a recently described experiment by Yu et al. (2022), to illustrate some of the challenges with recording 1Hα relaxation rates that must be overcome in the design of a robust pulse scheme. The initial element of the pulse sequence (A, in Fig. 1) is of interest to the relaxation experiment described here and in what follows we provide a brief overview of the magnetization pathway during this interval. Focusing on A.1, after the creation of longitudinal order (\(2{I}_{z}^{\alpha }{C}_{z}^{\alpha }\)) by the first INEPT element, where Ij and Cj are the proton and carbon spins that are one-bond coupled and j = α denotes either the 1Hα or 13Cα spin, the water magnetization is dephased by application of a pulsed field gradient to minimize the radiation damping field (gradient g4); failure to do so can lead to apparent 1Hα PREs that are significantly elevated, by 1.5 to 2-fold, in applications involving CAPRIN1 (see below). Subsequently, the 1Hα spins are locked along their respective effective fields in a manner that is efficiently achieved for the narrow 1Hα chemical shift range for IDPs (~ 4–4.8 ppm for CAPRIN1) using a 1 kHz 1H continuous-wave (cw) field applied in the center of the 1Hα spectrum (along the x-axis; a 1H 180y pulse is included in the center of the cw element), and the magnetization subsequently restored to the z-axis prior to magnetization transfers to 13CO(t1) and 15N(t2) that are identical to those in a regular haCONHA experiment (Wong et al. 2020a).
Although the haCONHA approach circumvents issues with hydrogen exchange, other problems are introduced when using aliphatic proton spins, such as 1Hα, and Scheme A.1 of Fig. 1 is our best attempt to minimize these. For example, 1Hα protons are three-bond scalar coupled to 1HN and 1Hβ spins and evolution of 1Hα transverse magnetization from 3JHα-Hβ couplings is not as readily refocused as for 3JHN-Hα couplings in the context of 1HN-based measurements, for example. In a simple spin-echo scheme that might be considered for measurement of transverse relaxation rates (Fig. 1, Scheme A.3, starting from \({I}_{x/y}^{\alpha }\)) this evolution would proceed for the complete Trelax period, modulating the signal, and complicating extraction of accurate transverse relaxation rates. To illustrate this, as well as our solution to the problem (Scheme A.1, starting from \(2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\)), in more detail, we consider the “generic” spin system shown in Fig. 2A, top left, and simulate the evolution of transverse 1Hα x-magnetization during the spin-echo element shown in Fig. 2A, top right. We consider the case where 1Hα spins are locked along their respective effective fields or when the spin lock field is removed. In the simulations shown in Fig. 2A (bottom) ωHα = 4.4 ppm and ωHβ is varied from 2 to 3.5 ppm with the orange (navy) profiles obtained with (without) the 1 kHz spin-lock field centered at 4.35 ppm. Notably, for ωHβ = 2 ppm (bottom left) or 3.0 ppm (bottom center) the orange profiles are flat, as if the scalar couplings involving 1Hα were “turned off” (3JHα,NH = 7, 3JHα,Hβ1 = 5.5, and 3JHα,Hβ2 = 7.5 Hz are used in the simulation). In contrast, if the spin-lock field is omitted the navy curves result, clearly showing modulation from 1Hα–1Hβ and 1Hα–1HHN scalar couplings. When the chemical shifts of the 1Hβ protons are increased to 3.5 ppm (bottom right), closer to the position of the spin lock, a slight amount of modulation (approximately 1–2%—between 1 and 0.98) is obtained for the spin lock case.
It is noteworthy that in IDPs all 1Hβ protons resonate upfield of 3.5 ppm with the exception of those from Ser and Thr (Wishart et al. 1995), so that flat profiles (i.e., unmodulated by homonuclear scalar couplings) would be expected for all non-Ser/Thr 1Hα protons using a spin lock scheme to measure relaxation. As might be expected, the presence of more spins on the side-chain does not affect the trajectory of x-magnetization, as shown in the simulation for a Met spin system containing 6 spins, where random coil chemical shifts are taken from Wishart et al. (1995) (Fig. 2B, left). The proximity of 1Hα and 1Hβ chemical shifts in Ser (ωHα = 4.47 ppm, ωHβ = {3.87, 3.89} ppm) leads to significant modulation that cannot be suppressed by the 1H spin-lock field (Fig. 2B, center), with a similar situation occurring for Thr (Fig. 2B, right). For both of these residues homonuclear J-modulation simply decreases magnetization intensity; there is no net transfer of observable magnetization between spins, as there would be in a Hartmann-Hahn scheme where in-phase 1Hα/β… magnetization is initially created. This is because the initial magnetization for each proton is anti-phase with respect to its attached carbon and 13C–1H scalar coupled evolution is largely suppressed by the 1H cw field. Thus, while the transfer, \(2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\stackrel{{J}_{H\alpha H\beta }}{\to }2{I}_{x}^{\beta }{C}_{z}^{\alpha }\), does occur, the transfer, \(2{I}_{x}^{\beta }{C}_{z}^{\beta }\stackrel{{J}_{H\alpha H\beta }}{\to }2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\), does not, with only antiphase magnetization of the form \(2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\) ultimately detected. This ensures that the measured 1Hα relaxation rates are not ‘contaminated’ by contributions from relaxation of other scalar coupled protons in the case of Ser and Thr. For 1Hα spins from these residues, however, the scalar coupled evolution, \(2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\stackrel{{J}_{H\alpha H\beta }}{\to }2{I}_{x}^{\beta }{C}_{z}^{\alpha }\), prohibits extraction of accurate relaxation rates from exponential fits of the “decay” curves. In contrast, as J-coupled modulation of non-Ser/Thr 1Hα protons does not occur, there is no “leakage” of magnetization from \(2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\) to \(2{I}_{x}^{\beta }{C}_{z}^{\alpha }\) in these cases, and exponential decays are expected. Finally, for Gly, the 1Hα spins become very strongly coupled in the presence of the cw field (i.e., essentially equivalent), and the sum of 1Hα magnetization does not evolve under scalar coupling in this limiting case since \(\left[{I}_{x}^{\alpha 1}+{I}_{x}^{\alpha 2},{{\varvec{I}}}^{\alpha 1}\cdot {{\varvec{I}}}^{\alpha 2}\right]=0\), where [] denotes the commutator operation. Thus, flat profiles are observed for the 1Hα spins of Gly in simulations even when scalar coupled evolution is considered. Of course, even in the case where the two 1Hα Gly spins are resolved (typically with chemical shift differences of 0.05–0.1 ppm), the observed relaxation rates would represent the average of the values from the two Hα positions, which are expected to be very similar, as magnetization is very efficiently transferred between the 1Hα spins during the application of the spin-lock. It is worth noting that complications from J-modulation can be prevalent in non-1H homonuclear spin systems and similar strategies involving band-selective locking schemes have been used previously to measure 13Cα relaxation rates in uniformly 13C-labeled proteins (Yamazaki et al. 1994).
Figure 2 illustrates the importance of the 1H cw spin-lock field in the suppression of homonuclear J-modulation of magnetization for the majority of the spin systems in IDPs. Spin-locking of magnetization can, however, potentially lead to non-exponential relaxation from magnetization transfer mediated by dipolar cross-relaxation (spin diffusion). As described above, in the context of magnetization transfer through scalar couplings, the dipolar transfer can also be minimized by recording relaxation rates of 1Hα magnetization that is anti-phase with respect to the one-bond coupled 13C (Sekhar et al. 2016). Thus, in the case of a pair of dipolar coupled 1H spins, I and M, relaxation proceeds as
where ρI' and σI'M' are auto- and cross-relaxation rates of the aligned magnetization in the tilted spin-lock frame that is germane for spin-locked magnetization considered in our experiments (the primes in \({I}_{z}^{^{\prime}}\) and \({M}_{z}^{^{\prime}}\) denote ‘tilted’ magnetization). As hetero-spins M and CI are not scalar coupled (CI is one bond coupled to proton spin I) there is no longitudinal order of the form \(2{M}_{z}^{^{\prime}}{C}_{z}^{I}\) initially so that the decay of the magnetization of interest is essentially single exponential, with magnetization transfer between \(2{M}_{z}^{^{\prime}}{C}_{z}^{I}\) and \(2{I}_{z}^{^{\prime}}{C}_{z}^{I}\) effectively suppressed over the relatively short range of relaxation times considered (Trelax_max = 30 ms in our experiments). This is in contrast to what would be expected if the relaxation of in-phase 1H magnetization was quantified. Figure 3A illustrates the evolution of anti-phase I spin magnetization during the relaxation element for a 3-spin {I, C, M}-spin system, with the chemical shift of the M spin varied between 2 and 4 ppm; cross-relaxation introduces a negligible effect, using an effective I–M distance of 1.9 Å for relaxation times extending to 120 ms and for a rotational correlation time of 2 ns, appropriate for the experimental system considered here (Kim et al. 2021). Note that the evolution of magnetization is decidedly non-exponential when ωHβ = 4 ppm, due to I–M scalar coupled evolution.
The importance of “water” management, even in 1Hα-based experiments is illustrated in Fig. 3B. Here, by means of example, we consider the relaxation of longitudinal order, \(2{I}_{z}^{\alpha }{C}_{z}^{\alpha }\), during an interval, Trelax, where a gradient that dephases water is applied either at the start or the end of the relaxation period. If the water transverse magnetization is not dephased initially, its precession induces an oscillating current in the receiver coil, and hence, a magnetic field, oscillating at the frequency of precession. This induced field rotates water magnetization and other spins resonating close to the water line back to their equilibrium (Krishnan and Murali 2013). Thus, the effect of the induced magnetic field would be expected to be more pronounced for 1Hα spins whose chemical shifts are closer to the water line. With this in mind, the measured longitudinal order relaxation rate for each 1Hα–13Cα spin pair in CAPRIN1 (described below) is plotted as a function of 1Hα chemical shift, showing a clear elevation in rates for spins resonating near the water line when water is not dephased. As the water is initially in the transverse plane in this experiment, as it would be at point a in Schemes A.1–A.3 of Fig. 1 the radiation-damping field from bulk water can be considerable, unless water magnetization is initially dephased (Saturation of the water line significantly attenuates sensitivity of the experiment and is not a good option). Similar experiments, starting from anti-phase 1Hα magnetization (\(2{I}_{x}^{\alpha }{C}_{z}^{\alpha }\)), show a 1.5- to 2-fold increase in measured 1Hα PRE rates, in the absence of dephasing. Water magnetization is therefore dephased in schemes A.1 and A.2 immediately after the initial INEPT transfer and prior to the Trelax period.
Experimental validation
The RNA binding protein CAPRIN1 has been shown to play an essential role in the formation of neuronal and stress granules in cells (Kedersha et al. 2016; Nakayama et al. 2017), and the C-terminal low complexity disordered region comprising residues 607–709, and referred to in what follows as CAPRIN1, phase separates in vitro (Kim et al. 2019). Because of the small size of CAPRIN1 it has been used as a model system in our laboratory, both for the development of NMR methodology for characterizing IDPs in condensates, and, importantly, to understand the interactions that give rise to phase separation in the first place (Kim et al. 2021). CAPRIN1 has a pI of 11.5 and a charge of + 13 under the conditions of our experiments and the resulting unfavorable electrostatic interactions between proximal molecules must be screened before phase separation can occur. This can be achieved typically by the addition of negatively charged molecules or by adding salt (Kim et al. 2019; Wong et al. 2020a). Here we have used low salt buffers (25 mM MES-NaOH (pH 5.5) or 25 mM HEPES–NaOH (pH 7.4)) to ensure that the protein solutions studied are fully mixed (i.e., not phase separated), and, therefore, CAPRIN1 is expected to have a positive electrostatic potential, as established below. Figure 4A, top, shows the 13CO–15N projection of a 3D haCONHA dataset recorded with the pulse sequence of Fig. 1 (Scheme A.1), Trelax = 0 ms, along with the magnetization transfer pathway that gives rise to the spectrum (bottom). Three peaks are highlighted, along with the residues from where the correlations originate; analysis of these peaks in a series of 3D datasets recorded as a function of Trelax, generates the decay curves in Fig. 4B. As our goal is to calculate the near surface electrostatic potential (Yu et al. 2021) of CAPRIN1, we have measured 1Hα transverse relaxation rates in the presence or absence (Diamagnetic, purple) of 5 mM negative (Carboxy-PROXYL, red) or neutral (Carbamoyl-PROXYL, grey) solvent spin labels. A comparison of intensity profiles using Schemes A.3 and A.1 (titled Scheme A.3 and Scheme A.1 in the figure) clearly shows the effects of scalar coupling on the evolution of 1Hα magnetization when recording data with the spin-echo scheme (Fig. 1A.3) relative to the spin-lock element of Fig. 1A.1. Notably, J-modulation gives rise to decidedly non-exponential decays of 1Hα magnetization (left column, Scheme A.3), as is particularly apparent in the profile of G609, where the magnetization becomes negative for Trelax values in excess of approximately 20 ms. The effective intensity decays are slower when using the spin-lock, including for T705, despite the fact that scalar coupling effects are not completely eliminated for 1Hα spins of this residue when magnetization is locked (see above).
Suppression of J-modulation via spin locking of 1Hα magnetization. A 13CO-15N projection of a 3D haCONHA dataset recorded with the pulse sequence of Fig. 1A.1, Trelax = 0 ms. Several peaks are highlighted from which the 1Hα relaxation profiles shown are derived. The magnetization transfer pathway is indicated at the bottom. B Decay curves of selected residues measured in the presence or absence (Diamagnetic, purple) of 5 mM negative (carboxy-PROXYL, red) or neutral (carbamoyl-PROXYL, grey) solvent spin labels. Solid lines connect the experimental points in the panels titled Scheme A.3 and Scheme A.1. Ratio of corresponding peak intensities in spectra recorded with the sequences of either Fig. 1A.3 (left) or Fig. 1A.1 (right) and either with, \({I}^{para.i}({T}_{relax})\), or without, \({I}^{dia.}({T}_{relax})\), solvent spin-labels, along with exponential fits of the data and extracted PRE rates, are shown. All measurements were performed on a 300 μM U-13C, 15N CAPRIN1 sample at pH 5.5, 25 °C and 14.0 Tesla
Recognizing the deleterious effects of homonuclear scalar couplings to the measurement of 1Hα relaxation rates using spin-echo type experiments (Fig. 1A.3), Iwahara and co-workers determined PRE rates by simultaneous analysis of the ratios of signal intensities in paramagnetic and diamagnetic samples in spectra recorded with identical Trelax values (Iwahara et al. 2004; Clore and Iwahara 2009; Yu et al. 2022). In this way the scalar coupling terms cancel, and ratios are sensitive only to the PRE. However, the signal intensities themselves are reduced by the modulation making this approach more error prone than if coupled evolution was not present in the first place. For example, the large germinal 1Hα coupling in Gly residues results in low peak intensities for Trelax values greater than approximately 15 ms (Fig. 4B); in our applications Trelax values between 16 and 25 ms had to be omitted when data were recorded using a spin-echo based sequence (Fig. 1A.3). For other residue-types (non-Gly residues), the reduction in intensities of resonances is less severe, on average a ratio of 0.46 ± 0.12 for Trelax = 30 ms is obtained, when comparing the schemes shown in Fig. 1A.1 and A.3. Also shown in Fig. 4B are exponential fits of intensity ratios, Ipara.,i(Trelax)/Idia.(Trelax), of cross-peaks from spectra recorded with the spin-echo and spin-lock schemes. Notably, while the PRE rates from Schemes A.1 and A.3 are somewhat different, the ratio of rates recorded with different combinations of solvent spin labels tends to be similar (with the exception of a number of Gly residues, for which the germinal coupling is particularly detrimental for the spin-echo scheme).
The simulations and experimental data presented in Figs. 2, 3, and 4 strongly suggest that the spin-lock scheme of Fig. 1A.1 suppresses J-modulation (except for 1Hα from Ser and Thr) without introducing magnetization transfer via the ROE. A more rigorous evaluation of the robustness of the experiment can be made through comparison to an analogous yet distinct approach, illustrated in Fig. 1A.2 where the relaxation of transverse (not spin-locked) 1Hα magnetization is measured. In this pulse scheme suppression of 1Hα-1H scalar coupled evolution is achieved for the majority of amino acids through the use of band-selective 1H adiabatic decoupling (Kupce and Wagner 1996) that is carefully adjusted so as to minimally perturb the 1Hα signals of interest, while decoupling 1Hβ and 1HN proton spins. Since adiabatic decoupling of 1Hβ is applied over a chemical shift range of ~ 2.2 ± 1.1 ppm, Ser, and Thr, whose 1Hβ chemical shifts do not fall within this region (and overlap with those of 1Hα) are not effectively decoupled. In addition, the large germinal (two-bond) 1Hα1–1Hα2 coupling (~ −15 Hz) for Gly results in a severe modulation of the 1Hα signals for this residue, as is observed in experiments recorded with Fig. 1A.3. Figure 5 compares carboxy-PROXYL PRE values obtained via the schemes of Fig. 1A.1 and A.2 omitting Gly residues, and the agreement is excellent (RMSD = 0.69 s−1 for \({\Gamma }_{2,-}\), significantly better than when PRE rates are compared between spin-echo (Fig. 1A.3) and spin lock (Fig. 1A.1) schemes (RMSD = 2.21 s−1). Thus, accurate PRE values are obtained by using the 1H cw spin-lock field in Fig. 1A.1, without deleterious effects from residual J-evolution or cross-relaxation. As the 1Hα signals from Gly residues are not modulated by geminal scalar couplings using the sequence of Fig. 1A.1, we prefer it, and in what follows all results were obtained using this experiment.
Correlation plot of carboxy-PROXYL 1Hα PRE rates using schemes A.1 and A.2 of Fig. 1. The carboxy-PROXYL 1Hα PRE rates (Γ2,− = paramagnetic − diamagnetic) measured using two schemes of Fig. 1 are plotted. The PRE rates plotted along the y-axis were measured using a 1H adiabatic decoupling approach (A.2) and those plotted along the x-axis were quantified via a spin-lock element (A.1). PRE measurements were performed on a 300 μM U-13C, 15N CAPRIN1 sample at pH 5.5, 25 °C and 14.0 Tesla, with and without 5 mM 3-carboxy-PROXYL. R2 is the squared Pearson correlation coefficient
Figure 6A shows 15N–1HN TROSY-HSQC spectra of CAPRIN1 recorded at pH 5.5 (left) and at pH 7.4 (center); all other experimental conditions are identical. The degradation of spectral quality with pH is obvious. However, the 13CO–15N projection of the 3D haCONHA dataset (non-TROSY acquisition in the 15N dimension) recorded on the pH 7.4 sample is of high quality (right), so that electrostatic potentials (ϕENS) can be obtained at neutral pH values using relaxation rates measured with the sequence of Fig. 1A.1. A comparison of ϕENS values measured at pH 5.5 using haCONHA and 15N-1HN TROSY pulse schemes is presented in Fig. 6B, C (left), with good agreement between the two methods. A strong correlation between ϕENS values measured at pH 5.5 and pH 7.4 is also found (Fig. 6B, C, right), as expected, since CAPRIN1 does not contain His residues. Slightly higher errors in the haCONHA based ϕENS measurements at pH 7.4 are noted compared to those at pH 5.5. This reflects the fact that solvent exchange is close to two orders of magnitude more rapid at the higher pH so that the amide protons are more effectively saturated through exchange with water (that is saturated using this pulse scheme). In turn, this leads to saturation transfer to the 1Hα protons via spin-diffusion, decreasing the initial 1Hα polarization and hence the resulting signal intensities in 3D datasets.
Validation of the methodology. A 15N-1HN TROSY-HSQC spectra of CAPRIN1 recorded at pH 5.5 (left) and at pH 7.4 (center) with all other experimental conditions identical (25 °C, 23.5 T), along with a 13CO-15N projection of the 3D haCONHA dataset (right, pH 7.4, 14.0 T) B, C comparison of ϕENS values measured at pH 5.5 using haCONHA and 15N-1HN TROSY pulse schemes (left) and between ϕENS values measured at pH 5.5 and pH 7.4 using the haCONHA experiment (right)
Concluding remarks
Herein we have described a robust method for the measurement of backbone 1Hα relaxation rates in IDPs, a first step for obtaining near surface electrostatic potentials of IDPs at neutral pH values. The experiment avoids 1HN magnetization, leading to high quality IDP spectra even when recorded at high pH where solvent exchange can often be a limiting factor. A number of issues associated with the measurement of 1Hα relaxation in fully protonated protein systems are discussed and solutions presented so that robust rates can be obtained. Notably, the use of a band-selective spin-lock significantly suppresses homonuclear scalar coupling modulation for all 1Hα protons, except those from Ser and Thr, improving the accuracy of measured PRE values, since signal decay is attenuated only by relaxation. The excellent agreement between PRE rates measured using 1H spin-lock and 1H adiabatic decoupling schemes, the close correlation between ϕENS values measured on CAPRIN1 at pH 5.5 using 1HN- and 1Hα-based experiments, where amide exchange is not limiting, and the good agreement for CAPRIN1 ϕENS calculated from experiments on samples at pH 5.5 and 7.4 (where exchange is severe), provides strong confidence in the developed methodology. During the completion of this study we became aware of related work by Yu et al (2022) where 1Hα transverse relaxation rates were used to establish the surface potential of ubiquitin using 2D (HCACO)NH-based experiments. This approach is most clearly appropriate for studies of folded proteins where solvent exchange is not limiting, although it seems likely that here, too, there would be considerable benefit with spin-locking of 1Hα magnetization during the relaxation measurement. This work sets the stage for the measurement of electrostatic potentials in CAPRIN1 condensates, in order to establish the role of electrostatics in phase separation.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Abragam A (1961) Principles of nuclear magnetism. Clarendon Press, Oxford
Allard P, Helgstrand M, Härd T (1997) A method for simulation of NOESY, ROESY, and off-resonance ROESY spectra. J Magn Reson 129:19–29. https://doi.org/10.1006/jmre.1997.1252
Allard P, Helgstrand M, Härd T (1998) The complete homogeneous master equation for a heteronuclear two-spin system in the basis of cartesian product operators. J Magn Reson 134:7–16. https://doi.org/10.1006/jmre.1998.1509
Anthis NJ, Clore GM (2015) Visualizing transient dark states by NMR spectroscopy. Q Rev Biophys 1:35–116. https://doi.org/10.1017/S0033583514000122
Cavanagh J, Fairbrother WJ, Palmer AG III et al (2007) Protein NMR spectroscopy: principles and practice, 2nd edn. Academic Press
Clore GM, Iwahara J (2009) Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes. Chem Rev 109:4108–4139. https://doi.org/10.1021/cr900033p
Delaglio F, Grzesiek S, Vuister G et al (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293. https://doi.org/10.1007/BF00197809
Desvaux H, Berthault P, Birlirakis N, Goldman M (1994) Off-resonance ROESY for the study of dynamic processes. J Magn Reson Ser A 108:219–229. https://doi.org/10.1006/jmra.1994.1114
Donaldson LW, Skrynnikov NR, Choy W-Y et al (2001) Structural Characterization of proteins with an attached ATCUN motif by paramagnetic relaxation enhancement NMR spectroscopy. J Am Chem Soc 123:9843–9847. https://doi.org/10.1021/ja011241p
Ernst RR, Bodenhausen G, Wokaun A (1987) Principles of nuclear magnetic resonance in one and two dimensions. Clarendon Press, Oxford
Geen H, Freeman R (1991) Band-selective radiofrequency pulses. J Magn Reson 93:93–141. https://doi.org/10.1016/0022-2364(91)90034-Q
Goto NK, Gardner KH, Mueller GA et al (1999) A robust and cost-effective method for the production of Val, Leu, Ile (δ1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J Biomol NMR 13:369–374. https://doi.org/10.1023/A:1008393201236
Grzesiek S, Anglister J, Bax A (1993) Correlation of backbone amide and aliphatic side-chain resonances in 13C/15N-enriched proteins by isotropic mixing of 13C magnetization. J Magn Reson Ser B 101:114–119. https://doi.org/10.1006/jmrb.1993.1019
Hähnke MJ, Richter C, Heinicke F, Schwalbe H (2010) The HN(COCA)HAHB NMR experiment for the stereospecific assignment of Hβ-protons in non-native states of proteins. J Am Chem Soc 132:918–919. https://doi.org/10.1021/ja909239w
Hansen DF, Kay LE (2007) Improved magnetization alignment schemes for spin-lock relaxation experiments. J Biomol NMR 37:245–255. https://doi.org/10.1007/s10858-006-9126-6
Hansen AL, Lundström P, Velyvis A, Kay LE (2012) Quantifying millisecond exchange dynamics in proteins by CPMG relaxation dispersion NMR using side-chain 1H probes. J Am Chem Soc 134:3178–3189. https://doi.org/10.1021/ja210711v
Helmus JJ, Jaroniec CP (2013) Nmrglue: an open source Python package for the analysis of multidimensional NMR data. J Biomol NMR 55:355–367. https://doi.org/10.1007/s10858-013-9718-x
Hyberts SG, Takeuchi K, Wagner G (2010) Poisson-gap sampling and forward maximum entropy reconstruction for enhancing the resolution and sensitivity of protein NMR data. J Am Chem Soc 132:2145–2147. https://doi.org/10.1021/ja908004w
Ishima R, Wingfield PT, Stahl SJ et al (1998) Using amide 1H and 15N transverse relaxation to detect millisecond time-scale motions in perdeuterated proteins: application to HIV-1 protease. J Am Chem Soc 120:10534–10542. https://doi.org/10.1021/ja981546c
Ishima R, Louis JM, Torchia DA (1999) Transverse 13C relaxation of CHD2 methyl isotopmers to detect slow Conformational changes of protein side chains. J Am Chem Soc 121:11589–11590. https://doi.org/10.1021/ja992836b
Iwahara J, Schwieters CD, Clore GM (2004) Ensemble approach for NMR structure refinement against 1H paramagnetic relaxation enhancement data arising from a flexible paramagnetic group attached to a macromolecule. J Am Chem Soc 126:5879–5896. https://doi.org/10.1021/ja031580d
Iwahara J, Tang C, Marius Clore G (2007) Practical aspects of 1H transverse paramagnetic relaxation enhancement measurements on macromolecules. J Magn Reson 184:185–195. https://doi.org/10.1016/j.jmr.2006.10.003
Kainosho M, Torizawa T, Iwashita Y et al (2006) Optimal isotope labelling for NMR protein structure determinations. Nature 440:52–57. https://doi.org/10.1038/nature04525
Kasinath V, Valentine KG, Wand AJ (2013) A 13C labeling strategy reveals a range of aromatic side chain motion in calmodulin. J Am Chem Soc 135:9560–9563. https://doi.org/10.1021/ja4001129
Kay LE, Torchia DA (1991) The effects of dipolar cross correlation on 13C methyl-carbon T1, T2, and NOE measurements in macromolecules. J Magn Reson 95:536–547. https://doi.org/10.1016/0022-2364(91)90167-R
Kay LE, Torchia DA, Bax A (1989) Backbone dynamics of proteins AS studied by 15N inverse detected heteranuckar. Biochemistry 28:8972–8979
Kay LE, Ikura M, Tschudin R, Bax A (1990) Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson 89:496–514. https://doi.org/10.1016/0022-2364(90)90333-5
Kay LE, Muhandiram DR, Wolf G et al (1998) Correlation between binding and dynamics at SH2 domain interfaces. Nat Struct Biol 5:156–163. https://doi.org/10.1038/nsb0298-156
Kedersha N, Panas MD, Achorn CA et al (2016) G3BP–Caprin1–USP10 complexes mediate stress granule condensation and associate with 40S subunits. J Cell Biol. https://doi.org/10.1083/jcb.201508028
Kim TH, Tsang B, Vernon RM et al (2019) Phospho-dependent phase separation of FMRP and CAPRIN1 recapitulates regulation of translation and deadenylation. Science 365:825–829. https://doi.org/10.1126/science.aax4240
Kim TH, Payliss BJ, Nosella ML et al (2021) Interaction hot spots for phase separation revealed by NMR studies of a CAPRIN1 condensed phase. Proc Natl Acad Sci 118:e2104897118. https://doi.org/10.1073/pnas.2104897118
Krishnan VV, Murali N (2013) Radiation damping in modern NMR experiments: progress and challenges. Prog Nucl Magn Reson Spectrosc 68:41–57. https://doi.org/10.1016/j.pnmrs.2012.06.001
Kupce E, Freeman R (1995) Adiabatic pulses for wideband inversion and broadband decoupling. J Magn Reson Ser A 115:273–276
Kupce E, Wagner G (1996) Multisite band-selective decoupling in proteins. J Magn Reson 110:309–312. https://doi.org/10.1006/jmrb.1996.0048
Lipari G, Szabo A (1982a) Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J Am Chem Soc 104:4546–4559. https://doi.org/10.1021/ja00381a009
Lipari G, Szabo A (1982b) Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 2. Analysis of experimental results. J Am Chem Soc 104:4559–4570. https://doi.org/10.1021/ja00381a010
Logan TM, Olejniczak ET, Xu RX, Fesik SW (1993) A general method for assigning NMR spectra of denatured proteins using 3D HC(CO)NH-TOCSY triple resonance experiments. J Biomol NMR 3:225–231. https://doi.org/10.1007/BF00178264
Long D, Delaglio F, Sekhar A, Kay LE (2015) Probing invisible, excited protein states by non-uniformly sampled pseudo-4D CEST spectroscopy. Angew Chemie Int Ed 54:10507–10511. https://doi.org/10.1002/anie.201504070
Lundström P, Teilum K, Carstensen T et al (2007) Fractional 13C enrichment of isolated carbons using [1-13C]- or [2-13C]-glucose facilitates the accurate measurement of dynamics at backbone Cα and side-chain methyl positions in proteins. J Biomol NMR 38:199–212. https://doi.org/10.1007/s10858-007-9158-6
Lundström P, Hansen DF, Vallurupalli P, Kay LE (2009) Accurate measurement of alpha proton chemical shifts of excited protein states by relaxation dispersion NMR spectroscopy. J Am Chem Soc 131:1915–1926. https://doi.org/10.1021/ja807796a
Mäntylahti S, Hellman M, Permi P (2011) Extension of the HA-detection based approach: (HCA)CON(CA)H and (HCA)NCO(CA)H experiments for the main-chain assignment of intrinsically disordered proteins. J Biomol NMR 49:99–109. https://doi.org/10.1007/s10858-011-9470-z
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange in proteins. J Magn Reson 85:393–399. https://doi.org/10.1016/0022-2364(89)90152-2
Millet O, Muhandiram DR, Skrynnikov NR, Kay LE (2002) Deuterium spin probes of side-chain dynamics in proteins. 1. Measurement of five relaxation rates per deuteron in 13 C-labeled and fractionally 2H-enriched proteins in solution. J Am Chem Soc 124:6439–6448. https://doi.org/10.1021/ja012497y
Mittermaier A, Kay LE (2006) New tools provide new insights in NMR studies of protein dynamics. Science 312:224–228. https://doi.org/10.1126/science.1124964
Muhandiram DR, Yamazaki T, Sykes BD, Kay LE (1995) Measurement of 2H T1 and T1ρ. relaxation times in uniformly 13C-labeled and fractionally 2H-labeled proteins in solution. J Am Chem Soc 117:11536–11544. https://doi.org/10.1021/ja00151a018
Nakayama K, Ohashi R, Shinoda Y et al (2017) RNG105/caprin1, an RNA granule protein for dendritic mRNA localization, is essential for long-term memory formation. Elife. https://doi.org/10.7554/eLife.29677
Okuno Y, Szabo A, Clore GM (2020) Quantitative interpretation of solvent paramagnetic relaxation for probing protein-cosolute interactions. J Am Chem Soc 142:8281–8290. https://doi.org/10.1021/jacs.0c00747
Palmer AG (2014) Chemical exchange in biomacromolecules: past, present, and future. J Magn Reson 241:3–17. https://doi.org/10.1016/j.jmr.2014.01.008
Pervushin K, Riek R, Wider G, Wüthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA 94:12366–12371. https://doi.org/10.1073/pnas.94.23.12366
Sekhar A, Rosenzweig R, Bouvignies G, Kay LE (2016) Hsp70 biases the folding pathways of client proteins. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1601846113
Sørensen OW, Eich GW, Levitt MH et al (1984) Product operator formalism for the description of NMR pulse experiments. Prog Nucl Magn Reson Spectrosc 16:163–192. https://doi.org/10.1016/0079-6565(84)80005-9
Sun H, Kay LE, Tugarinov V (2011) An optimized relaxation-based coherence transfer NMR experiment for the measurement of side-chain order in methyl-protonated, highly deuterated proteins. J Phys Chem B 115:14878–14884. https://doi.org/10.1021/jp209049k
Teilum K, Brath U, Lundström P, Akke M (2006) Biosynthetic 13C labeling of aromatic side chains in proteins for NMR relaxation measurements. J Am Chem Soc 128:2506–2507. https://doi.org/10.1021/ja055660o
Tugarinov V, Clore G (2021) The measurement of relaxation rates of degenerate 1H transitions in methyl groups of proteins using acute angle radiofrequency pulses. J Magn Reson 330:107034. https://doi.org/10.1016/j.jmr.2021.107034
Tugarinov V, Kay LE (2005) Methyl groups as probes of structure and dynamics in NMR studies of high-molecular-weight proteins. ChemBioChem 6:1567–1577. https://doi.org/10.1002/cbic.200500110
Vallurupalli P, Bouvignies G, Kay LE (2013) A computational study of the effects of 13C–13C scalar couplings on 13C CEST NMR spectra: towards studies on a uniformly 13C-labeled protein. ChemBioChem 14:1709–1713. https://doi.org/10.1002/cbic.201300230
Vold RR, Vold RL (1976) Transverse relaxation in heteronuclear coupled spin systems: AX, AX2, AX3, and AXY. J Chem Phys 64:320–332. https://doi.org/10.1063/1.431924
Werbelow LG, Grant DM (1977) Intramolecular dipolar relaxation in multispin systems. In: Advances in magnetic and optical resonance, pp 189–299
Wishart DS, Bigam CG, Holm A et al (1995) 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J Biomol NMR 5:67–81. https://doi.org/10.1007/BF00227471
Wong LE, Kim TH, Muhandiram DR et al (2020a) NMR experiments for studies of dilute and condensed protein phases: application to the phase-separating protein CAPRIN1. J Am Chem Soc 142:2471–2489. https://doi.org/10.1021/jacs.9b12208
Wong LE, Kim TH, Rennella E et al (2020b) Confronting the invisible: assignment of protein 1HN chemical shifts in cases of extreme broadening. J Phys Chem Lett 11:3384–3389. https://doi.org/10.1021/acs.jpclett.0c00747
Yamazaki T, Muhandiram R, Kay LE (1994) NMR experiments for the measurement of carbon relaxation properties in highly enriched, uniformly 13C,15N-labeled proteins: application to 13Cα carbons. J Am Chem Soc 116:8266–8278. https://doi.org/10.1021/ja00097a037
Ying J, Delaglio F, Torchia DA, Bax A (2017) Sparse multidimensional iterative lineshape-enhanced (SMILE) reconstruction of both non-uniformly sampled and conventional NMR data. J Biomol NMR 68:101–118. https://doi.org/10.1007/s10858-016-0072-7
Yu B, Pletka CC, Pettitt BM, Iwahara J (2021) De novo determination of near-surface electrostatic potentials by NMR. Proc Natl Acad Sci 118:e2104020118. https://doi.org/10.1073/pnas.2104020118
Yu B, Pletka CC, Iwahara J (2022) Protein electrostatics investigated through paramagnetic NMR for nonpolar groups. J Phys Chem B 126:2196–2202. https://doi.org/10.1021/acs.jpcb.1c10930
Yuwen T, Sekhar A, Kay LE (2016) Evaluating the influence of initial magnetization conditions on extracted exchange parameters in NMR relaxation experiments: applications to CPMG and CEST. J Biomol NMR 65:143–156. https://doi.org/10.1007/s10858-016-0045-x
Acknowledgements
We thank Dr. Enrico Rennella (University of Toronto) for help with time-domain fitting of pseudo-4D datasets. Y.T. is supported through a Japan Society for the Promotion of Science Overseas Research Fellowship, an Uehara Memorial Foundation postdoctoral fellowship, and a fellowship from the Canadian Institutes of Health Research (CIHR). A.K.R is grateful to the CIHR for post-doctoral support. This research was funded through grants from the CIHR and the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toyama, Y., Rangadurai, A.K. & Kay, L.E. Measurement of 1Hα transverse relaxation rates in proteins: application to solvent PREs. J Biomol NMR 76, 137–152 (2022). https://doi.org/10.1007/s10858-022-00401-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-022-00401-4