Abstract
The effects of W6+ ion substitution at the Mn site on the structure, vacancy characteristics, and magnetic and dielectric properties of GdMnO3 ceramics synthesized using the solid-state method were investigated. Structural measurements indicate that all GdMn1-xWxO3 ceramics exhibit a single-phase structure, and that the introduction of W6+ ions induces structural distortion. W6+ substitution changes the valence states of Mn and the oxygen vacancy concentration in GdMn1-xWxO3. Experimental results of positron annihilation indicate that the concentration of cation vacancies increases with increasing W6+ concentration. The evolution of temperature- and magnetic field-dependent magnetization curves indicates that appropriate W6+ substitution could evidently improve the magnetization of GdMn1-xWxO3. Dielectric measurements reveal that the W6+-substituted samples exhibit giant dielectric characteristics over a broad frequency range. It was found that the magnetization of GdMn1-xWxO3 has a close relationship with vacancy concentration, structural distortion, and the dilution effect. Further, the giant dielectric constant behavior of GdMn1-xWxO3 ceramics is related to the mixed-valent structure of Mn and the resistivity of grain and grain boundary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
GdMnO3 (GMO) is a typical single-phase multiferroic material with a distorted orthorhombic perovskite structure, which has attracted worldwide attention owing to its special magnetic and ferroelectric structure, and intrinsically strong magnetoelectric coupling [1,2,3,4]. As temperature decreases, GMO shows multiple magnetic transitions owing to the interactions of Mn–Mn, Gd–Gd, and Mn–Gd. It is found that GMO transitions from the paramagnetic to the antiferromagnetic phase at ~ 43 K, followed by a weak ferromagnetic transition at ~ 25 K. A ferroelectric state appears below 25 K owing to the spin canting of Mn3+ and Gd3+ moments [5, 6]. Although the electric and magnetic properties of GMO have been investigated by researchers, the physical mechanism of ferroelectricity generation and complex spin states is still unclear, and the electric and magnetic transition temperature is relatively low.
Recently, various methods have been employed to comprehend the origin of physical properties and enhance the properties of GMO material, such as (1) synthesizing GMO with a novel method [7, 8]; (2) applying external pressures to GMO systems [9]; (3) substituting the Gd site with Y3+, Sr2+, etc., or replacing the Mn site with Fe3+, Cr3+, etc. [10,11,12,13]; (4) introducing cationic vacancies in the crystal lattice [14]; (5) synthesizing GMO nanoparticles [15]; and (6) depositing a GMO film [16]. Among these, ion substitution is an efficient method to modulate the structure and performances of GMO. The Ho3+-substituted Gd1-xHoxMnO3 has been investigated by Zhang et al. [17], who showed that Ho substitution induced a decrease in Mn–O–Mn bond angle and, hence, improved electric polarization. Tiwari et al. [13] explored the influences of Cr substitution on the structure and magnetic performances of GdMn1-xCrxO3, and found that Cr doping induced a structural transformation and a decrease of Jahn–Teller distortion. Further, an increase in the magnetic transition temperature along with magnetization reversal with spin reorientation and magnetic switching was observed in Cr-doped GMO owing to the magnetic interactions between Gd3+, Mn3+, and Cr3+ ions. Herein, the W6+ ion is selected as a dopant owing to its similar ionic radius to Gd3+ [18], and its potential effects on the structural, magnetic, and dielectric properties of GMO.
Vacancy defects can observably influence the electric and magnetic performances of perovskite oxide materials [19]. Therefore, probing the evolution of vacancy characteristics in perovskite oxides is beneficial for exploring inherent physical mechanisms and improving the performances of perovskite oxides. Positron annihilation technology is often used to investigate the vacancies information in material due to its high sensitivity to atomic scale defects [20, 21]. Unfortunately, research on the vacancies characteristics of GMO systems investigated using positron annihilation technology is still lacking. Therefore, using positron annihilation technology to detect vacancy and reveals vacancy evolution in the performance of GMO will be extremely helpful for fundamental research and material applications. Herein, the GdMn1-xWxO3 multiferroic samples were prepared using the solid-state method, and the vacancy information in GdMn1-xWxO3 compounds was characterized using the positron annihilation technology. The influence of W6+ ion replacement on the structural, vacancy, and magnetic and dielectric performance of GdMn1-xWxO3 samples was studied.
2 Experimental details
The GdMn1-xWxO3 (x = 0.00–0.20) ceramics were synthesized using the solid-state synthesis method with Gd2O3 (4 N), Mn3O4 (4 N), and WO3 (4 N) precursors. The starting materials with stoichiometric ratio were mixed and thoroughly ground using an agate mortar to form a homogeneous mixture. The mixtures were calcined in air at 1273 K for 24 h followed by several intermittent grindings for 2 h and then calcined in air again at 1523 K for 24 h. Finally, the calcined mixtures were pressed into cylindrical pellets under 10 MPa and sintered in air at 1623 K for 24 h.
The structural details of the GdMn1-xWxO3 samples were collected using a Brucker D8 Advance X-ray diffractometer (CuKα radiation). The valence states of the specimens were analyzed using X-ray photoelectron spectroscopy (XPS, XSAM800) at room temperature. Raman experiments were conducted using a Renishaw inVia Raman spectrometer using an excitation wavelength of 532 nm. The microstructural characterization of GdMn1-xWxO3 ceramics was detected using a Quanta 250 FEG scanning electron microscope (SEM). The positron annihilation spectroscopy data were collected using a fast-low coincidence system at ~ 298 K. The positron annihilation data could be obtained using the LT 9.0 program. Magnetic measurements between − 5 and 5 kOe were performed using a SQUID magnetometer (Quantum Design, USA) over a temperature range of 5–300 K. The dielectric properties and impedance spectrum of the ceramics were recorded with an Agilent HP 4194 A impedance analyzer in the 100–1 × 107 Hz frequency range.
3 Results and discussion
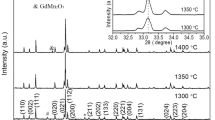
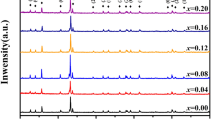
The X-ray diffraction (XRD) patterns of GdMn1-xWxO3 samples are shown in Fig. 1. The peaks of GdMn1-xWxO3 samples can be indexed by orthorhombic crystal structures with the space group Pbnm. This means that the substitution of the W6+ ion at the Mn site does not change the crystal structure of GMO. No secondary phases can be detected in the GdMn1-xWxO3 compounds under XRD resolution, confirming the successful preparation of GdMn1-xWxO3 with a single-phase structure using the solid-state synthesis method. The expansion of XRD patterns in the vicinity of 2θ = 33.1° for GdMn1-xWxO3 ceramics is shown in the inset of Fig. 1. The peak position of 112 for GdMn1-xWxO3 gradually shifts to higher 2θ angles as the substitution of W6+ ions increases, indicating that the W6+ ion has successfully replaced the Mn ion in GMO, causing lattice distortion. The above phenomena could be attributed to the radius of W6+ ion (0.060 Å) being smaller than that of the Mn3+ ion (0.0645 Å) [18].
To understand the charge compensation induced by W6+ substitution, the element valence states and content in the GdMn1-xWxO3 samples were analyzed using XPS. Figure 2a shows the core-level XPS spectra of Mn 2p3/2 for GdMn1-xWxO3 compounds. The symmetrical Mn 2p3/2 band for the unsubstituted GMO implies that only Mn3+ is present in the unsubstituted sample, while the asymmetrical Mn 2p3/2 spectra for the W6+-substituted GMO indicate that Mn exists in several oxidation states in the W6+-substituted samples. It has been reported that the binding energy of Mn2+ and Mn3+ in the Mn 2p3/2 XPS spectrum is located at ~ 640.0 eV and ~ 642.2 eV, respectively [14]. Therefore, the first deconvoluted peak in W6+-substituted ceramics is attributed to Mn2+ (red line), and the other deconvoluted peak corresponds to Mn3+ (blue line). The Mn2+ concentration can be obtained using the following formula:
where A is the corresponding peak area. The Mn2+ concentration in the GdMn0.95W0.05O3, GdMn0.90W0.10O3, and GdMn0.80W0.20O3 samples is ~ 10.0%, 21.8%, and 22.5%, respectively. This means that a certain amount of W6+ substitution introduces more Mn2+ in GdMn1-xWxO3 samples, while the Mn2+ content remains unchanged when x is higher than 0.10. The charge compensation induced by the addition of W6+ ions to GMO at the Mn site may be realized by the conversion of Mn3+ to Mn2+. Therefore, W6+ substitution could increase Mn2+ concentration. Figure 2b depicts the O 1 s XPS spectra for GdMn1-xWxO3 samples. Each spectrum of O 1 s can be deconvoluted into two components: the low binding energy component (OI) at ~ 529.5 eV, attributed to the oxygen ion in the crystal structure, and the higher one (OII) at ~ 531.6 eV that corresponds to oxygen vacancies [22]. The ratio of OII/OI is ~ 0.352, 0.311, 0.306, and 0.185 for the unsubstituted GMO, GdMn0.95W0.05O3, GdMn0.90W0.10O3, and GdMn0.80W0.20O3 samples, respectively. This indicates that the oxygen vacancy content is roughly the same in samples with x values between 0.00 and 0.10, it decreases for the x = 0.20 sample. Charge compensation induced by the addition of W6+ ions to GMO at the Mn site may be realized by a decrease in cation valency (i.e., the formation of Mn2+) and/or anion vacancies. Therefore, W6+ ion substitution can modulate the valence state of Mn ions and the concentration of oxygen vacancies. The change of Mn2+ ions and oxygen vacancy concentrations demonstrates that the predominant charge compensation mechanism in GdMn0.95W0.05O3 and GdMn0.90W0.10O3 samples is the conversion of Mn3+ to Mn2+, while the conversion of Mn3+ to Mn2+ and the elimination of oxygen vacancies are found to be the dominant charge compensation mechanism in GdMn0.80W0.20O3 sample.
Figure 3 shows the SEM images of the unsubstituted and W6+-substituted GMO compounds. The prepared ceramics exhibit a dense microstructure with plump, irregular hexagonal grains, and clear grain boundaries. The grain size of all the samples is not uniformly distributed. The grain size for the GdMn0.95W0.05O3 and GdMn0.90W0.10O3 samples is similar to that of the unsubstituted GMO; it becomes smaller for the x = 0.20 samples. This indicates that a larger concentration of W6+ substitution could hinder the grain growth of GMO. This may be related to the decrease in the oxygen vacancy concentration caused by W6+ substitution, which results in the slower movement of oxygen ions and, hence, inhibits the grain growth rate [23].
Positron annihilation lifetime spectrum measurement was performed to investigate the defects information in the GdMn1-xWxO3 samples. The fitted positron annihilation lifetime spectrum comprises three lifetime components τ1, τ2, and τ3 with relative intensities I1, I2, and I3 (I1 + I2 + I3 = 1), respectively. The longest lifetime τ3 (~ 1200 ps) is considered to be the result of positrons annihilated at the grain surface of GMO-based ceramics [24,25,26]. Owing to the lower intensity (I3 < 0.8%), τ3 and I3 are neglected herein, while I1 and I2 are renormalized. The values of the positron parameters τ1, τ2, I2, and τav in GdMn1-xWxO3 ceramics are shown in Fig. 4; τ1 is the reduced bulk positron lifetime, which is attributed to the annihilation of positrons in the perfect lattice; τ2 is mainly related to the lifetime of positrons annihilated at vacancy type defects within the samples, representing the information of defect size [24,25,26,27,28,29]. The intensity I2 is the probability of positrons annihilated at the localized defect state, which reflects the defect concentration in the experimental materials. As shown in Fig. 4, τ2 increases for x = 0.00–0.10 and then decreases. Because a higher value of τ2 represents a bigger size of vacancy in solid materials, the change in τ2 indicates that the vacancy size increases for the x = 0.00–0.10 samples, while it decreases with further increase in the W6+ content. The intensity I2 increases monotonously as the W6+ content x increases. This suggests that W6+ replacement can introduce considerable cation vacancy defects in GdMn1-xWxO3, and that the vacancy concentration increases with increasing W6+ content. The substitution of W6+ ion for Mn3+ in GMO causes the structural distortion, which might introduce cation vacancies in GdMn1-xWxO3. Furthermore, on substituting Mn3+ with W6+ ions, cationic vacancy defects with a negative charge are created owing to charge compensation, which can be described by Eq. (2):
The above two factors induce the increase of cationic vacancy concentration in W6+-substituted ceramics. The average lifetime τav can also be used to judge the defect characteristics in a solid, which can be expressed by the following formula [28, 29]:
It can be found from Fig. 4 that the change of τav with W6+ concentration x has the similar trend as that of I2. Therefore, the correlation between vacancies concentration and W6+ substitution content is further verified by the change of τav.
The temperature dependence of magnetization (M-T) for GdMn1-xWxO3 ceramics measured under zero-field-cooled (ZFC) and field-cooled (FC) modes at 1 kOe is displayed in Fig. 5. According to previous literature [8, 30], GMO undergoes three magnetic transitions at low temperature, namely, paramagnetism to antiferromagnetism at ~ 43 K attributed to the appearance of Mn3+ AFM ordering, antiferromagnetism to ferromagnetism at 25 K resulting from the canted AFM of Mn3+, and ferromagnetism to antiferromagnetism at 8 K owing to Gd3+ AFM ordering. The ZFC and FC plots shown in Fig. 5 are almost equal in the temperature range of 300–50 K for GdMn1-xWxO3 samples; however, the magnetic transition at ~ 43 K disappears in GdMn1-xWxO3 owing to the strong paramagnetic moment of Gd. For the x = 0.00–0.10 ceramics, a remarkable improvement of magnetization in the ZFC curve at ~ 23 K marks the onset of ferromagnetic ordering, then the magnetic transition at ~ 8 K appears, and finally magnetization increases with a decrease in temperature. For the GdMn0.80W0.20O3 samples, a magnetic transition at ~ 8 K in the ZFC curve cannot be observed, which may shift to a lower temperature. After a field cooling measurement, the magnetization for GdMn1-xWxO3 increases with a decrease in temperature, while the magnetic transition at ~ 8 K in the ZFC curves disappears owing to the enhancement of the local magnetic field at Gd, which can be attributed to the ferromagnetic ordering of the Mn moment. To study the magnetic interactions in GdMn1-xWxO3, the temperature dependence of inverse susceptibility (1/χ–T) for all samples is displayed in the illustration in Fig. 5. All 1/χ–T curves obey the Curie–Weiss law at a higher temperature, and the calculated Curie–Weiss temperature (Tcw) for GdMn1-xWxO3 samples is negative, indicating that antiferromagnetic interaction is predominant in GdMn1-xWxO3 compounds.
Figure 6 depicts the magnetic field dependence of the magnetization (M-H) for GdMn1-xWxO3 ceramics measured at 5 K, 30 K, and 40 K. The M-H curves for GdMn1-xWxO3 samples recorded at 30 K and 40 K exhibit linear characteristics, indicating antiferromagnetic behavior; meanwhile, the curve measured at 5 K shows non-linear characteristics, demonstrating that weak ferromagnetic/ferrimagnetic behavior is presented. The presence of weak ferromagnetism/ferrimagnetism at 5 K should be the result of canted Gd and Mn moments [31, 32]. The M–H loops for GdMn1-xWxO3 samples at low temperatures are unsaturated demonstrating the coexistence of antiferromagnetic and weak ferromagnetic/ferrimagnetic properties in the prepared compounds. The remnant magnetization (Mr) at 5 K is ~ 7.38, 18.25, 3.18, and 2.95 emu/g for unsubstituted GMO, GdMn0.95W0.05O3, GdMn0.90W0.10O3, and GdMn0.80W0.20O3, respectively. It can be observed that Mr initially increases with increasing W6+ amount from 0.00 to 0.05 but decreases as the W6+ content increases from 0.05 to 0.20. It can be observed from Fig. 6 that GdMn1-xWxO3 ceramics show ferromagnetic/ferrimagnetic properties at 5 K, and the insertion of cationic vacancies in ferromagnetic/ferrimagnetic orderings would improve magnetization. Meanwhile, GMO exhibits a spiral spin magnetic structure at low temperatures; the structural distortion caused by W6+ substitution could suppress the magnetic spiral spin and increase magnetization. Therefore, the magnetization increases with increasing W6+ content x from 0.00 to 0.05. The substitution of non-magnetic W6+ ions will cause magnetic dilution and thus a decrease in magnetization [33]. Therefore, magnetization decreases with increasing x from 0.05 to 0.20. Moreover, the change in the lattice structure, and the interaction of the Mn3+–Mn4+, Gd3+–Mn3+/Mn4+, and Gd3+–Gd3+ induced by W6+ substitution also affect the magnetic behavior of the GMO system [8, 14, 30].
The electrical properties of the grains and the grain boundaries of all the samples were investigated using impedance spectroscopy. The impedance plots (Z* plots, Z′′ vs. Z′) for GdMn1-xWxO3 ceramics at room temperature are shown in Fig. 7, and the expended view of high-frequency data close to the origin for GdMn1-xWxO3 ceramics is illustrated in Fig. 7. The grain resistance (Rg) and grain boundary resistance (Rgb) can generally be determined from the diameter of two semicircular arcs observed from the impedance spectrum at high- and low-frequency ranges, respectively [34]. The nonzero intercept on the Z′ axis presents the resistance value of the grains. Further, the resistance value of grain boundary can be directly obtained by the other intercept on the Z′ axis from the low-frequency impedance data [34]. It can be seen from Fig. 7 that only a part of the grain boundary semicircles or arcs is observed because of the limit of the measured frequency range. The nonzero intercept of the large arc at high frequencies is ~ 62, 80, 88, and 303 kΩ.cm corresponding to x = 0.00, 0.05, 0.10, and 0.20 samples, respectively. This means that the value of Rg changes little as the concentration of W6+ increases from 0.00 to 0.10; however, Rg for the x = 0.20 sample is clearly greater than that of the other samples. Although the Rgb of the samples cannot be obtained due to the nonzero intercept of large arc at low frequencies, it can be inferred that the values of Rgb increase with increasing W6+ substitution concentration according to the curvature of circular arcs. Therefore, W6+ substitution can improve the resistivity of grain and grain boundary.
Figure 8 displays the variation of dielectric constant (εr) with frequency at room temperature for the GdMn1-xWxO3 samples. εr for all these samples exhibits the same increasing trend with increasing frequency; it decreases considerably in the low-frequency range (< 104) and remains almost constant as the frequency exceeds 104. This phenomenon may be related to the relaxation of space charge polarization, which cannot keep up with the change of frequency at high frequencies. From Fig. 8, it can be clearly seen that the value of εr is enhanced considerably by W6+ ion substitution in the high-frequency range, which is ~ 125.8, 8369.7, 9216.1, and 11,023.2 measured at 1 × 107 Hz for unsubstituted GMO, GdMn0.95W0.05O3, GdMn0.90W0.10O3, and GdMn0.80W0.20O3, respectively. This shows that W6+ ion substitution makes the material display giant dielectric properties. Till date, few studies have reported the giant dielectric constant of this material at higher frequencies, and thus, the mechanism of giant dielectric properties remains unknown. In the typical giant dielectric CaCu3Ti4O12 ceramics, the polar arrangement of electrons on mixed-valence Ti3+/Ti4+ and/or Cu+/Cu2+ is the physical origin of giant dielectric behavior [35]. The giant dielectric response in LuFe2O4 ceramics also supports this mechanism [36]. The XPS results in Fig. 2 demonstrate that the mixed valence of Mn2+/Mn3+ exists in the W6+-substituted GMO. Therefore, the giant dielectric properties of GdMn1-xWxO3 may be related to the polar arrangement of electrons on the mixed-valence structure of Mn2+/Mn3+. Meanwhile, grain and grain boundary having large resistivity are conducive to the improvement of the dielectric constant. The results of impedance spectroscopy show that W6+ substitution can improve the resistivity of grain and grain boundary, so W6+ substitution enhances the dielectric constant of GMO. Therefore, the formation mechanism of the giant dielectric properties in GdMn1-xWxO3 may have risen from the polar arrangement of electrons in the mixed-valence structure of Mn2+/Mn3+ and the increase in grain and grain boundary resistivity induced by W6+ substitution. The room-temperature dielectric loss (tan δ) as a function of frequency for GdMn1-xWxO3 ceramics is shown in Fig. 9. The change of tan δ with frequency shows a similar trend to that of the dielectric constant. tan δ for unsubstituted GMO, GdMn0.95W0.05O3, and GdMn0.90W0.10O3 samples is almost the same, while GdMn0.80W0.20O3 samples have a lower dielectric loss than that of other samples, which may be related to the leakage current in the prepared ceramics. As shown in Fig. 2, the concentration of oxygen vacancy in the GdMn0.80W0.20O3 sample is lower than that of other samples, which will decrease electronic conduction in the material and, thus, considerably influence the electronic properties of GdMn1-xWxO3 [37]. Therefore, tan δ of GdMn0.80W0.20O3 is lower than that of other samples.
4 Conclusion
The structural characterstics and magnetic and dielectric performance of GdMn1-xWxO3 polycrystalline ceramics synthesized using the solid-state reaction method were studied. The substitution of W6+ at the Gd site induces structural distortion. W6+ ion substitution induces the conversion of Mn3+ to Mn2+ and the elimination of oxygen vacancies in GdMn1-xWxO3. The positron annihilation studies reveal that W6+ ion substitution can change the cation vacancy size and that the vacancy concentration increases with an increasing amount of W6+ ions. M–T curves show that antiferromagnetic interaction is dominant in GdMn1-xWxO3 samples. M-H curves show that all samples display weak ferromagnetic/ferrimagnetic behavior at 5 K, and the GdMn0.95W0.05O3 sample shows optimized magnetic properties. Furthermore, the W6+-substituted samples exhibit giant dielectric characteristics over a broad frequency range. The above results suggest that the magnetic properties of the GdMnO3 system are mainly modulated by vacancy concentration, structural distortion, and the magnetic dilution effect; the mixed-valence structure of Mn2+/Mn3+ and the increase in grain and grain boundary resistivity are the main factors in the appearance of giant dielectric properties. Present findings show that the physical properties of GdMnO3 materials can be tailored by W6+ substitution.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
J. Stein, M. Baum, S. Holbein, T. Finger, T. Cronert, C. Tolzer, T. Frohlich, S. Biesenkamp, K. Schmalzl, P. Steffens, C.H. Lee, M. Braden, Phys. Rev. Lett. 119, 177201 (2017)
P.J. Xue, H. Wu, Y. Lu, X.H. Zhu, J. Mater. Sci. Technol. 34, 914 (2018)
C. Chakrabarti, Q.S. Fu, X.H. Chen, Y. Qiu, S.L. Yuan, C.L. Li, Ceram. Int. 46, 212 (2020)
F. Wan, X.J. Bai, K.K. Song, J.B. Zheng, X. Lin, C.D. Cao, J. Mater. Sci. Technol. 33, 1061 (2017)
T. Kimura, G. Lawes, T. Goto, Y. Tokura, A.P. Ramirez, Phys. Rev. B 71, 224425 (2005)
K. Noda, S. Nakamura, J. Nagayama, H. Kuwahara, J. Appl. Phys. 97, 10C103 (2005)
N.R. Qu, Z.P. Li, J. Supercond. Nov. Magn. 31, 2869 (2018)
M.M. Wang, R.M. Wang, H.Y. Dai, T. Li, Y. Sun, D.W. Liu, F.F. Yan, X.B. Xing, Ceram. Int. 48, 3685 (2022)
L.L. Xu, J.L. Meng, Q.S. Liu, J. Meng, X.J. Liu, H.J. Zhang, Phys. Chem. Chem. Phys. 22, 4905 (2020)
P. Pant, H. Agarwal, S. Bharadwaj, M.A. Shaz, J. Mater. Sci.: Mater. Electron 33, 18871 (2022)
A. Ahmed, D. Mazumdar, K. Das, I. Das, J. Magn. Magn. Mater. 551, 169133 (2022)
F. Bzour, A. Gismelseed, I.Z. Al-Yahmadi, F. Al-Ma’Mari, A. Al-Rawas, S. Al-Harthi, A. Yousif, H. Widatallah, M. ElZain, M.T.Z. Myint, Hyperfine Interact. (2021). https://doi.org/10.1007/s10751-021-01782-y
P. Tiwari, M. Yadav, A. Bastia, G.C. Pandey, C. Rath, New J. Chem. 45, 22396 (2021)
A. Pal, P. Murugavel, J. Appl. Phys. 123, 234102 (2018)
T. Tajiri, H. Deguchi, M. Mito, A. Kohno, J. Phys. Chem. C 125, 14474 (2021)
Y. Romaguera-Barcelay, S. Talu, R.S. Matos, R.M.P.B. Oliveira, J.A. Moreira, J.P. Cruz, H.D.F. Filho, Appl. Sci. 11, 3886 (2021)
G.Q. Zhang, S.J. Luo, S. Dong, Y.J. Gao, K.F. Wang, J.M. Liu, J. Appl. Phys. 109, 07D901 (2011)
J.A. Dean, Lange’s Handbook of Chemistry, 15th edn. (McGraw-Hill, New York, 1999), p.4.30-4.43
D.J. Keeble, S. Singh, R.A. Mackie, M. Morozov, S. McGuire, D. Damjanovic, Phys. Rev. B 76, 144109 (2007)
S.X. Jin, P. Zhang, E.Y. Lu, L.P. Guo, B.Y. Wang, X.Z. Cao, Acta Mater. 103, 658 (2016)
H.Y. Dai, F.J. Ye, T. Li, Z.P. Chen, X.Z. Cao, B.Y. Wang, Ceram. Int. 45, 24570 (2019)
F.Z. Huang, X.M. Lu, Z. Wang, W.W. Lin, Y. Kan, H.F. Bo, W. Cai, J.S. Zhu, Appl. Phys. A 97, 699 (2009)
X.L. Wen, Z. Chen, E.H. Liu, X. Lin, C.L. Chen, J. Alloy. Compd. 678, 511 (2016)
Y. Tang, Y. Zhang, P.Y. Du, W. Deng, J. Am. Ceram. Soc. 96, 2537 (2013)
W.N. Ge, A. Rahman, H.R. Cheng, M. Zhang, J.D. Liu, Z.M. Zhang, B.J. Ye, J. Magn. Magn. Mater. 449, 401 (2018)
M.F. Zeng, Y.D. Wang, Q. Liu, X. Yuan, S.F. Zuo, R.K. Feng, J. Yang, B.Y. Wang, C.Z. Qi, Y. Lin, A.C.S. Appl, Mater. Inter. 8, 33157 (2016)
W. Brandt, R. Paulin, Phys. Rev. B 5, 2430 (1972)
H.Y. Dai, F.J. Ye, Z.P. Chen, T. Li, D.W. Liu, J. Alloys Compd. 734, 60 (2018)
D.D. Wang, N. Qi, M. Jiang, Z.Q. Chen, Appl. Phys. Lett. 102, 042407 (2013)
M.H. Ghozza, I.S. Yahia, J. Mater. Sci.: Mater. Electron. 32, 11628 (2021)
Y.N. Zhang, J.J. Li, Z.Q. Zhang, F.Y. Liu, X.D. Zhao, X.Y. Liu, Chem. Res. Chin. Univ. 31, 699 (2015)
M.M. Wang, H.Y. Dai, T. Li, J. Chen, F.F. Yan, R.Z. Xue, X.B. Xing, D.Y. Chen, T.D. Ping, J.J. He, Mater. Sci.: Mater. Electron. 32, 27348 (2021)
K. Yadagiri, R. Nithya, S. Neeraj, A.T. Satya, AIP Adv. 7, 035003 (2017)
T. Li, H.F. He, T. Zhang, B. Zhao, Z.Q. Chen, H.Y. Dai, R.Z. Xue, Z.P. Chen, J. Alloys Compd. 684, 315 (2016)
L. Ni, X.M. Chen, Appl. Phys. Lett. 91, 122905 (2007)
N. Ikeda, H. Ohsumi, K. Ohwada, K. Ishii, T. Inami, K. Kakurai, Y. Murakami, K. Yoshii, S. Mori, Y. Horibe, H. Kito, Nature 436, 1136 (2005)
H.Y. Dai, T. Li, Z.P. Cen, D.W. Liu, R.Z. Xue, C.Z. Zhao, H.Z. Liu, N.K. Huang, J. Alloys Compd. 672, 182 (2016)
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant Nos. 12275242, 11975211, and 12005194), and the Natural Science Foundation of Henan Province (Grant Nos. 212300410092, 212102210489).
Funding
This work is supported by the National Natural Science Foundation of China (Grant Nos. 12275242, 11975211, and 12005194), and the Natural Science Foundation of Henan Province (Grant Nos. 212300410092, 212102210489).
Author information
Authors and Affiliations
Contributions
TL contributed to material preparation, investigation, and writing-original draft; HL contributed to material preparation, data collection, and validation; JC contributed to validation and analysis; DL contributed to formal analysis and validation; and HD contributed to resources, conceptualization, investigation, and writing-original draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, T., Liu, H., Chen, J. et al. The structure, vacancy characteristics, and magnetic and dielectric properties of GdMn1-xWxO3 ceramics. J Mater Sci: Mater Electron 33, 25920–25929 (2022). https://doi.org/10.1007/s10854-022-09282-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09282-7