Abstract
A novel ternary heterojunction composite photocatalyst g-C3N4/TiO2/NiWO4 was fabricated using a simple hydrothermal method. The synthesized samples were characterized using X-ray diffraction (XRD), scanning electronic microscopy (SEM), energy-dispersive spectrum (EDS), Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), ultraviolet–visible (UV–Vis) absorption spectra, photoluminescence (PL) spectra, transient photocurrent responses, and electrochemical impedance spectroscopies (EIS). The results indicated that the composite of g-C3N4/TiO2/NiWO4 had been successfully synthesized. By constructing a ternary heterojunction, the electron migration rate and light absorption of the material are further improved; the photogenerated electron–hole recombination is inhibited. The ternary composite photocatalyst shows the highest photocatalytic activity for the degradation of rhodamine B (RhB) than that of g-C3N4, TiO2, NiWO4, and g-C3N4/TiO2 photocatalyst. The degradation efficiency of RhB using g-C3N4/TiO2/NiWO4 can reach 99% after visible-light irradiation for 40 min. Finally, the migration mechanism of charge carriers in the ternary system has been schematically illustrated by the active species capture experiment. Our research can pave the way for the fabrication of ternary heterojunction composite photocatalyst with high photocatalytic activity for the environmental contaminants treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As a traditional photocatalyst, TiO2 has been widely researched due to its high chemical and thermal stability, non-toxicity, higher activity, lower cost and environmentally friendly advantages [1,2,3]. However, the practical applications of TiO2 was limited due to its some intrinsic disadvantages, such as lower quantum efficiency, relatively wide bandgap only being excited by ultraviolet light and rapid recombination of photogenerated electron–hole pairs on the surface or in the lattice of TiO2, etc., so many efforts have been made to improve its photocatalytic performance and photoelectric conversion efficiency [4, 5], including hydrogenation modified TiO2, energy band modulation by doping with some metal elements and non-metal elements [6], etc. Combining TiO2 with other semiconductor which possesses narrow bandgap to fabricate heterojunction composite photocatalyst is considered to be a simple and effective method to improve the photocatalytic activity [7].
In recent years, the development of visible light-driven composite materials has become a hot spot in solar photocatalysis research field. g-C3N4 is a kind of non-metal polymerized semiconductor with a narrow bandgap (2.7 eV) which can be excited by visible light, and it has great application potential in the fields of photocatalytic hydrogen production, carbon dioxide conversion, and degradation of organic pollutants retained in the environment [8, 9]. Unfortunately, pure g-C3N4 exhibits poor photocatalytic performance due to lower separation efficiency of photogenerated electron–hole pairs. But fortunately, g-C3N4 has a suitable band position which matches well with that of TiO2.
Constructing heterojunction, doping and loading catalyst are modification strategy for g-C3N4 and TiO2 [10, 11]. To construct TiO2/g-C3N4 heterojunction not only can broaden the light response range of TiO2-based photocatalyst but also can effectively suppress recombination of photogenerated electron–hole pairs [12]. Up to now, there are many researches on the synthesis and modification of the composite g-C3N4/TiO2. Zhang et al. loaded g-C3N4 onto a TiO2 nanotube array by a simple electrodeposition method and exhibited excellent photocatalytic hydrogen evolution activity [13]. Wang et al. prepared g-C3N4/TiO2 nanomaterials by using high-voltage electrospinning and hydrothermal methods. They treated the surface of pure nano-TiO2 by acidification before it was combined with g-C3N4. The results showed that the photocatalytic activity of the g-C3N4/TiO2 nanocomposite treated by acidification is much higher than that of the untreated catalyst [14]. Tan et al. prepared nanostructured g-C3N4/TiO2 through a one-step method, which can improve hydrogen production efficiency under visible light [15]. Zhang et al. prepared ternary MoS2/g-C3N4/TiO2 nanosheet composites by liquid stripping and solvothermal methods [16]. Compared with binary photocatalyst, the ternary composites have higher photogenerated carrier separation rates and faster electron migration rates [17]. Wu et al. synthetized heterojunction photocatalyst g-C3N4/TiO2/HNTs which can efficiently catalyze the degradation of ciprofloxacin in water [18]. However, the reports on ternary photocatalysts with high stability, low cost, and excellent catalytic performance are still scarce.
Recently, a series of low-cost metal tungstates such as Bi2WO6, CuWO4, NiWO4, and CoWO4 have been widely studied due to their excellent optical, electrical, magnetic, and catalytic properties [19,20,21,22]. Among them, NiWO4, as a simple and common tungstate material, possess the narrow bandgap and can make full use of the solar radiation energy, has been applied in many fields, including sensors, catalysts and supercapacitors [23]. Many researchers have used NiWO4 as photocatalyst for the degradation of organic pollutants in the environments [20].
In the present paper, we designed and synthesized a novel ternary heterojunction composite photocatalyst g-C3N4/TiO2/NiWO4 by using a simple hydrothermal method for the degradation of rhodamine B (RhB) in aqueous solutions. The electron migration rate and light absorption of the material prepared are improved, and the photogenerated electron–hole recombination is inhibited, compared with that of the photocatalyst g-C3N4, TiO2, NiWO4, and g-C3N4/TiO2. This work provides a new way for the preparation of ternary heterojunction composite photocatalyst with high photocatalytic activity and for the treatment of contaminants in the environment.
2 Experimental section
2.1 Materials employed
Melamine, absolute ethanol, tetra-n-butyl titanate, nickel nitrate (Ni(NO3)2·6H2O), sodium tungstate (Na2WO4·2H2O), and rhodamine B were all purchased from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai China). All the chemicals employed were of analytical grade.
2.2 Synthesis of the photocatalysts
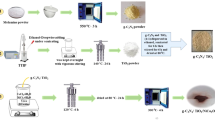
The g-C3N4 was prepared by the thermal polycondensation method. In a typical synthesis, 1 g of melamine was heated at 500 °C for 120 min at the rate of 10 °C/min in a crucible with a cover, and then cooled to room temperature. The sample was then ground into the powder for further use.
The g-C3N4/TiO2 was synthesized by the hydrothermal method. Firstly, 50 mg of the as-prepared g-C3N4 were dispersed into 70 mL of absolute ethanol; the mixture was then stirred by ultrasound for 5 min. Then, under the condition of continuous magnetic stirring, 1 mL of tetra-n-butyl titanate reagent was slowly added dropwise to the g-C3N4/absolute ethanol mixture, and then continued to stir the mixture for 2 h. After that, the resulting mixture was transferred into a Teflon-lined stainless steel vessel with a capacity of 100 mL and heated at 200 °C in an oven for 20 h. Then the product was cooled to room temperature, the obtained solid samples were collected by centrifugation with distilled water and ethanol for several times. Finally, dried in a vacuum oven at 80 °C for 24 h, the resultant pale-yellow solid powder was g-C3N4/TiO2 labeled as CT.
For synthesis of the ternary composite g-C3N4/TiO2/NiWO4, 0.13 g of Ni(NO3)2·6H2O and 0.15 g of Na2WO4·2H2O were separately dispersed in 30 mL deionized water, and remarked as solution A and solution B, respectively. Then, a known quantity of the prepared g-C3N4/TiO2 solid powder was dispersed in the solution A, and stirred for 1 h. After that, solution B was slowly added dropwise to the above mixture, then heated to 80 °C and stirred for 2 h. The resulting mixture was transferred into a Teflon-lined stainless steel vessel with a capacity of 100 mL and heated at 200 °C for 20 h. The product was then cooled to room temperature; the obtained solid samples were collected by centrifugation with distilled water and ethanol for several times. Finally, dried in a vacuum oven at 60 °C for 24 h, the resultant green solid powder was g-C3N4/TiO2/NiWO4.
2.3 Characterization of catalysts
2.3.1 XRD
At room temperature, small angle diffraction was performed using an X’Pert-Pro MPD (Holland) D/max-γA X-ray diffractometer with Cu Kα radiation (λ = 0.154178 nm), and the scanning rate was 1°/min, with 2θ range of 10–70°.
2.3.2 FT-IR
The infrared spectra of the samples were determined by a Nicolet-360 Fourier transform infrared spectroscopy (FT-IR) by diffuse reflectance scanning technique from 4000 to 500 cm−1.
2.3.3 SEM
The morphology and structure of the as-prepared samples were examined by Scanning electron microscopy (SEM, JSM-6360LV, JEOL Japan).
2.3.4 EDS
The energy-dispersive X-ray spectra (EDS) were carried out by the JSM-6360LV microscope.
2.3.5 PL spectra
Photoluminescence (PL) spectra of the as-prepared samples were recorded by a RF-5301PC luminescence spectrometer excited with 320 nm wavelength.
2.3.6 XPS
X-ray photoelectron spectroscopy (XPS) were acquired on an ARL Quant X-ray photoelectron spectrometer using Al Kα X-ray (hv = 1486.6 eV).
2.3.7 UV–Vis
UV–Vis absorption spectra of the samples were recorded on a Lambda 750 (Perking Elmer) spectrophotometer in the tested range of 200–800 nm.
2.3.8 Electrochemical test
The electrochemical test was use a typical three-electrode system and carried out by a CHI 660b workstation.
2.3.9 Photocatalytic activity test
First, 50 mg of photocatalyst was placed into a 100 mL of RhB aqueous solutions (10 mg/L) and stirred for 30 min in a dark environment to achieve adsorption–desorption equilibrium. Then, a 300 W xenon lamp with a 420 nm cut filter was chosen as a visible-light source. At certain time intervals, 5 mL of aliquots were extracted and centrifuged, and then, analyzed by using UV–Vis spectrophotometer at a maximum absorption wavelength of 553 nm. The degradation efficiency (DE) of RhB was calculated using the formula: DE (100%) = (1 − c/c0) ×100%, where, c is the concentration of RhB at each irradiation time interval and c0 is the initial concentration.
3 Result and discussion
3.1 Crystal structure and morphology
The XRD patterns of the as-prepared samples g-C3N4, g-C3N4/TiO2 and g-C3N4/TiO2/NiWO4 are shown in Fig. 1.
It can be seen from Fig. 1 that g-C3N4 show two distinct characteristic peaks at 12.9° and 27.44°, corresponding to the (100) crystal plane of the hexazine heterocyclic unit and the (002) graphite layer stacked crystal plane, respectively, which is consistent with previous literature report [24]. g-C3N4/TiO2 exhibit the overlapped characteristic peaks of both g-C3N4 and TiO2, indicating the successful synthesis of the binary composite [25]. As for g-C3N4/TiO2/NiWO4, the characteristic peak of g-C3N4 at 27.44° disappeared, and meanwhile, three new diffraction peak appeared at 35.4°, 40.7°, and 53.6° [26]; compared with the standard XRD card of NiWO4, the three new diffraction peaks correspond to the characteristic peaks of NiWO4 at 36.5°, 41.8°, and 54.7° respectively, which demonstrated that the characteristic peak of g-C3N4 is covered after loading NiWO4. It also shows that a new ternary composite g-C3N4/TiO2/NiWO4 was successfully fabricated via compounding NiWO4 and g-C3N4/TiO2. All of characteristic peaks of NiWO4 are shifted about 1.1° to the small angle of 2θ, while the positions of other characteristic peaks remain unchanged, indicating that the residual stress exist in the material during the material compounding process. Therefore, the crystal lattice of NiWO4 is distorted, and the cell parameters and crystal surface spacing become larger. So that the characteristic peaks of NiWO4 are collectively shift to the negative position.
The morphology of g-C3N4, g-C3N4/TiO2, and g-C3N4/TiO2/NiWO4 was investigated by means of SEM technique, respectively. g-C3N4 shown in Fig. 2a presents an irregular stacked sheet-like structure with a diameter range from 100 nm to several micrometers. As can be seen from Fig. 2b and c, g-C3N4/TiO2 shows the peanut-like structure. From Fig. 2d, we can see that, a small layer of nanoparticles, namely NiWO4 nanoparticles, is newly loaded on the surface of composite g-C3N4/TiO2 to form a ternary heterojunction composite g-C3N4/TiO2/NiWO4. Meanwhile, EDS spectrum of the as-prepared g-C3N4/TiO2/NiWO4 show the existence of C, N, O, W, Ti, and Ni in the composite, indicating that the uniform dispersion of these five elements (Fig. 3). These results together with XRD analysis can demonstrate that the ternary heterojunction photocatalyst was successfully prepared.
3.2 FT-IR spectra
FT-IR spectra of the samples g-C3N4, g-C3N4/TiO2 and g-C3N4/TiO2/NiWO4 are shown in Fig. 4.
From Fig. 4, we can see that, the peak at around 3430 cm−1 is related to hydroxyl groups, which can be attributed to the tensile vibration of adsorbed water molecules [27, 28]. The broad absorption peaks between 2900 and 3300 cm−1 correspond to the –NH2 and =NH stretching vibrations [29]. It can also easily be seen from Fig. 4, g-C3N4 contains a small amount of amino groups, while amino groups cannot be seen from the FT-IR spectra of composites g-C3N4/TiO2 and g-C3N4/TiO2/NiWO4. This is mainly related to the low content of g-C3N4 in composite g-C3N4/TiO2 and g-C3N4/TiO2/NiWO4. The absorption peak in the range of 1200 cm−1 to 1650 cm−1 is caused by the stretching vibration of the C–N heterocycle [30]. After being combined with TiO2, the characteristic peak of C–N heterocycle in composite g-C3N4/TiO2 is still obvious. After being combined with NiWO4, the strength of C–N heterocycle in composite g-C3N4/TiO2/NiWO4 is weakened, but still obvious, indicating that the C–N heterocycle in the composite is relatively stable. The peak at 807 cm−1 can correspond to the stretching vibration of the triazine ring [31]; it is worth noting that the triazine ring characteristic peaks cannot be observed in FT-IR spectra of the composites g-C3N4/TiO2 and g-C3N4/TiO2/NiWO4, indicating that the triazine ring structure in g-C3N4 is destroyed during the combined process with TiO2.
3.3 XPS analysis
X-ray photoelectron spectroscopy (XPS) technique was employed to investigate the surface chemical compositions and valence state of g-C3N4/TiO2/NiWO4. The C 1s spectra (Fig. 5a) showed 2 peaks. The peak at 284.8 eV can be assigned to C=C bond or indefinite-form carbon, which originate from instrument itself. The peaks at 288.4 eV can be ascribed to the N–C=N bond [32].
From Fig. 5b, we can see that, the N 1s region can be divided into two different peaks at 399.6 and 400.8 eV, which correspond to N–(C)3 and C–N–H groups, respectively [33].
In the Ti 2p spectra shown in Fig. 5c, the two peaks of 2p3/2 and 2p1/2 at binding energies of 458.4 and 464.3 eV, respectively, can be attributed to Ti4+ in the composite [34].
As shown in Fig. 5d, the dominant peak of O 1s locates at binding energy of 529.6 eV, which suggests the lattice oxygen O2−. The other peak at 531.6 eV is assigned hydroxyl groups absorbed in the surface.
There are six peaks at binding energies of 880.5, 873.5, 869.7, 861.8, 855.6, and 853.0 eV for Ni 2p element (Fig. 5e), respectively. The two peaks located at 855.6 and 873.5 eV, respectively, indicate that there exist Ni ions in bivalent status.
From Fig. 5f we can see that, the W 4f spectra were fitted by two peaks centered around 34.9 eV and 37.1 eV, which can be ascribed to W 4f7/2 and W 4f5/2, respectively, representing the presence of W6+, that formed NiWO4 together with Ni2+ and O2− [35].
Based on the above-mentioned XPS results, it is reasonable to assert that the sample is indeed a ternary heterojunction composite photocatalyst g-C3N4/TiO2/NiWO4.
3.4 UV–Vis spectra analysis
The optical absorption properties of g-C3N4, TiO2, NiWO4, g-C3N4/TiO2, and g-C3N4/TiO2/NiWO4 was studied by UV–Vis spectra and displayed in Fig. 6a. The absorption band edges of g-C3N4, TiO2, and NiWO4 are located at around 445 nm, 378 nm, and 430 nm, respectively. However, g-C3N4/TiO2/NiWO4 exhibits a red shift toward longer wavelength compared with g-C3N4 and TiO2, indicating that the introduction of NiWO4 can promote the light absorption of the photocatalyst under visible light.
The Tauc’s bandgap plots of the samples g-C3N4, TiO2, and NiWO4 is converted from the UV–Vis diffuse reflectance absorption spectra (DRS) according to the Kubelka–Munk function. As shown in Fig. 6b–d, the bandgap energy of g-C3N4, TiO2, and NiWO4 is estimated to be 2.78 eV, 3.29 eV, and 2.89 eV respectively.
3.5 Photocatalytic degradation of RhB and reusability of as-prepared catalyst
RhB in aqueous solutions were selected as the target pollutant to evaluate the photocatalytic performance of the as-prepared catalysts under visible-light irradiation.
From Fig. 7a we can see that, after visible-light irradiation for 60 min, 12%, 72%, and 41% of RhB are degraded by using pure NiWO4, g-C3N4, and P25 TiO2 as photocatalyst, respectively. Both binary and ternary photocatalysts exhibit enhanced photocatalytic activity, and the degradation efficiencies of RhB can all reach 99% after visible-light irradiation for 40 min. But, obviously, the degradation rate of ternary photocatalyst g-C3N4/TiO2/NiWO4 is faster than that of binary photocatalyst g-C3N4/TiO2.
The stability of the ternary composite photocatalyst was a significant factor for its practical application. In order to assess the photocatalytic stability of g-C3N4/TiO2/NiWO4, a cycling experiment for photocatalytic degradation of RhB was carried out.
As shown in Fig. 7b, after five cycles, the composite photocatalyst g-C3N4/TiO2/NiWO4 shows a little or even no deactivation, indicating that the composite photocatalyst g-C3N4/TiO2/NiWO4 is stable during the photocatalytic reaction. Therefore, the as-prepared ternary heterojunction composite g-C3N4/TiO2/NiWO4 could be employed as an efficient photocatalyst for application in organic pollutant removal.
3.6 Photoluminescence spectra analysis
In order to further investigate the reasons for the improvement of photocatalytic performance of the as-prepared g-C3N4/TiO2/NiWO4, the fluorescence spectra of g-C3N4, g-C3N4/TiO2 and g-C3N4/TiO2/NiWO4 were analyzed.
As seen in Fig. 8, for pure g-C3N4, fluorescence intensity was relatively higher, indicating a lower separation rate of photogenerated carriers. The composite g-C3N4/TiO2 exhibited lower PL intensity; g-C3N4/TiO2/NiWO4 displayed the lowest fluorescence signal, suggesting that the construction of the ternary heterojunction photocatalyst can effectively suppress the recombination of the photogenerated electron–hole pairs and improve the photocatalytic activity of the catalyst [36].
3.7 Photocurrent and electrochemical impedance measurements
The surface charge separation and transfer efficiencies of the as-prepared materials were further investigated by transient photocurrent responses and electrochemical impedance spectroscopy. As shown in Fig. 9a, g-C3N4/TiO2/NiWO4 possesses the highest photocurrent intensity. The semicircular radius in the impedance spectra of g-C3N4/TiO2/NiWO4 was obviously smaller than that of binary photocatalyst, suggesting an efficient interfacial charge transport resistance (Fig. 9b) [37,38,39].
The above-mentioned results demonstrate that the construction of the ternary heterojunction photocatalyst can accelerate the interfacial transfer and separation of charge carriers in hybrid photocatalyst.
3.8 Active species trapping experiments and photocatalytic mechanism for the as-prepared composite
At present research, radical species trapping experiments were carried out to study the photocatalytic mechanism of the composite g-C3N4/TiO2/NiWO4 for the degradation of RhB solution. In the trapping experiments, isopropyl alcohol (IPA), ethylenediaminetetraacetic acid disodium (EDTA-2Na), and ascorbic acid (VC) were separately used as scavenger of ·OH, h+, and ·O2−, respectively.
As shown in Fig. 10a, 99% of RhB in solutions can be removed by the g-C3N4/TiO2/NiWO4 without scavengers during 30 min. When IPA is added into the RhB solutions, there was no dramatic decrease in the photocatalytic activity, the degradation efficiency of RhB decreased from 99 to 90% during 30 min, implying that ·OH contributes just a little during photocatalysis. While the introduction of EDTA-2Na and VC to RhB solutions made the degradation efficiencies dropped from 99 to 41% and 22%, respectively, indicating that the h+ and ·O2− play an important role in RhB degradation process.
Based on the above analysis, a possible mechanism for the degradation of RhB is proposed as illustrated in Fig. 10b. Under visible-light irradiation, both g-C3N4 and NiWO4 in the composite photocatalyst g-C3N4/TiO2/NiWO4 absorb photons of energy greater than the corresponding bandgap energy, which excite the electrons in the VB to the CB and then leave holes in the VB. The bandgap of TiO2 is too wide to be excited by visible light to produce photogenerated electron–hole pairs. The conduction band position of TiO2 (− 0.51 eV) is more positive than that of g-C3N4 (− 1.15 eV) and NiWO4 (− 1.05 eV), so the photoexcited electrons (e−) will transfer from the CB of g-C3N4 and NiWO4 to the CB of TiO2. Thereby, the recombination of photogenerated electron–hole pairs in g-C3N4 and NiWO4 can be effectively suppressed. The dissolved oxygen (O2) in RhB solutions can be trapped by electrons (e−) accumulated in the CB of TiO2 and generated ·O2− to degrade RhB, while the holes (h+) remaining on the VB of g-C3N4 and NiWO4 can directly oxidize RhB. The degradation process of RhB is as follows:
4 Conclusions
In conclusion, a novel photocatalyst g-C3N4/TiO2/NiWO4 could be successfully fabricated by using a simple hydrothermal method. By constructing the ternary heterojunction composite photocatalyst, the electron migration rate and light absorption of the composite material are further improved; the photogenerated electron–hole recombination is inhibited. The resulting g-C3N4/TiO2/NiWO4 exhibit enhanced photocatalytic activity for the degradation of RhB under visible light compared with the photocatalyst g-C3N4, TiO2, NiWO4, and g-C3N4/TiO2. Moreover, the as-prepared composite shows a very strong stability and reusability. In addition, we concluded that the h+ and ·O2− radicals are the main active species of the composite g-C3N4/TiO2/NiWO4 in aqueous solution under visible-light irradiation by trapping experiments for radicals and holes. Our work provides a new perception on designing ternary heterojunction composite photocatalyst with superior photocatalytic performance to deal with the environmental pollution issues.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
A. Ojha, M. Thakker, D.O. Shah, P. Thareja, Flow-directed assembly of non-spherical titania nanoparticles into superhydrophilic thin films, Front. Mater. Sci. 10, 1–7 (2016)
Z.P. Tshabalala, K. Shingange, B.P. Dhonge, O.M. Ntwaeaborwa, G.H. Mhlongo, D.E. Motaung, Fabrication of ultra-high sensitive and selective CH4 room temperature gas sensing of TiO2 nanorods: detailed study on the annealing temperature. Sens. Actuators B 238, 402–419 (2017)
J.M. Meichtry, N. Quici, G. Mailhot, M.I. Litter, Heterogeneous photocatalytic degradation of citric acid over TiO2. I: mechanism of 3-oxoglutaric acid degradation. Appl. Catal. B 102, 454–463 (2011)
W.T. Sun, Y. Yu, H.Y. Pan, X.F. Gao, Q. Chen, L.M. Peng, CdS quantum dots sensitized TiO2 nanotube-array photoelectrodes. J. Am. Chem. Soc. 130, 1124–1125 (2008)
G.L. Chen, W.T. Zhong, Y.S. Li, Q. Deng, X. Ou, Q.C. Pan, X.W. Wang, X.H. Xiong, C.H. Yang, M.L. Liu, Rational design of TiO–TiO2 heterostructure/polypyrrole as a multifunctional sulfur host for advanced lithium–sulfur batteries. ACS Appl. Mater. Interface 11, 5055–5063 (2019)
L. Zhang, D.W. Jing, X.L. She, H.W. Liu, D.J. Yang, Y. Lu, J. Li, Z.F. Zheng, L.J. Guo, Heterojunctions in g-C3N4/TiO2 (B) nanofibres with exposed (001) plane and enhanced visible-light photoactivity. J. Mater. Chem. A 2, 2071–2078 (2014)
R. Plugaru, A. Cremades, J. Piqueras, The effect of annealing in different atmospheres on the luminescence of polycrystalline TiO2. J. Phys. Condens. Mater. 16, S261–S268 (2004)
H.Q. Xu, J.H. Hu, D.K. Wang, Z.H. Li, Q. Zhang, Y. Luo, S.H. Yu, H.L. Jiang, Visible-light photoreduction of CO2 in a metal-organic framework: boosting electron–hole separation via electron trap states. J. Am. Chem. Soc. 137, 13440–13443 (2015)
Y. Zheng, J. Liu, J. Liang, M. Jaroniec, Graphitic carbon nitride materials: controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 5, 6717–6731 (2012)
H.J. Wang, X. Li, X.X. Zhao, C.Y. Li, X.H. Song, P. Zhang, P.W. Huo, X. Li, A review on heterogeneous photocatalysis for environmental remediation: from semiconductors to modification strategies. Chin. J. Catal. 43, 178–214 (2022)
Z. Zhu, Z.X. Liu, X. Tang, K. Reeti, P.W. Huo, J.W.C. Wong, J. Zhao, Sulfur-doped g-C3N4 for efficient photocatalytic CO2 reduction: insights by experiment and first-principles calculations. Catal. Sci. Technol. 11, 1725–1736 (2021)
J.Y. Lei, B. Chen, W.J. Lv, L. Zhou, L.Z. Wang, Y.D. Liu, J.L. Zhang, An inverse opal TiO2/g-C3N4 composite with a heterojunction for enhanced visible light-driven photocatalytic activity. Dalton Trans. 48, 3486–3495 (2019)
Q. Zhang, H. Wang, S. Chen, Y. Su, X. Quan, Three-dimensional TiO2 nanotube arrays combined with g-C3N4 quantum dots for visible light-driven photocatalytic hydrogen production. RSC Adv. 7, 13223–13227 (2017)
T. Wang, T.Y. Sun, J.H. Xu, H. Xiao, Z.M. Zhang, H.Q. Bian, H.H. Ding, The pre-acidizing corrosion on the surface of TiO2 enhanced the photocatalytic activity of g-C3N4/TiO2. J. Mater. Sci. 32, 21083–21092 (2021)
Y.G. Tan, Z. Shu, J. Zhou, T.T. Li, W.B. Wang, Z.L. Zhao, One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B 230, 260–268 (2018)
W.P. Zhang, X.Y. Xiao, Y. Li, X.Y. Zeng, L.L. Zheng, C.X. Wan, Liquid-exfoliation of layered MoS2 for enhancing photocatalytic activity of TiO2/g-C3N4 photocatalyst and DFT study. Appl. Surf. Sci. 389, 496–506 (2016)
M. Mohamed Jaffer Sadiq, U. Sandhya Shenoy, D. Krishna Bhat, Novel RGO-ZnWO4-Fe3O4 nanocomposite as high performance visible light photocatalyst. RSC Adv. 6, 61821–61829 (2016)
D.Y. Wu, J.Z. Li, J.R. Guan, C.Y. Liu, X.X. Zhao, Z. Zhu, C.C. Ma, P.W. Huo, C.X. Li, Y.S. Yan, Improved photoelectric performance via fabricated heterojunction g-C3N4/TiO2/HNTs loaded photocatalysts for photodegradation of ciprofloxacin. J. Ind. Eng. Chem. 64, 206–218 (2018)
A.A. Kaminskii, H.J. Eichler, K.I. Ueda, N.V. Klassen, B.S. Redkin, L.E. Li, J. Findeisen, D. Jaque, J. García-Sole, J. Fernández, R. Balda, Properties of Nd3+-doped and undoped tetragonal PbWO4, NaY(WO4)2, CaWO4, and undoped monoclinic ZnWO4 and CdWO4 as laser-active and stimulated raman scattering-active crystals. Appl. Opt. 38, 4533–4547 (1999)
M. Hao, X. Meng, Y. Miao, Synthesis of NiWO4 powder crystals of polyhedron for photocatalytic degradation of rhodamine. Solid State Sci. 72, 103–108 (2017)
M. Eghbali-Arani, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, F. Ahmadi, S. Pourmasoud, Ultrasound-assisted synthesis of YbVO4 nanostructure and YbVO4/CuWO4 nanocomposites for enhanced photocatalytic degradation of organic dyes under visible light. Ultrason. Sonochem. 43, 120–135 (2018)
J. Wan, X. Du, R.M. Wang, E.Z. Liu, J. Jia, X. Bai, X.Y. Hu, J. Fan, Mesoporous nanoplate multi-directional assembled Bi2WO6 for high efficient photocatalytic oxidation of NO. Chemosphere 193, 737–744 (2018)
M.M. Mohamed, S.A. Ahmed, K.S. Khairou, Unprecedented high photocatalytic activity of nanocrystalline WO3/NiWO4 hetero-junction towards dye degradation: effect of template and synthesis conditions. Appl. Catal. B 150–151, 63–73 (2014)
Y.J. Cui, G.G. Zhang, Z.Z. Lin, X.C. Wang, Condensed and low-defected graphitic carbon nitride with enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B 181, 413–419 (2015)
C.Q. Li, Z.M. Sun, W.Z. Zhang, C.H. Yu, S.L. Zheng, Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B 220, 272–282 (2018)
M.I. Ahmed, A. Adam, A. Khan, M.N. Siddiqui, Z.H. Yamani, M. Qamar, Synthesis of mesoporous NiWO4 nanocrystals for enhanced photoelectrochemical water oxidation. Mater. Lett. 177, 135–138 (2016)
Y.P. Zhang, C.X. Pan, TiO2/graphene composite from thermal reaction of graphene oxide and its photocatalytic activity in visible light. J. Mater. Sci. 46, 2622–2626 (2010)
D.J. Cooke, D. Eder, J.A. Elliott, Role of benzyl alcohol in controlling the growth of TiO2 on carbon nanotubes. J. Phys. Chem. C 114, 2462–2470 (2010)
Q.H. Liang, Z. Li, Y. Bai, Z.H. Huang, F.Y. Kang, Q.H. Yang, A composite polymeric carbon nitride with in situ formed isotype heterojunctions for highly improved photocatalysis under visible light. Small 13, 1603182 (2017)
Y. Wang, X. Wang, M. Antonietti, Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry. Angew Chem. Int. Ed. Engl. 51, 68–89 (2012)
X.F. Chen, J.S. Zhang, X.Z. Fu, M. Antonietti, X.C. Wang, Fe-g-C3N4-catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light. J. Am. Chem. Soc. 131, 11658–11659 (2009)
F. Dong, L.W. Wu, Y.J. Sun, M. Fu, Z.B. Wu, S.C. Lee, Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mate Chem. 21, 15171–15174 (2011)
H.H. Ji, F. Chang, X.F. Hu, W. Qin, J.W. Shen, Photocatalytic degradation of 2,4,6-trichlorophenol over g-C3N4 under visible light irradiation. Chem. Eng. J. 218, 183–190 (2013)
R.R. Hao, G.H. Wang, H. Tang, L.L. Sun, C. Xu, D.Y. Han, Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl. Catal. B 187, 47–58 (2016)
X.S. Feng, Y. Huang, M.H. Chen, X.F. Chen, C. Li, S.H. Zhou, X.G. Gao, Self-assembly of 3D hierarchical MnMoO4/NiWO4 microspheres for high-performance supercapacitor. J. Alloy Compd. 763, 801–807 (2018)
Y. Liu, Z.H. Yang, P.P. Song, R. Xu, H. Wang, Facile synthesis of Bi2MoO6/ZnSnO3 heterojunction with enhanced visible light photocatalytic degradation of methylene blue. Appl. Surf. Sci. 430, 561–570 (2018)
M.Y. Li, Y.B. Tang, W.L. Shi, F.Y. Chen, Y. Shi, H.C. Gu, Design of visible-light-response core–shell Fe2O3/CuBi2O4 heterojunctions with enhanced photocatalytic activity towards the degradation of tetracycline: Z-scheme photocatalytic mechanism insight. Inorg. Chem. Front. 5, 3148–3154 (2018)
J. Xu, B.F. Luo, W. Gu, Y.P. Jian, F.L. Wu, Y.B. Tang, H. Shen, Fabrication of In2S3/NaTaO3 composites for enhancing the photocatalytic activity toward the degradation of tetracycline. New. J. Chem. 42, 5052–5058 (2018)
F.Y. Chen, X. Zhang, Y.B. Tang, X.G. Wang, K.K. Shu, Facile and rapid synthesis of a novel spindle-like heterojunction BiVO4 showing enhanced visible-light-driven photoactivity. RSC Adv. 10, 5234–5240 (2020)
Acknowledgements
This work was supported by Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX18_2340).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by H-CG, Y-BT and F-YC. The first draft of the manuscript was written by H-CG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, Hc., Tang, Yb., Chen, Fy. et al. Preparation of a novel composite g-C3N4/TiO2/NiWO4 with enhanced photocatalytic activity toward the degradation of rhodamine B. J Mater Sci: Mater Electron 33, 14581–14592 (2022). https://doi.org/10.1007/s10854-022-08379-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08379-3