Abstract
The Bi6B10O24 microwave dielectric ceramics for ultra-low temperature co-fired ceramics application were prepared by solid-state reaction method. The process conditions, phase composition, and microwave dielectric properties of the ceramics were investigated. The results indicated that boron volatilization was effectively avoided by dry ball milling of the raw materials and calcined in sealed environment for the powder preparation and then a single Bi6B10O24 phase ceramic was successfully obtained. The Bi6B10O24 ceramics exhibited microwave dielectric properties: εr = 13.2 ± 0.1, Q × f = 25,000 ± 200 GHz, and τf = − 65 ± 2 ppm/°C. The effect of small excess B2O3 on the phase, microstructure, and microwave dielectric properties on the Bi6B10O24 ceramics were discussed. Bi6B10O24–xB2O3 ceramics (x = 0–0.12 mol%) were sintered at 670–710 °C for 2 h. The XRD patterns of specimens illustrated that only the Bi6B10O24 phase was observed for all the ceramics with varying x. The microwave dielectric properties of the ceramics were found to strongly correlate with the x values and sintering process. The appropriate excess B2O3 is beneficial to densification of the ceramics, effectively reducing the dielectric loss and increasing Q × f. The excellent microwave dielectric properties of the Bi6B10O24 ceramics were obtained for x = 0.1 mol, εr = 12.5 ± 0.1, Q × f = 38,200 ± 300 GHz, and τf = − 62 ± 1 ppm/°C. The high-performance Bi6B10O24 ceramics are promising candidates for ULTCC integration applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of wireless communication, global positioning system (GPS), Internet of Things, and other information industries, the corresponding systems and devices are developing into miniaturization, multi-function, and low cost [1]. Low-temperature co-fired ceramic (LTCC) technology is an important way to realize the miniaturization, integration, and high reliability of components [2, 3]. In practical applications, excellent properties of microwave dielectric materials are required, for example, the appropriate dielectric constant (εr), high quality factor (Q × f), and the near-zero temperature coefficient of the resonant frequency (τf). The research for LTCCs has accelerated in recent years, and ultra-low temperature co-fired ceramics (ULTCC) technology comes up and becomes one of hotspots. ULTCC requires lower sintering to co-firing with other low melting point electrode materials, which is beneficial to reduce energy consumption and improve the compatibility with economical and effective metal electrodes [4].
At current, a large number of excellent microwave dielectric materials have been reported in the literature; however, most of them have high sintering temperatures [5]. Usually, the sintering temperature can be reduced by adding low melting glass, but this method often cause the deterioration of microwave dielectric properties [6]. Therefore, some low melting oxides such as Li2O-, TeO2-, B2O3-, WO3-, and MoO3-based compounds with intrinsically low sintering temperature and promising microwave dielectric properties have been extensively studied [4, 7,8,9]. However, most of these materials have fetal defects, such as hygroscopic, unstable, poisonous, high cost and so on. As we known, B2O3-based microwave dielectric materials have stable chemical properties, and there are few researches on them at present. Ohashi et al. [10] prepared Li3AlB2O6 ceramics by conventional solid-phase reaction method and obtained the microwave dielectric properties of εr = 4.2, Q × f = 13,027 GHz, and τf = 10 ppm/°C. CuO–B2O3–Li2O (CBL) glass–ceramic [11] showed microwave dielectric properties with εr = 5.84, Q × f = 10,120 GHz (at 13.44 GHz), and τf = − 33 ppm/°C.
Until now, borate ceramics with ultra-low sintering temperatures and excellent microwave dielectric properties are limited, so it is necessary to develop new borate ceramics. The B2O3–Bi2O3 system was a low melting point system, and previous researchers were focus on single crystal and glass materials for the optical properties [12,13,14,15,16]. The research on the ceramics was few. Bi4B2O9 and Bi6B10O24 ceramics in the B2O3–Bi2O3 system were firstly prepared as sintering aids by Chen et al. [17] through solid-state reaction and their microwave dielectric properties were reported, εr = 39, Q × f = 2600 GHz, and τf = − 203 ppm/°C for Bi4B2O9 ceramics and εr = 10, Q × f = 10,800 GHz, and τf = − 41 ppm/°C for Bi6B10O24 ceramics. Recently, Wang et al. [18] stoichiometrically weighted Bi2O3 and H3BO3 as raw powders to prepare Bi6B10O24 ceramics, but the Bi4B2O9 phase as second phase was appeared in the Bi6B10O24 ceramics. The volatile of boron caused the second phase to occur in the ceramics. For this reason, excess boron (over 80% of the stoichiometric amount) was added to compensate for boron volatilization in order to obtain a single-phase Bi6B10O24 ceramics. The microwave dielectric properties of the ceramics were εr = 12.14, Q × f = 14,800 GHz, and τf = − 72 ppm/°C. It is obviously that microwave dielectric properties of Bi6B10O24 ceramics are poor and a high amount of excess boron has to be added.
The Bi6B10O24 ceramics exhibit low sintering temperature and are potential for ULTCCs. However, the properties obtained from the current researchers are not excellent enough. In this study, the massive volatilization of boron was avoided by dry ball milling and calcined in sealed environment for the powders preparation, making the actual composition of the ceramics to be consistent with the nominal composition. The effect of small excess B2O3 on the Bi6B10O24 ceramics was discussed. The process conditions, microstructures, and microwave dielectric properties of Bi6B10O24–xB2O3 ceramics (x = 0–0.12 mol%) as a microwave dielectric material for ultra-low temperature sintering are investigated.
2 Experimental
Bi6B10O24–xB2O3 ceramics (x = 0–0.12 mol%) were prepared by the solid-state reaction route. Raw powders of B2O3 and Bi2O3 (AR grade, Sinopharm Chemical Reagent) were weighed as ratio of Bi6B10O24–x B2O3 (x = 0–0.12 mol%). In the ball milling of raw materials, two ball milling methods were used for comparison, wet milling, and dry milling. The wet milling was ball milled for 8 h using agate balls with isopropanol as medium and dried at 85 °C for 8 h. The dry milling was ball milled for 2 h using agate balls without isopropanol as medium. After milling, both powders calcined at 660 °C for 2 h in sealed environment, respectively. The calcined powders were re-milled for 6 h with isopropanol as the dispersive media, dried at 85 °C for 8 h, and pressed into pellets (diameter 12 mm) using 5 wt% PVA as a binder. These pellets were heated at 400 °C for 10 h to eliminate the PVA and sintered at 660–710 °C for 2 h in air.
The crystalline phase of samples were identified by X-ray diffraction (XRD: PANalytical X'Pert PW3050/60 Philips, Netherlands) using Cu Kα (λ = 1.54056 Å) range of 10°–80° (at a speed of 0.2°/s). The microstructures of samples were observed using a scanning electron microscope (SEM: Zeiss Ultra Plus). The particle size was examined by the analysis of the images obtained using SEM. The FT-IR and Raman spectra were collected through Fourier transform infrared spectrometer (Thermo Nicolet) at room temperature. The bulk densities of the sintered pellets were measured by the Archimedes method. The microwave dielectric properties (the εr and the Q × f value) of samples were measured in the TE011 mode using a network analyzer (Agilent HPB8722ET; Agilent Technologies, Santa Clara, CA) and parallel boards. The temperature coefficient of resonant frequency
was experimentally measured in the temperature range of 25–85 °C.
3 Results and discussion
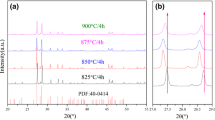
Figure 1a shows the XRD pattern of the Bi/B = 3/5 powder by wet ball milling calcined in the sealed environment. The main phase of the ceramic is the Bi4B2O9 phase (PDF: 01-070-1458), and the Bi6B10O24 (PDF: 01-070-0154) is indexed to the second phase. Figure 1b shows the XRD pattern of the Bi/B = 3/5 powder by dry ball milling calcined in the sealed environment, figuring that a single Bi6B10O24 phase occurs without Bi4B2O9 phase. The cell parameters of Bi6B10O24 are a = 6.5320 Å, b = 7.7330 Å, c = 18.5660 Å, α = β = γ = 90°, and V = 937.80 Å3 with orthorhombic structure in space group Pnma (62) (PDF: 01-070-0154). This result indicates that the volatilization of B2O3 occurs in the drying process after wet ball milling. It reveals that boron volatilization during drying for wet ball milling can effectively avoid by dry ball milling for powders preparation, making the actual composition of the powders to be consistent with the nominal composition, which is an important improvement on previous research results.
Figure 2 shows the XRD patterns for the Bi/B = 3/5 powders calcined for 2 h at 600–680 °C by dry ball milling. At 600–620 °C, Bi4B2O9 and Bi6B10O24 are observed. With the temperature increasing to 640–680 °C, all the peaks are matched with the Bi6B10O24, suggesting the following reaction sequence:
It reveals that the pure Bi6B10O24 phase powders can be prepared at suitable calcined temperature. The calcined temperature of powders in this study is chosen at 660 °C.
Figure 3 shows the relative density and microwave dielectric properties of the Bi6B10O24 ceramics at different sintering temperatures. The εr and τf are basically stable as the sintering temperature changes, because all samples are with pure Bi6B10O24 phase and high density. The Q × f reaches maximum of 25,000 ± 200 GHz at 660–670 °C and decreases significantly as sintering temperature further increasing, indicating that the microwave properties of the ceramics are affected by densification and sintering temperature. As the sintering temperature increases, it is gradually approaching the melting point of Bi6B10O24, resulting in the appearance of excess glass content, which deteriorates microwave dielectric properties of the ceramics.
By improving the powders preparation processes, i.e., dry ball milling and calcining in sealed environment, single Bi6B10O24 phase ceramics sintered at 660–670 °C are obtained in the study: εr = 13.2 ± 0.1, Q × f = 25,000 ± 200 GHz, and τf = − 65 ± 2 ppm/°C. In the previous research of the low melting point B2O3–Bi2O3 system [18], a high amount of excess B2O3 affects the phase composition, microstructure, and microwave dielectric properties of the ceramics. In this work, the low melting point and volatility of B2O3 may cause a very small amount of volatilization during the preparation process even if above improved powders preparation processes are adopted to obtain the pure Bi6B10O24 ceramics. Therefore, a small amount of extra B2O3 is added to compensate for possible volatilization and as sintering additive to promote sintering process.
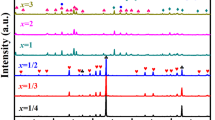
The XRD patterns for Bi6B10O24–xB2O3 ceramics (x = 0–0.12 mol%) sintered at 700 °C for 2 h in sealed environment are shown in Fig. 4. All the peaks of the ceramics with the various boron contents are indexed as Bi6B10O24 phase, and no other phases are detected. It reveals that small excess B2O3 does not change the phase composition of the ceramics.
Bi6B10O24–xB2O3 ceramics (x = 0, 0.1 mol%) were also characterized by FT-IR and Raman spectroscopy. Figure 5 shows the FT-IR absorption spectra and Raman spectra of the Bi6B10O24 ceramics sintered at 700 °C, respectively. The peaks of both samples are compared with the characteristic peaks of the known materials in Bi2O3–B2O3 system [19, 20]. All the peaks in the FT-IR and Raman spectrum of Bi6B10O24–xB2O3 ceramics (x = 0, 0.1 mol%) are well matched with previous reports, and all agree well with the groups of the Bi6B10O24 phase. It can be considered that only the Bi6B10O24 phase is formed in both ceramics. These results also agree well with the above XRD analysis.
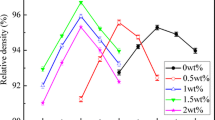
Figure 6 shows the variations of sintering temperature, εr, Q × f, and τf of Bi6B10O24–xB2O3 ceramics (x = 0–0.12 mol%). As x increases from 0 to 0.12, the variations of the εr and τf are not significant. The optimal sintering temperature of specimens increases. The Q × f increases with increasing x values, reaches a maximum of 38,200 ± 300 GHz for x = 0.1, and then decreases when x = 0.12. The εr and τf are mainly determined by the phase composition. According to the above XRD analysis, there is no secondary phase in all the samples. The phase composition of the ceramics is only Bi6B10O24, therefore the εr and τf values of the ceramics have little change and are about 12.5 ± 0.1 and − 62 ± 1 ppm/°C, respectively. The Q × f depends on extrinsic and intrinsic factor. The intrinsic losses are associated with lattice vibration modes while the extrinsic losses are associated with density, second phase, and so on [21, 22]. Therefore, higher density means lower porosity and higher Q × f value. When x = 0, the average grain size of the ceramic is 4.2 μm. With the x increases from 0.03 to 0.1, the average grain sizes of the samples increased from 2.1 to 3.6 μm, as shown in Fig. 7a–e. Obviously, their average grain sizes are smaller than the size of Bi6B10O24–xB2O3 ceramics (x = 0). The grain size of the ceramics decreased with the content of glass increase in it [23]. The excess of B2O3 (x) results in the increase the glass in the ceramics. The average grain size is obviously affected by the x and sintering temperature. The appropriate excess B2O3 makes porosity decreases and densification of the ceramic at the optimum sintering temperature. The best microwave dielectric properties are obtained when x = 0.1, εr = 12.5 ± 0.1, Q × f = 38,200 ± 300 GHz, and τf = − 62 ± 1 ppm/°C. This phenomenon is mainly due to the liquid phase formed by the excess B2O3, which reduces the porosity of the ceramic, promotes the release of stress, and makes the ceramic sintered densely, thereby reducing the dielectric loss and increasing Q × f. Almost all the samples could be densified at their optimal sintering temperature. At x = 0.12, more glasses exist, resulting in the grain size decrease with average size of 1.6 μm and the porosity increase (Fig. 7f) and the Q × f decrease. The more extra B2O3 deteriorates the microwave dielectric properties of the ceramics. It illustrates that an appropriate excess B2O3 promotes the densification of ceramics and improves their microwave dielectric properties.
The relative density and microwave dielectric properties of ceramics (x = 0.1) at different sintering temperatures are exhibited in Fig. 8. It shows that the microwave dielectric properties are greatly affected by the sintering temperature. The ceramics can be densified at 700–710 °C, and the microwave dielectric properties is significantly improved. With the sintering temperature from 660 to 710 °C, the average grain sizes increase (Fig. 9a–f), and the average grain sizes are 1.5, 1.5, 1.8, 2.5, 2.5, and 3.6 μm, respectively. The best microwave dielectric properties are obtained for 2 h, εr = 12.5 ± 0.1, Q × f = 38,200 ± 300 GHz, and τf = − 62 ± 1 ppm/°C.
The microwave dielectric properties of the ceramics studied in the Bi2O3–B2O3 system are listed in Table 1. In the previous studies of Bi2O3–B2O3 system, Bi6B10O24 has better microwave dielectric properties. However, previous studies have failed to obtain excellent properties of the pure Bi6B10O24 ceramic which limit their applications. The enhanced microwave dielectric properties of the ceramics with pure Bi6B10O24 phase are obtained in this study, with an εr of 12.5 ± 0.1, a high Q × f of 38,200 ± 300 GHz, and a τf of − 62 ± 1 ppm/°C, and the consistency of the raw material ratio and the target Bi6B10O24 phase are guaranteed in our work, showing the potential of the Bi6B10O24 ceramics in the field of excellent ULTCC microwave dielectric materials.
4 Conclusion
Bi6B10O24 ceramics in the Bi2O3–B2O3 system with high Q × f were investigated. The boron volatilization was effectively avoided by dry ball milling of the raw materials and calcined in sealed environment for the powder preparation, and a single Bi6B10O24 phase ceramic was successfully obtained. Comparing with previous work, the Bi6B10O24 ceramics exhibited enhanced microwave dielectric properties of εr = 13.2 ± 0.1, Q × f = 25,000 ± 200 GHz, and τf = − 65 ± 2 ppm/°C. The effect of small excess B2O3 on the Bi6B10O24–xB2O3 ceramics (x = 0–0.12 mol%) was discussed. As x changed from 0 to 0.12, the excess B2O3 does not change the phase composition of the ceramics, while the microwave dielectric properties of Bi6B10O24 ceramics changed significantly. Appropriate excess B2O3 promotes the densification of ceramics and improves their microwave dielectric properties. When x = 0.1, sintered at 710 °C for 2 h, Bi6B10O24 ceramics possess excellent microwave dielectric properties of εr = 12.5 ± 0.1, Q × f = 38,200 ± 300 GHz, and τf = − 62 ± 1 ppm/°C. The ultra-low sintering temperature and excellent properties reveal the potential of Bi6B10O24 ceramics for applications as a new ULTCC material.
Data availability
All data analyzed are used to support this study and are included in the submitted article.
References
J. Xi, G.H. Chen, F. Liu, F. Shang, J.W. Xu, C.R. Zhou, C.L. Yuan, Synthesis, microstructure and characterization of ultra-low permittivity CuO–ZnO–B2O3–Li2O glass/Al2O3 composites for ULTCC application. Ceram. Int. 45, 24431–24436 (2019)
J. Dhanya, E.K. Suresh, R. Naveenraj, R. Ratheesh, Synthesis and characterization of Na5M(MoO4)4 (M = Y, Yb) microwave ceramics for ULTCC applications. Ceram. Int. 44, 6699–6704 (2018)
J. Zhou, Towards rational design of low-temperature co-fired ceramic (LTCC) materials. J. Adv. Ceram. 1, 89–99 (2012)
H.C. Xiang, Y. Bai, J. Varghese, C.C. Li, L. Fang, H. Jantunen, Ultralow temperature cofired BiZn2VO6 dielectric ceramics doped with B2O3 and Li2CO3 for ULTCC applications. J. Am. Ceram. Soc. 102, 1218–1226 (2018)
M.T. Sebastian, H. Wang, H. Jantunen, Low temperature co-fired ceramics with ultra-low sintering temperature: a review. Curr. Opin. Solid State Mater. Sci. 20, 151–170 (2016)
D. Zhou, L.X. Pang, H.D. Xie, J. Guo, B. He, Z.M. Qi, T. Shao, X. Yao, C.A. Randall, Crystal structure and microwave dielectric properties of an ultralow-temperature-fired (AgBi)0.5WO4 ceramic. Eur. J. Inorg. Chem. 2014, 296–301 (2014)
H.D. Xie, H.H. Xi, F. Li, C. Chen, X.C. Wang, D. Zhou, Microwave dielectric properties of Pb2MoO5 ceramic with ultra-low sintering temperature. J. Eur. Ceram. Soc. 34, 4089–4093 (2014)
H.T. Yu, J.S. Liu, W.L. Zhang, S.R. Zhang, Ultra-low sintering temperature ceramics for LTCC applications: a review. J. Mater. Sci. Mater. Electron. 26, 9414–9423 (2015)
M.Y. Yu, Y. Tang, J. Li, W.S. Fang, L. Duan, L. Fang, Microwave dielectric properties and chemical compatibility with alumina electrode of two novel ultra-low temperature firing ATeMoO6 (A = Mg, Zn) ceramics. Ceram. Int. 46, 25619–25625 (2020)
M. Ohashi, H. Ogawa, A. Kan, E. Tanaka, Microwave dielectric properties of low-temperature sintered Li3AlB2O6 ceramic. J. Eur. Ceram. Soc. 25, 2877–2881 (2005)
M.S. Ma, Z.Q. Fu, Z.F. Liu, Y.X. Li, Fabrication and microwave dielectric properties of CuO–B2O3–Li2O glass ceramic with ultra-low sintering temperature. Ceram. Int. 43, S292–S295 (2017)
P. Becker, Thermal and optical properties of glasses of the system Bi2O3–B2O3. Cryst. Res. Technol. 38, 74–82 (2003)
M. Burianek, M. Mühlberg, Crystal growth of boron sillenite Bi24B2O39. Cryst. Res. Technol. 32, 1023–1027 (1997)
N.M. Bobkova, Properties and structure of bismuth–borate glasses (review). Glass Ceram. 72, 360–365 (2016)
G.M. Kuzmicheva, T.I. Mel’nikova, Structural features of bismuth borates in the system nBi2O3–mB2O3. Russ. J. Inorg. Chem. 54, 73–80 (2009)
A. Bajaj, A. Khanna, B.H. Chen, J.G. Longstaffe, U.W. Zwanziger, J.W. Zwanziger, Y. Gomez, F. Gonzalez, Structural investigation of bismuth borate glasses and crystalline phases. J. Non-Cryst. Solids 355, 45–53 (2009)
X.Y. Chen, W. Zhang, B. Zalinska, I. Sterianou, S. Bai, I.M. Reaney, Low sintering temperature microwave dielectric ceramics and composites based on Bi2O3–B2O3. J. Am. Ceram. Soc. 95, 3207–3213 (2012)
K.G. Wang, T.T. Yin, H.F. Zhou, X.B. Liu, J.J. Deng, S.X. Li, C.M. Lu, X.L. Chen, Bismuth borate composite microwave ceramics synthesised by different ratios of H3BO3 for ULTCC technology. J. Eur. Ceram. Soc. 40, 381–385 (2020)
A.V. Egorysheva, V.I. Burkov, Y.F. Kargin, V.G. Plotnichenko, V.V. Koltashev, Vibrational spectra of crystals of bismuth borates. Crystallogr. Rep. 50, 127–136 (2005)
A.V. Egorysheva, V.I. Burkov, V.S. Gorelik, Y.F. Kargin, V.V. Koltashev, V.G. Plotnichenko, Raman scattering in monocrystal Bi3B5O12. Phys. Solid State 43, 1655–1658 (2001)
C.F. Tseng, P.J. Tseng, C.M. Chan, Y.C. Kao, Novel temperature stable Li2MnO3 dielectric ceramics with high Q for LTCC applications. J. Am. Ceram. Soc. 97, 1918–1922 (2014)
A.V. Trukhanov, N.A. Algarou, Y. Slimani, M.A. Almessiere, A. Baykal, D.I. Tishkevich, D.A. Vinnik, M.G. Vakhitov, D.S. Klygach, M.V. Silibin, Peculiarities of the microwave properties of hard–soft functional composites SrTb0.01Tm0.01Fe11.98O19–AFe2O4 (A = Co, Ni, Zn, Cu, or Mn). RSC Adv. 10, 32638–32651 (2020)
D.S. Liu, C.W. Zhong, T.Y. Qin, Q. Yang, B. Tang, S.R. Zhang, Low-temperature sintering of CaMgSi2O6–KBS composites with ultralow dielectric constant. Ceram. Int. 46, 17818–17824 (2020)
D. Zhou, C.A. Randall, H. Wang, L.X. Pang, X. Yao, Microwave dielectric ceramics in Li2O–Bi2O3–MoO3 system with ultra-low sintering temperatures. J. Am. Ceram. Soc. 93, 1096–1100 (2010)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51772225).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
SS and QZ contributed to all the experimental work, completed the data analysis, and wrote the manuscript. YD guided all the experimental design and led the manuscript revision work. XP approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, S., Zhang, Q., Dai, Y. et al. Enhanced microwave dielectric properties of Bi6B10O24 ceramics as ultra-low temperature co-fired ceramics materials. J Mater Sci: Mater Electron 33, 13604–13613 (2022). https://doi.org/10.1007/s10854-022-08295-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08295-6