Abstract
NaBi(MoO4)2 mesoporous material was prepared by a simple solvothermal method. Co3O4 and CuO doped NaBi(MoO4)2 composites were prepared by sintering NaBi(MoO4)2 nanoparticles covered with the corresponding nitrate. The NaBi(MoO4)2 and the composites were characterized by XRD, SEM, TEM and nitrogen adsorption–desorption analysis. The results show that NaBi(MoO4)2 was mesoporous structures assembled by random aggregation of nanoparticles with an average grain size of about 31 nm. In the composite, the Co3O4 was obtained and uniformly distributed on the surfaces of NaBi(MoO4)2 nanoparticles to form heterojunctions. The sensor based on pure NaBi(MoO4)2 mesoporous material showed a response value of up to 44.8 for 100 ppm n-butanol at an optimum operating temperature of 260 °C with a fast response and recovery time. The Co3O4-doped NaBi(MoO4)2 composite exhibited superior gas-sensitive properties over the pure NaBi(MoO4)2 mesoporous material. The sensor based on 1.0 wt% Co3O4-doped NaBi(MoO4)2 showed a response value of 63 for 100 ppm n-butanol at 260 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Industrial production activities and building renovation materials release harmful volatile organic compounds (VOCs), including formaldehyde, toluene, acetone, n-butanol and hundreds of other identifiable volatile organic compounds [1]. When the concentration of VOCs is too high, it can endanger human health and life. Therefore, there is a need for effective methods to monitor and detect these harmful gases in real time [2]. Although gas-sensitive sensors made of simple metal oxides such as ZnO, TiO2, SnO2, In2O3 and WO3 have a high response, the poor selectivity possibly leads to false alarms [3]. Therefore, an increasing number of researchers have devoted themselves to studying gas-sensitive sensors based on multi-metal oxide semiconductors [4]. Among them, NiGa2O4 [5], BiVO4, CuBi2O4, Fe2(MoO4)3 [6] and other gallium-based multi-metal oxides, bismuth-based multi-metal oxides and molybdates have been proved to be valuable for gas sensing applications. Qiao et al. [7] found that doping BiVO4 with Mo6+ improved the response value and stability of gas-sensitive sensors based on BiVO4. As a result, gas-sensitive sensors based on Bi2MoO6 and NaBi(MoO4)2 have been gradually developed [8].
NaBi(MoO4)2 has certain catalytic properties and has been investigated for promising applications in the field of luminescent materials and photocatalysis [9, 10]. In recent years, another researcher has discovered the application potential of NaBi(MoO4)2 in the field of gas sensing. Lin et al. [11] first observed the specific response of lamellar NaBi(MoO4)2 to alcohols, especially n-butanol and ethanol. Subsequent studies have reported the gas-sensitive properties of different forms of NaBi(MoO4)2, including doped NaBi(MoO4)2.
Metal ion doping and the construction of heterojunctions are common methods to enhance the gas-sensitive properties of materials [12, 13]. NaBi(MoO4)2 exhibits the gas-sensitive properties of an n-type semiconductor, and metal ion doping or compounding with a p-type semiconductor is expected to enhance the gas response of NaBi(MoO4)2. CuO and Co3O4 are commonly used p-type semiconductors [14]. Sun et al. [15] demonstrated that the CuO-doped In2O3 hollow spheres increased the gas-sensitive response to NO2 from 4.7 to 859 at 100 °C. Yuan et al. [16] prepared 3.0 wt% Co–ZnO porous nanosheets, which exhibited a response value of 27.93 to toluene at 400 °C, 2.5 times higher than that of pure ZnO.
In this paper, NaBi(MoO4)2 mesoporous materials were prepared by the solvothermal method, and CuO doped and Co3O4 doped NaBi(MoO4)2 composites were synthesized through annealing with doping with the corresponding salts. The gas-sensing performances of the sensors fabricated from these three materials were investigated, revealing that the Co3O4 doping effectively improved the response of NaBi(MoO4)2.
2 Experimental section
2.1 Reagents
Bismuth nitrate pentahydrate, cobalt nitrate hexahydrate, cupric nitrate dihydrate, sodium molybdate dihydrate, nitric acid, ethanol and ethylene glycol were purchased from Sinopharm Chemical Reagent Co. All chemicals are of analytical grade and were used without further treatment.
2.2 Preparation of NaBi(MoO4)2 nanorods and their composites
0.001 mol of Bi(NO3)3 and Na2MoO4 were dissolved in a mixture of 8 mL of ethylene glycol and 10 mL of anhydrous ethanol, respectively. The Bi(NO3)3 solution was added drop by drop to the Na2MoO4 solution under magnetic stirring to obtain a milky white suspension. Then 20 mL of 0.18 mol/L dilute nitric acid was added and transferred to an autoclave with sufficient stirring and the reaction was carried out hydrothermally at 170 °C for 12 h. After cooling to room temperature, the product was washed, dried and transferred to a muffle furnace and calcined at 400 °C for 30 min to produce NaBi(MoO4)2. When doping, a certain mass ratio of cobalt nitrate or copper nitrate is weighed before sintering, a small amount of deionized water is dissolved and dropped into 100 mg of NaBi(MoO4)2 respectively, ground well, dried sufficiently and sintered at 450 °C for 30 min to obtain NaBi(MoO4)2 doped with 1.0 wt% Co3O4 and 1.0 wt% CuO.
2.3 Characterization
The crystallite phase of the NaBi(MoO4)2 was characterized by XRD, the microscopic morphology was characterized by SEM and TEM, and the pore size distribution and specific surface area of the material were analyzed by BET. A Bruker D8 Advance X-ray diffraction was used to characterize the composition of the material with a scan width of 20–80° and a scan speed of 4°/min. The average particle size of the samples was calculated using the Debye Scherrer formula d = kλ/Bcosθ, here, k is constant with the value of 0.9, λ is the wavelength of X-ray of Cu kα1 of 0.15418 nm, B is the corrected full width at half maximum (FWHM) of the X-ray diffraction peak, obtained by subtracting the instrumental contribution from the measured FWHM, and θ is Bragg diffraction angle. A Hitachi S4800X scanning electron microscope was used for the scanning electron microscopy (SEM). Transmission electron microscope (TEM) and specific surface and pore size tester (BET) were adopted from JEOL, Japan, Model JEM-2100 transmission electron microscope and Conta, USA, Model NOVA 2000e BET tester, respectively.
2.4 Fabrication and measurement of gas sensor
The sensing properties were tested and recorded using WS-30 A static gas-sensing system (Zhengzhou Winsen Electronics Technology, China) under room temperature and a humid range of 35–60%. The sensor was a side-heated structure as shown in Fig. 1a, and Fig. 1b shows a schematic diagram of the gas-sensing test setup. The ceramic tubes had a length of 4 mm, an external diameter of 1.2 mm and an internal diameter of 0.8 mm. Au electrodes were ringed to both ends of the ceramic tubes, and two platinum wires were attached to each electrode. 0.02 g of the three materials were weighed separately, ground to a thick consistency with anhydrous ethanol, applied dropwise to the ceramic tube and then soldered to the base. A heating wire was threaded through the middle of the ceramic tube to ensure that the resistance at both ends of the heating wire was approximately 28 Ω. To obtain good stability, the devices were aged at 350 °C for 12 h prior to testing. NaBi(MoO4)2 as an n-type semiconductor, its response was expressed by \(S={{Ra} \mathord{\left/ {\vphantom {{Ra} {Rg}}} \right. \kern-0pt} {Rg}}\), where Ra denotes the resistance of the sensor in air, and Rg denotes the resistance when exposed to a specific concentration of the target gas. The time required to reach a 90% change in the resistance of the element was defined as the response time or recovery time in the case of the target gas.
3 Results and discussion
3.1 Structure and morphology
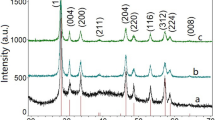
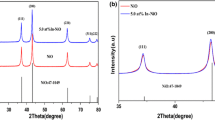
The XRD pattern of the produced materials (as shown in Fig. 2) clearly show that the diffraction peaks closely match the JCPD standard card for NaBi(MoO4)2 (JCPDS:51-1508). The diffraction peaks of CuO doped and Co3O4 doped NaBi(MoO4)2 are basically identical to the pure NaBi(MoO4)2, indicating that the crystalline of NaBi(MoO4)2 was not significantly changed when doped by Cu or Co ion through sintering. The average grain size of the pure NaBi(MoO4)2, CuO doped NaBi(MoO4)2 and Co3O4 doped NaBi(MoO4)2 were 31 nm, 32 nm, and 36 nm, respectively, calculated by the Debye Scherrer formula with the peak of (112), (004), (200) and (204). In the XRD patterns, the diffraction peaks of CuO and Co3O4 were not detected, which should be attributed to the relatively low doping concentration beyond the detection range of XRD.
The morphology of the pure NaBi(MoO4)2 and Co-doped NaBi(MoO4)2 are shown in Fig. 3. It can be found that the NaBi(MoO4)2 nanoparticles exhibit random distribution. The nanoparticle size of Co-doped NaBi(MoO4)2 ranges from 25 to 50 nm, which is larger than the size of particles in pure NaBi(MoO4)2 ranging from 15 to 40 nm. It should be ascribed to the annealing treatment for Co3O4 formation at 450 °C for 30 min. The NaBi(MoO4)2 nanoparticles grew up in the process. Figure 3c shows the EDS-mapping of Co3O4 doped NaBi(MoO4)2, it can be found that Co, Bi and O are uniformly distributed in the composite. It implies the Co3O4 are dispersed evenly and covered on the surfaces of NaBi(MoO4)2 nanoparticles. Since the distribution maps of Bi and Co do not overlap, it indicates the existence of Co3O4 phases in the doped NaBi(MoO4)2 composite, rather than a solid solution.
The TEM images of Co3O4 doped NaBi(MoO4)2 and pure NaBi(MoO4)2 are shown in Fig. 4. It’s clear that the NaBi(MoO4)2 nanoparticles possess well-defined edges and exhibit a diverse range of shapes, including round, oval, and short rods. These findings suggest that the synthesis process resulted in the formation of NaBi(MoO4)2 particles with distinct morphological features and size variations. Furthermore, the particles maintained their individual characteristics throughout the synthesis process. In the Fig. 4a and b, there are some small black spots (less than 5 nm) on the surfaces of NaBi(MoO4)2, it should be attributed to the Co3O4 formation in the Co3O4 doped NaBi(MoO4)2 composite, which are not found in pure NaBi(MoO4)2 (Fig. 4c and d).
The nitrogen adsorption–desorption isotherm and pore size distribution curve of the undoped NaBi(MoO4)2 nanomaterials are shown in Fig. 5. The isotherm exhibits a “type IV” isotherm, and the hysteresis line P/P0 of the samples is within the range of 0.80–0.99, which correspond to the IUPAC classification H3 [17]. These results indicate that the NaBi(MoO4)2 mesoporous material possesses a significant porosity. The pore size distribution analysis reveals that the pores in the NaBi(MoO4)2 mesoporous material range from 5 to 61 nm, with an average pore size of 21.5 nm. Furthermore, the nanoparticle material exhibits a pore volume of 0.125 cm3/g. The specific surface area of NaBi(MoO4)2 mesoporous material is determined to be 42.37 m2/g using the multi-point BET method. This indicates a relatively high surface area, which can contribute to increased surface interactions and adsorption capabilities of the material.
3.2 Gas-sensing properties of NaBi(MoO4)2 mesoporous material
Figure 6a shows the resistance of the NaBi(MoO4)2 and doped NaBi(MoO4)2 based sensors as a function of temperature in an air atmosphere. All sensors showed high resistance (> 109 Ω) at low temperatures, which decreased by about an order of magnitude as the operating temperature increases from 260 to 370 °C. Both Cu-doping and Co-doping increase the resistance of NaBi(MoO4)2. This may be attributed to the CuO, Co3O4 formation on the surfaces of NaBi(MoO4)2 nanoparticles after annealing treatment. Since CuO and Co3O4 are p-type semiconductors and NaBi(MoO4)2 is an n-type semiconductor, the p–n junction will form when the two types of nanoparticles contact. The p–n junctions will prevent electron migration, so the resistances of the doped sensors increase.
From Fig. 6b and c, it is evident that the sensors based on pure NaBi(MoO4)2 and Co3O4-doped NaBi(MoO4)2 show high response to both 100 ppm n-butanol and ethanol. The highest response values for pure NaBi(MoO4)2 were 37.7 and 44.8 for ethanol and n-butanol, respectively. The highest response values for Co3O4-doped NaBi(MoO4)2 were 41.7 and 63.0 for ethanol and n-butanol, respectively. The response showed a decrease with increasing temperature and did not follow the typical trend of increasing to a maximum and then decreasing with increasing temperature as for gas-sensitive sensors [18]. The reason is that the resistance of the sensors in the air is beyond the detection range of the test system when the temperature is below 260 °C, which makes it difficult to measure the response value below 260 °C. Therefore, the optimum operating temperature for pure NaBi(MoO4)2 and doped NaBi(MoO4)2 is noted as 260 °C. The curves of the sensors to formaldehyde and acetone conform to the variation of the response of the gas-sensitive sensor with temperature, as shown in Fig. 6b. Compared to the reported NaBi(MoO4)2 based sensors, the responses and working temperature are listed in Table 1, the Co3O4-doped NaBi(MoO4)2 prepared in this paper demonstrates the highest response to n-butanol and worked at the lowest temperature.
As can be seen from Fig. 6d, although the optimum operating temperature of the CuO-doped NaBi(MoO4)2 for alcohol gases remains unchanged, the response values to all gases are substantially reduced. The highest response values for CuO-doped NaBi(MoO4)2 were 5.4 and 8.7 for ethanol and n-butanol, which were significantly less than pure NaBi(MoO4)2.
Figure 7 shows the response of the three sensors for 20–2000 ppm ethanol and 20–1300 ppm n-butanol at 260 °C as a function of concentration. The response shows a curvilinear increase with increasing test concentration and presents an unsaturated state. This can be described by the equation \(S=a{\left( {Cg+1} \right)^b}\), where Cg represents the concentration of the test gas, and a and b are constants [21]. Table 2 shows the values of constants a and b of the concentration fitting curves for the response of the three materials to the two gases. For the ethanol concentration-response curves, the b values of the pure NaBi(MoO4)2, Co3O4-doped NaBi(MoO4)2 and CuO-doped NaBi(MoO4)2 were 0.49928, 0.51422 and 1.56748, while for the n-butanol concentration, the b values were 0.4905, 0.5298 and 0.85267, respectively. According to the quantitative interpretation of b-values proposed by Yamazoe and Barsan, when the b-values are 0.5 and 1, the oxygen species adsorbed on the surface of the corresponding materials predominantly are O2− and O−, respectively [22]. It implies that the oxygen species were O2− in the pure NaBi(MoO4)2 and Co3O4-doped NaBi(MoO4)2 sensors. The CuO-doped NaBi(MoO4)2 sensor were different gas reaction mechanisms, and the responses of the CuO-doped NaBi(MoO4)2 sensors were significantly lower than those of the pure NaBi(MoO4)2 sensor.
Figure 8a shows the response of the sensors made from the three materials for eight different gases at 260 °C. The sensors based on pure NaBi(MoO4)2 and Co3O4-doped NaBi(MoO4)2 showed higher responses to alcohols, especially n-butanol and ethanol, and lower responses to non-alcohols such as formaldehyde, acetone, toluene and xylene. The response to 100 ppm n-butanol (S = 63.0) was approximately 1.5, 2.8, 5.7, 5.4, 7.5, 8.2 and 2.3 times higher than that to ethanol (41.7), methanol (22.5), formaldehyde (11), acetone (11.6), toluene (8.4), xylene (7.7) and iso-propanol (18.2). Figure 8b shows the response-recovery behavior of the sensors to 100 ppm n-butanol. The Co3O4-doped sensor shows very fast response (7 s) and recovery (10 s) properties. Moreover, sensors based on pure NaBi(MoO4)2, CuO-doped and Co3O4-doped NaBi(MoO4)2, whether positively or negatively doped, exhibit fast response and recovery rates.
4 Conclusions
In summary, the NaBi(MoO4)2 mesoporous material was successfully synthesized by a solvothermal method and doped with Co and Cu oxide. The NaBi(MoO4)2 mesoporous material exhibited random aggregation with an average particle size of approximately 31 nm. The introduction of copper oxide led to a decrease in the response of the NaBi(MoO4)2 sensor. On the other hand, the addition of a small amount of cobalt as a dopant increased the response of the NaBi(MoO4)2 sensor. Specifically, the 1.0 wt% Co3O4-doped NaBi(MoO4)2 nanoparticles sensor showed a high response to n-butanol (S = 63) at 260 °C.
Data Availability
All data generated or analysed during this study are included in this published article.
References
H. Wang, L. Nie, J. Li et al., Chin. Sci. Bull. 58, 724 (2013). https://doi.org/10.1007/s11434-012-5345-2
F. Teimoori, K. Khojier, N.Z. Dehnavi, J. Theor. Appl. Phys. 11, 157 (2017). https://doi.org/10.1007/s40094-017-0253-0
N. Bârsan, J. Phys. Condens. Matter 15, R813 (2003). https://doi.org/10.1088/0953-8984/15/20/201
J.W. Fergus, Sens. Actuators B 123, 1169 (2007). https://doi.org/10.1016/j.snb.2006.10.051
H. Chen, S. Ao, G.-D. Li, Q. Gao, X. Zou, C. Wei, Sens. Actuators B 282, 331 (2019). https://doi.org/10.1016/j.snb.2018.11.072
Y.-Y. Wu, Y. Teng, M. Zhang et al., Sens. Actuators B 329, 129126 (2021). https://doi.org/10.1016/j.snb.2020.129126
X. Qiao, Y. Xu, K. Yang et al., Chem. Eng. J. 395, 125144 (2020). https://doi.org/10.1016/j.cej.2020.125144
S. Liu, Y. Qin, Y. Bai, Electrochim. Acta. 427, 140863 (2022). https://doi.org/10.1016/j.electacta.2022.140863
M. Isik, I. Guler, Mat. Sci. Semicon. Proc. 108, 104875 (2023)
J.H. Xie, L.Q. Cheng, H. Tang et al., Opt. Laser Technol. (2022). https://doi.org/10.1016/j.optlastec.2021.107659
Z. Lin, M. Xu, Y. Hong, X. Wang, P. Fu, Mater. Lett. 168, 72 (2016). https://doi.org/10.1016/j.matlet.2016.01.038
A. Moumen, G.C.W. Kumarage, E. Comini, Sensors Basel (2022). https://doi.org/10.3390/S22041359
L.M. Maebana, R.G. Motsoeneng, Z.P. Tshabalala et al., J. Alloy Compd. (2023). https://doi.org/10.1016/j.jallcom.2023.170683
H.-J. Kim, J.-H. Lee, Sens. Actuators B 192, 607 (2014). https://doi.org/10.1016/j.snb.2013.11.005
C. Zhang, Y. Huan, D. Sun, Y. Lu, J. Alloys Compd. 842, 155857 (2020). https://doi.org/10.1016/j.jallcom.2020.155857
Z. Yuan, Z. Feng, L. Kong, J. Zhan, X. Ma, J. Alloys Compd. 865, 158890 (2021). https://doi.org/10.1016/j.jallcom.2021.158890
C. Zhu, F. Du, P. Fu, S. Wang, Z. Lin, Mater. Res. Express. 5, 125016 (2018). https://doi.org/10.1088/2053-1591/aae17a
L. Liu, H. Wang, X. Zhang, Z. Lin, Sens. Actuators B 237, 275 (2016). https://doi.org/10.1016/j.snb.2016.06.095
C. Zhu, W. Guo, F. Du, P. Fu, Q. Deng, Z. Lin, Mater. Lett. 220, 172 (2018). https://doi.org/10.1016/j.matlet.2018.03.043
Z. Shang, S. Wang, Z. Lin, L. Liu, F. Chi, X. Zhang, J. Mater. Sci. Mater. Electron. 31, 22143 (2020). https://doi.org/10.1007/s10854-020-04716-6
W. Wang, Y. Tian, X. Wang et al., J. Mater. Sci. 48, 3232 (2013). https://doi.org/10.1007/s10853-012-7103-x
N. Barsan, U. Weimar, J. Electroceram. 7, 143 (2001). https://doi.org/10.1023/A:1014405811371
Funding
This work was financially supported by the National Natural Science Foundation of China (62075035), the Guangdong Basic and Applied Basic Research Foundation, (2021B1515420001), the Engineering Technology Research Center of Colleges and Universities in Guangdong Province (2021GCZX005) and the Innovation Team of Colleges and Universities in Guang-dong Province (2021KCXTD040)
Author information
Authors and Affiliations
Contributions
XL: Conceptualization, Experimental, Data curation, writing—Original Draft. ZL: Project administration, Formal analysis, Review & Editing, Supervision. LL: Funding acquisition; Resources, Review & Editing. XZ: Resources, review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest or competing interests and have no relevant financial or non-financial interests to disclose
Research involving human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Lin, Z., Liu, L. et al. Preparation and gas sensing properties of NaBi(MoO4)2 mesoporous material and its Co3O4 doped composites. J Mater Sci: Mater Electron 34, 1839 (2023). https://doi.org/10.1007/s10854-023-11248-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11248-2