Abstract
In this work, ordered mesoporous structures of In2O3-decorated NiO were prepared by a two-step process, comprising of the synthesis of ordered mesoporous NiO followed by injection of In3+ into their pores. The pore size distribution of the as prepared samples was between 4.1 and 21.1 nm. Furthermore, their sensing performances toward NO2 were tested systematically. The results showed the highest response about 3 towards 15 ppm NO2 sensing at room temperature for 5.0 at.% In2O3-decorated NiO compared to other decorated and pure samples. Moreover, the sensor displayed excellent selectivity towards NO2 in the presence of other interfering gases, such as carbon monoxide, ammonia, ethanol, methanol, formaldehyde, toluene, acetone. The exceptional NO2 sensing performance of the In2O3-decorated mesoporous NiO may be attributed to their high specific surface area and the formation of p–n junction with modified carrier concentration caused by In3+ doping. This method can act as an effective strategy for enhancement of gas-sensing properties of pure metal oxides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the current times, air pollution has caused a great deal of harm to human health. NO2 being a major air pollutant, causes adverse effects on plants and respiratory system of human beings and animals. Moreover, as a source of photochemical smog and acid rain, it causes serious environmental damages [1]. In addition, asthma diagnosis can be performed by detecting the concentration of NO2 in the air exhaled by patients [2]. Therefore, the detection of NO2 has attracted tremendous interest in the context of health and environment monitoring and thus, it is necessary to develop a NO2 gas sensor with excellent properties, including low detection limit concentration, high sensitivity, good selectivity, and low cost. Metal oxide semiconductors (MOSs) have been widely used in the detection of a variety of toxic and harmful gases owing to its high stability, low cost, and convenience [3].
Nickel oxide(NiO), a typical p-type semiconductor with a wide bandgap (3.6–4.0 eV), due to its high chemical and thermal stability, unique magnetic, optical, catalytic, and electrical properties, has been widely applied in many areas, such as chip fabrication [4], medical diagnosis [5], fabrication of electrochemical supercapacitors [6], catalytic combustion [7], and gas sensing [8]. As a matter of fact, studies on n-type semiconductors have been far more in number than those corresponding to p-type materials [9]. Therefore, it is necessary to explore and develop p-type oxide semiconductor gas sensors which possess advantages of high catalytic activity and enhanced stability.
However, to the best of our knowledge, there are only a few reports on gas sensing studies of NiO at room temperature and the response in these reports is limited [10,11,12]. Hence, it is still essential and challenging to develop a low cost and effective gas sensitive material based on NiO.
Morphology are very significant for the detection of materials. Afkhami et al. successfully attached NiFe2O4 nanoparticles to Gr for detecting tramadol and acetaminophen [13]. Bagheri et al. synthesized Fe3O4–SnO2–Gr ternary nanocomposite with the formation of uniform and monodispersed Fe3O4–SnO2 for the detection of dopamine, uric acid and ascorbic acid [14]. And Gas-sensitive materials also require proper structure.
In general, the gas sensing performance of metal oxide semiconductors is usually determined by three factors, namely, receptor function, transducer function, and utility factor [15]. In short, the first factor is related to the response of each crystal, the second to electronic transport between adjacent crystals, and the third to effective diffusion and recognition of gas molecules on the reactive surface. Therefore, enhancing adsorption, diffusion of target gas, and mobility of charge carriers would benefit the performance of these gas sensors. Li et al. synthesized 3D Ni(OH)2 and NiO nanowalls for HCHO and NO2 sensing [16]. Miao et al. reported the preparation of NiO flower-like microspheres with abundant nanoparticles adhering to the petals, enabling the detection of ethanol [17]. Nguyen et al. found that NO2 detection can be achieved by mesoporous NiO nanosheets [18]. Moreover, doping other metal elements is also an effective means to enhance the gas-sensing response. Sun and coworkers reported the fabrication of Fe-doped ordered mesoporous NiO based sensors for detecting VOCs (volatile organic compounds) [19]. Tian et al. demonstrated the synthesis of Lotus pollen derived 3D hierarchically porous NiO microspheres for NO2 sensing and Kim et al. discovered the superior ethanol sensing properties of In2O3-decorated NiO [20, 21]. Considering the high cost of noble metals, it makes more sense to improve gas sensing performance by doping base metals. Thirdly, composite reduced graphene and mesoporous carbon are favorable for electron transport [22, 23].
Based on the literature survey as presented above, in this work, we designed the synthesis of ordered mesoporous NiO with large specific surface area, small pore, and particle size, followed by the injection of In3+ ions. Hence, the In2O3-decorated ordered mesoporous NiO was successfully prepared via a facile two-step process. For the synthesis of ordered mesoporous NiO, 3D cubic mesoporous KIT-6 silica was used as a hard template. And it was found that it was In doping that greatly enhanced the sensitivity of pure NiO to NO2. Furthermore, we found that the 5.0 at.% In2O3-decorated NiO exhibited an excellent selectivity toward NO2 at room temperature.
2 Experimental
2.1 Material preparation
2.1.1 Synthesis of KIT-6
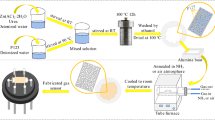
The synthesis of KIT-6 was carried out according to a previously reported procedure [24]. About 6 g of P-123 was dissolved in 11.8 g of HCl(35%) and 217 g of H2O. Then, 6 g of n-butanol was added and the solution was stirred for 1 h at 35 °C. Then, 12.9 g of tetraethyl orthosilicate (TEOS) was added, and the resultant solution was stirred for 24 h at 35 °C. It was then transferred to a Teflon-lined stainless steel autoclave and placed in an oven at 70 °C for 24 h. The resulting precipitate was filtered, washed, with deionized water, and dried at 550 °C at a ramp rate of 2 °C/min and calcined at this temperature in air for 5 h.
2.1.2 Synthesis of mesoporous NiO
The ordered mesoporous NiO was synthesized with the hard template named KIT-6, which was previously reported by Sun et al [25]. Typically,0.6 g of the KIT-6 silica template was dispersed in 20 ml of ethanol solution containing 1.45 g nickel nitrate hexahydrate (Ni(NO3)2·6H2O). The mixture under vigorous stirring was heated to 65 °C and kept at the temperature for 4 h. Due to the capillary condensation effect between the two substances, the Ni2+ can permeate into the template pores. Afterwards, the resulting sample was harvested by centrifugation and wash with distilled water for several times. Then, the as-obtained sample was transferred into a furnace and heated up to 500 °C at a ramp rate of 2 °C/min and calcined at this temperature in air for 3 h to vary the nickel nitrate precursors to NiO. To remove the silica template, the resulting NiO@KIT-6 powders were handled with 2 M NaOH solution. The precipitate was recovered by centrifugation and washing alternately with deionized water and ethanol for 3 times, respectively.
2.1.3 Synthesis of In-decorated mesoporous NiO
The periodic ordered NiO materials decorated with In2O3 were synthesized by an incipient-wetness impregnation method [26]. In a typical procedure, the as-prepared NiO materials were dispersed in 30 mL deionized water, then a certain amount of In(NO3)3·4.5H2O (the molar ratios of In/Ni were 2.0, 5.0, 10.0 at.%, respectively) were added into the dispersion. Following impregnation, the obtained powders were kept at room temperature for 24 h, dried at 80 °C in air overnight, and then calcined at 500 °C for 3 h. The calcination temperature was risen from room temperature at a heating rate of 2 °C/min. These specimens are referred to as “2.0 at.% In–NiO” and “5.0 at.% In–NiO” and “10.0 at.% In-NiO”.
2.2 Characterization
The crystalline phases of the powder samples was analyzed by the wide-angle X-ray diffraction(XRD, Shimadzu XRD-6000) using CuKα1 radiation (λ = 0.15406 nm) at 40 kV and 20 mA. The XRD pattern were obtained from 20° to 80° with a scanning rate of 5°/min. The morphology and crystal structure were performed by transmission electron microscopy(TEM, JEOL, JEM-2010). The doped compositions of the samples were examined by the energy dispersive X-ray spectrometers(EDS, JEOL, JSM-5510LV). Moreover, the mesoporous characteristics of the pure NiO and In-doped NiO were examined by nitrogen physisorption isotherm techniques on a Micromeritics ASAP2020 system. The specific surface areas of samples were evaluated using the Brunauer–Emmett–Teller (BET) method. The electronic structure of the surface of 5.0 at.% In-dope NiO was investigated by X-ray photoelectron spectroscopy(XPS, K-Alpha).
2.3 Fabrication and gas-sensing measurement
The as-prepared products were ground into fine powders and mixed with ethanol to form paste. Then, the paste was dropped onto an Al2O3 ceramic plate substrate installed previously with a pair of gold electrodes on the front side to form a sensing film. Afterwards, the as-prepared sensing layer was dried at 80 °C for 2 h, followed by annealing at 500 °C for another 3 h. The gas-sensing properties were examined in a steel-made chamber. A mixture of compressed dry air and target gas flowed at atmospheric pressure and room temperature (ca. 25 °C) on the condition that the relative humidity in the chamber was about 10%. The concentration of target gas was changed by controlling the flow ratio of the two gases using mass flowmeters. The tested gas with given concentration ran into the chamber and the response of sensors were measured. Due to hardly reach a steady equilibrium signal for the sensors exposed to the certain concentration of NO2 gas, thus every response event was fixed at 200 s. The gas response was defined as S = Ra/Rg when the gas was oxidizing. On the contrary, the gas response was defined as S = Rg/Ra when the gas was reductive. Ra is defined as the resistance of the gas sensor in the air, Rg is defined as the resistance of the gas sensor in the target gas.
3 Results and discussion
3.1 Morphology and structure
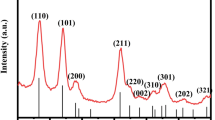
Figure 1a shows the wide-angle X-ray diffraction (XRD) patterns for the pure NiO and the 5.0 at.% In–NiO. All the diffraction peaks of the pure sample could be assigned to the face-centered cubic structure of NiO (JCPDS No.47-1049). Furthermore, the radius of In3+ is 0.08 nm and the radius of Ni2+ is 0.069 nm at a coordination number of 6, obviously, the former is larger than the latter. Therefore, the peaks shift can be attributed to In doping. Figure 1b shows the (111) and (200) diffraction peaks of 5.0 at.% In–NiO is shifted by about 0.05° to the left, which indicates the absence of In-related peaks can be explained by the entry of In3+ into the lattice of NiO or the existence of the secondary phase which is below the detection limit of XRD [19]. And the XRD patterns of 5.0 at.% In–NiO were almost same as those of pure NiO, suggesting that the In species might be homogenously dispersed in the NiO matrix.
Figure 2a, b show the transmission electron microscopy (TEM) images of the pure NiO, and the TEM image of the 5.0 at.% In–NiO is shown in Fig. 2c, respectively. TEM was used to analyze the mesoporous structure of the samples and the dispersion of the dopants on the support. The samples studied with TEM were suspended in ethanol and drop casted on a copper grid. The TEM studies reveal the presence of highly periodic cubic (Ia3d) mesostructures in the NiO samples. The energy dispersive spectra (EDS) (Fig. 2d) recorded on the 5.0 at.% In–NiO sample indicates the presence of the main elements i.e. Ni, O, and In.
X-ray photoelectron spectroscopy (XPS) was conducted to analyze the surface compositions and chemical states of the as-synthesized In2O3-decorated NiO, and the results are shown in Fig. 3. Figure 3a exhibits the XPS survey spectrum of the 5.0 at.% In–NiO nanocomposite, which clearly reflects the peaks corresponding to Ni, O, C, and In. Furthermore, the high-resolution scans of Ni 2p, In 3d, and O 1s are shown in Fig. 3b–d. As shown in Fig. 3b, the Ni 2p signal could be divided into five peaks. Consequently, the binding energies could be obtained as 854.0 and 855.8 eV corresponding to the Ni 2p3/2 peak, and its satellite peak could be observed at 861.0 eV. The peaks corresponding to 872.8 and 869.6 eV were assigned to Ni 2p1/2, and its satellite, respectively. The Ni 2p3/2 peak at 854.0 eV was assigned to Ni2+, while the one at 855.8 eV was attributed to the presence of Ni3+, caused by In doping [21, 27, 28]. In addition, the Ni 2p1/2 peaks were also attributed to Ni3+. The In 3d spectrum (Fig. 3c) was composed of two obvious peaks at 444.7 and 452.4 eV, corresponding to In 3d5/2 and In 3d3/2 of In2O3, respectively. Hence, combined with XRD results, it indicates that a part of In entered NiO lattice and the other part of In became In2O3. Figure 3d, the O 1s binding energy peaks whose shapes are asymmetric are located at 529.6 and 531.5 eV, respectively, corresponding to the lattice oxygen in crystalline NiO and chemisorbed oxygen species on the sample surface. The above results further confirmed that In3+ existed as the phase of In2O3 in the 5.0 at.% In–NiO sample.
Figure 4 displays the hysteresis loops recorded at relatively high pressures, which indicate large mesoporous diameters, and the pore size distributions calculated by the BJH method also confirmed the mesoporous structure. The detailed information on the pore structure is listed in Table 1. Two well-defined steps of capillary condensation corresponding to two pore size distributions can be clearly observed. The small one is caused by the hard template replica, substantiating the ordered uniform pore structure, and the bigger arises from the piled porosity caused by the aggregation of nanoparticles [29]. The 5.0 at.% In–NiO has the largest specific surface area and pore volume.
To further confirm the conversion of In(NO3)3 into In2O3, the thermal analysis was investigated. Figure 5 displays the thermogravimetric(TG) curve for the ordered mesoporous NiO after the impregnation with 5.0 at.% In(NO3)3, which reveals two stages of weight loss [30]. The first stage between 30 and 120 °C was ascribed to water from the In(NO3)3 sample, and the weight loss of this stage was about 4%. The second stage between 120 and 600 °C was attributed to the decomposition of In(NO3)3 into In2O3, and the weight loss of this stage was about 7.5%, which is close to the theoretical value (10.1%) within the error. Therefore, the formation of In2O3 phase can be completed by calcination of In(NO3)3.
3.2 Gas sensing properties
Figure 6 shows the room temperature dynamic response-recovery curves of the as-prepared sensor corresponding to 5.0 at.% In2O3-decorated mesoporous NiO as a function of different NO2 concentrations measured at regular intervals of 200 s. The sample displayed a quick response to NO2 before reaching saturation. However, it was observed that desorption of NO2 from the surface of the material was difficult. This could be ascribed to the strong interaction between NO2 and NiO, which is a common problem for NiO based NO2 sensors [31]. Moreover, it was found that the 5.0 at.% In–NiO performed much better compared to the other doped and pure samples. For the sake of comparison, sensors based on pure, 2.0 and 10.0 at.% In2O3-decorated mesoporous NiO were fabricated separately using the same synthesis procedure and their room temperature NO2 sensing properties were studied. The results are shown in Fig. S1 as a Supporting Information.
Furthermore, the reproducibility in sensing performance of the 5.0 at.% composites was investigated and the results have been presented in Fig. 7. Despite the strong adsorption between the NiO and NO2 which causes a considerably long recovery time in air, the sensor shows reproducible good performance under regular cycles of refreshment. All three cycles of response curves present very similar trends in the presence of a NO2 atmosphere.
Furthermore, good selectivity is a desirable characteristic of any sensing device. Hence, the gas response was examined in the presence of various target gases at room temperature, as shown in Fig. 8. The possible interferential gases include carbon monoxide (CO), ammonia(NH3), ethanol(C2H5OH), formaldehyde(HCHO), methanol (CH3OH), toluene (C7H8), and acetone (CH3COCH3). From the results, it was clear that the sensitivity towards NO2 was the highest, despite maintaining a sixfold concentration of other interfering gases. Consequently, 5.0 at.% In2O3-decorated mesoporous NiO showed excellent selectivity towards NO2 at room temperature.
The sensor’s stability was examined at room temperature for a month, and the result is given in Fig. 9. It indicated that the long term stability was also excellent.
3.3 Investigation of gas-sensing mechanism
At first, the sensing layer was composed of NiO and In2O3. The main body of sensing layer was NiO, and In2O3 in small amounts was present on the surface of NiO. Hence, the sensing layer as a whole showed the characteristics of NiO, the role of NiO was to form p-type semiconductor. And the role of In2O3 was to improve the surface activity and adjust the surface hole concentration of NiO, which was derived from the following specific mechanism analysis.
As a typical p-type semiconductor oxide, the widely accepted sensing model of NiO, is based on the modulation of the hole accumulation layer through absorption and desorption of gas molecules [9, 32, 33]. In air, the conduction electrons of NiO were captured via oxygen adsorption on the surface of NiO resulting in the formation of a hole accumulation layer. When the NiO is exposed to oxidizing gases (e.g. NO2 in our case), the surface reaction can be expressed by the following Eqs. (1) and (2) [18, 34]:
Equation 1 shows NO2 molecules capture electrons from the NiO surface, thereby increasing the hole concentration in the hole accumulation layer of NiO. Since hole is a major charge carrier in p-type semiconductor, their increasing concentration will lead to a decrease in the NiO’s resistance. Equation 2 shows adsorbed NO2 molecules can react with the absorbed oxygen species on the surface of NiO, which consumes more electrons.
The enhanced NO2 sensing can be attributed to the following aspects. Firstly, as previously mentioned, the specific surface area increased with raising In2O3 content when the modification amount of In2O3 was not too high. This means In doping improved the surface activity of the sensing material, which is favorable for the adsorption and penetration of NO2. Thus, the surface reactions of Eqs. (1) and (2) are facilitated. Therefore, the enhanced NO2 sensing can be partly explained by the increase of the specific surface area. Secondly, many previous studies have reported that the formation of p–n junctions can enhance the sensor response through changing the width of space-charge layer [35]. The effective electronic interaction of the p–n heterojunction actually occurred between the NiO matrix and In2O3 nanoparticles. As exhibited in Fig. 10, provided the Fermi level of In2O3 is higher than that of NiO [36], electrons will continue to flow from n-type In2O3 to p-type NiO, while the holes will flow in the opposite direction till an equilibrium is reached [37, 38]. Hence, the formation of nanoscale p-n junctions will lead to the creation of the hole depletion layer underneath the n-type In2O3, resulting in the narrowing of the hole accumulation layer for effective conduction. Because the In2O3 content in In-NiO is very low, the dominant NiO phase should determine the sensor resistance. Thus, the shrink of hole accumulation layer leads a higher sensor resistance in air. When the In–NiO sensors are exposed to NO2, the hole will be generated by the process that NO2 molecules capture the electrons on the surface of the composite, which will decrease height barrier of p–n junction, expand the hole accumulation layer and widen the conduction channel of NiO. Consequently, the conductivity of In–NiO is significantly increased and a high response is obtained. Further studies on deep understanding of In2O3-decorated NiO for gas sensing are still needed in view of the fact that there are few reports on this subject.
4 Conclusions
The ordered mesoporous NiO was prepared by utilizing the of mesoporous KIT-6 silica as a hard template in the nano-casting process followed by In doping. Consequently, the In2O3-decorated ordered mesoporous NiO was synthetized successfully. The results of gas sensing measurement manifested the sensor based on the 5.0 at.% In2O3-decorated NiO possessing significantly enhanced gas response for 3–15 ppm NO2 at room temperature. As compared to other In2O3-decorated and pure samples of NiO, this showed the best performance. In addition, it presented good selectivity and acceptable reproducibility towards NO2 sensing. The enhanced NO2 sensing properties of the mesoporous In2O3-decorated NiO sensor is attributed to its higher specific surface area and the formation of p–n junction which modifies the carrier concentration by In doping. This method can be used as an effective strategy to design metal-oxides-based gas sensors with enhanced gas sensing properties.
References
X. Xu, P. Zhao, D. Wang, P. Sun, L. You, Y. Sun, X. Liang, F. Liu, H. Chen, G. Lu, Sens. Actuators B 176, 405–412 (2013)
G.W. Hunter, J.C. Xu, A.M. Biaggi-Labiosa, D. Laskowski, P.K. Dutta, S.P. Mondal, B.J. Ward, D.B. Makel, C.C. Liu, C.W. Chang, R.A. Dweik, J. Breath Res. 5, 37111 (2011)
R.L. Vander Wal, G.W. Hunter, J.C. Xu, M.J. Kulis, G.M. Berger, T.M. Ticich, Sens. Actuators B 138, 113–119 (2009)
H.-M. Sung, C.-J. Chen, W.-S. KO, H.-C. Lin, IEEE Trans. Magn. 30, 4906–4908 (1994)
T.D. Schladt, K. Schneider, H. Schild, W. Tremel, Dalt. Trans. 40, 6315 (2011)
W. Li, Y. Bu, H. Jin, J. Wang, W. Zhang, S. Wang, J. Wang, Energy Fuels 27, 6304–6310 (2013)
G. Bai, H. Dai, J. Deng, Y. Liu, W. Qiu, Z. Zhao, X. Li, H. Yang, Chem. Eng. J. 219, 200–208 (2013)
I. Hotovy, V. Rehacek, P. Siciliano, S. Capone, L. Spiess, Thin Solid Films 418, 9–15 (2002)
H.-J. Kim, J.-H. Lee, Sens. Actuators B 192, 607 (2014)
J. Zhang, D. Zeng, Q. Zhu, J. Wu, K. Xu, T. Liao, G. Zhang, C. Xie, J. Phys. Chem. C 119, 17930–17939 (2015)
J. Zhang, D. Zeng, Q. Zhu, J. Wu, Q. Huang, C. Xie, J. Phys. Chem. C 120, 3936–3945 (2016)
Y.L.T. Ngo, S.H. Hur, Mater. Res. Bull 84, 168–178 (2016)
A. Afkhami, H. Khoshsafar, H. Bagheri, T. Madrakian, Anal. Chim. Acta 831, 50 (2014)
H. Bagheri, N. Pajooheshpour, B. Jamali, S. Amidi, A. Hajian, H. Khoshsafar, Microchem. J. 131, 120 (2017)
N. Yamazoe, K. Shimanoe, J. Sensors 2009, 1–21 (2009)
G. Li, X. Wang, L. Liu, R. Liu, F. Shen, Z. Cui, W. Chen, T. Zhang, Small 11, 731–739 (2015)
R. Miao, X. Yu, W. Zeng, Mater. Lett. 173, 107–110 (2016)
N.D. Hoa, S.A. El-Safty, Chem.-A Eur. J. 17, 12896–12901 (2011)
X. Li, X. Sun, X. Hu, Y. Wang, R. Xiong, X. Li, J. Liu, H. Ji, S. Cai, C. Zheng, J. Phys. Chem. C 119, 3228–3237 (2015)
K. Tian, X.X. Wang, H.Y. Li, R. Nadimicherla, X. Guo, Sens. Actuators B 227, 554–560 (2016)
H.J. Kim, H.M. Jeong, T.H. Kim, J.H. Chung, Y.C. Kang, J.H. Lee, ACS Appl. Mater. Interfaces 6, 18197–18204 (2014)
H. Bagheri, A. Hajian, M. Rezaei, A. Shirzadmehr, J. Hazard. Mater. 324, 762 (2017)
M.K. Rofouei, H. Khoshsafar, R.J. Kalbasi, H. Bagheri, RSC Adv. 6, 13160 (2016)
F. Kleitz, S.H. Choi, R. Ryoo, Chem. Comm. 17, 2136–2137 (2003)
X. Sun, H. Hao, H. Ji, X. Li, S. Cai, C. Zheng, ACS Appl. Mater. Interfaces 6, 401–409 (2014)
F. He, J. Luo, iS. Liu, Chem. Eng. J. 294, 362 (2016)
B. Zhao, X.K. Ke, J.H. Bao, C.L. Wang, L. Dong, Y.W. Chen, H.L. Chen, J. Phys. Chem. C 113, 14440–14447 (2009)
C. Wang, J. Liu, Q. Yang, P. Sun, Y. Gao, F. Liu, J. Zheng, G. Lu, Sens. Actuators B 220, 59–67 (2015)
H. Yang, D. Zhao, J. Mater. Chem. 15, 1217–1231 (2005)
T. Waitz, T. Wagner, T. Sauerwald, C.D. Kohl, M. Tiemann, Adv. Funct. Mater. 19, 653 (2009)
B. Wang, J. Nisar, R. Ahuja, ACS Appl. Mater. Interfaces 4, 5691–5697 (2012)
Y. Shimizu, M. Egashira, MRS Bull. 24, 18–24 (1999)
N. Barsan, C. Simion, T. Heine, S. Pokhrel, U. Weimar, J. Electroceram. 25, 11–19 (2010)
J. Zhao, T. Yang, Y. Liu, Z. Wang, X. Li, Y. Sun, Y. Du, Y. Li, G. Lu, Sens. Actuators B 191, 806–812 (2014)
D.R. Miller, S.A. Akbar, P.A. Morris, Sens. Actuators B 204, 250 (2014)
X. Yong, M.A.A. Schoonen, Am. Mineral. 85, 543–556 (2000)
G. Sun, H. Chen, Y. Li, Z. Chen, S. Zhang, G. Ma, T. Jia, J. Cao, H. Bala, X. Wang, Z. Zhang, Sens. Actuators B 233, 180–192 (2016)
C. Liu, B. Wang, T. Liu, P. Sun, Y. Gao, F. Liu, G. Lu, Sens. Actuators B 235, 294–301 (2016)
Acknowledgements
This work was supported by funding from the National Natural Science Foundation of China (No. 21471120), Natural Science Foundation of Hubei Province (2012IHA00201), Educational Commission of Hubei Province of China (T201306).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, Z., Liu, S. In2O3-decorated ordered mesoporous NiO for enhanced NO2 sensing at room temperature. J Mater Sci: Mater Electron 29, 2645–2653 (2018). https://doi.org/10.1007/s10854-017-8190-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-8190-x