Abstract
In this study, the phase structure, microstructure, and dielectric properties of BT-xKNN ceramic prepared by traditional solid-phase method were studied. The diffraction peaks of KNN-doped BaTiO3 ceramics shifted to high angles. Excessive KNN doping inhibited BaTiO3 grain growth. KNN doping improved the dielectric constant of BaTiO3 ceramics. When the amount of KNN doped was 0.5 wt%, the dielectric constant can reach 19,359.4. When KNN was doped at 1wt%, the dielectric peak was broadened and the dielectric temperature stability was good. However, the incorporation of KNN increased the dielectric loss of BaTiO3 ceramics, which may be caused by the increase in conduction loss caused by Nb5+. Moreover, the incorporation of KNN did not increase the Curie temperature of BaTiO3 ceramics, but decreased it, which may be related to the decomposition of KNN during high-temperature sintering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, with the rapid growth of the electronic information industry, it has promoted the rapid development of the electronic components industry [1]. As the base material of electronic components, electronic ceramics have increased demand for use and more and more applications [2, 3]. BaTiO3-based ceramics are a kind of electronic ceramics with many applications and are typical [4]. BaTiO3 replaces PbTiO3-based ferroelectric materials that pollute the environment in the early days and become an environmentally friendly ferroelectric ceramic material that meets the requirements of sustainable development [5], due to its excellent electrical properties, such as ferroelectricity, dielectric properties, piezoelectricity, and pyroelectricity. It has a wide range of applications in the fields of capacitors, energy storage devices, transducers, and actuators [6]. However, with the change of temperature, the dielectric constant of pure-phase BaTiO3 ceramics changes greatly. Therefore, it is necessary to optimize its performance to obtain ceramics with high dielectric constant and high-temperature stability.
The research on BaTiO3 mainly uses doped solid solution to improve the performance of BaTiO3 ceramics. Wen, Yu, and Liu used the solid-phase method and the sol–gel method to study and analyze the (1-x)BaTiO3-xBiAlO3 multiphase ceramic system. The study found that the samples all exhibited relaxation ferroelectric properties and with the increase of the amount of doping, the dielectric peak of the system gradually broadens and the relaxivity increases [7,8,9]. Khare studied the influence of holding time on the dielectric properties of 0.9BaTiO3-0.1CaCu3Ti4O12 ceramics and found that the dielectric constant of the sample held for 3 h under the condition of low frequency 1 kHz is the largest and the corresponding loss value is also the smallest [10]. Jiang used the solid-phase method of dual-ion Al3+ and Nb5+-co-doped BaTiO3 ceramics. Due to the difference in valence between substituted and substituted ions, more vacancy defects are formed in the system, which affect the final dielectric properties of the system [11]. Zhao and Zhang prepared (1-x) BaTiO3-xCuO ceramics by a solid-phase method. The study showed that with the increase of copper oxide doping content, the dielectric constant of the system showed a trend of first increasing and then decreasing. When it is 0.01, the relative dielectric constant at room temperature is 2040 and the dielectric loss value is 0.8% [12, 13].
In recent years, with the promulgation of environmental regulations, high-performance lead-free dielectric materials have been paid more and more attention in the context of overall research [14]. The most researched materials are K0.5Na0.5NbO3 (KNN) based, BaTiO3 (BT) based, Bi0.5Na0.5TiO3 (BNT) based, bismuth layered structure and tungsten bronze structure, and other ceramics [15,16,17,18]. Although BT-based ceramics have excellent dielectric properties, their lower Curie temperature severely limits its application at high temperatures [16]. KNN-based ceramics have a high Curie temperature (420 °C) and in a low-intensity driving electric field, KNN ceramics can obtain high unipolar strain and narrow strain hysteresis [15]. Therefore, this article intends to improve the performance of BT-based ceramics by doping KNN.
2 Experimental part
The experimental process of preparing BaTiO3 (BT) powder and K0.5Na0.5NbO3 (KNN) powder by solid-phase method is the same, only the pre-sintering temperature and time are different. Consulting the relevant literature and the related literature [19,20,21,22], BaTiO3 uses BaCO3 and TiO2 as raw materials in stoichiometric proportions, wet ball milling for 24 h, drying at 70 °C, and then sintering the mixture obtained after passing through a 100-mesh sieve at 1100 °C for 4 h to obtain BaTiO3 powder. K0.5Na0.5NbO3 (KNN) uses K2CO3, Na2CO3, and Nb2O5 as raw materials and mixes the materials according to the above method. After drying and sieving, they are pre-fired at 850 °C for 2 h to obtain the required powder. Through XRD pattern analysis, BaTiO3 powder and KNN powder with good crystallinity were obtained. According to the mass percentage ratio BT-xKNN (x = 0.1, 0.3, 0.5, 0.7, 1 wt%), ball milling for 12 h, after drying and sieving, adding an appropriate amount of 5 wt% PVA to the powder to carry out granulation, then forms a tablet under a pressure of 6–10 MPa, and then sinters it into porcelain at a certain sintering temperature. After polishing and cleaning, the silver paste is coated and fired and the obtained samples are tested for dielectric properties.
The phase structure test of the ceramic sample was carried out with the Rigaku Corporation D/max2500V/pc X-ray diffractometer and the surface of the ceramic sample was tested with the Hitachi S-4800 scanning electron microscope (SEM). Agilent E4980A high-temperature dielectric temperature spectrum test system performs dielectric temperature spectrum test on samples and uses 6500B impedance analyzer to analyze the influence of frequency on dielectric properties.
3 Results and discussions
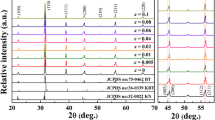
Figure 1 is the XRD pattern of the KNN powder material calcined at 850 °C for 12 h. Through the analysis of Jade software, it is found that the powder sample prepared in the experiment has the same spectrum as the NaNbO3 powder PDF card (NO.73-1106), which proves that the pure-phase KNN crystal is synthesized and the main crystal phase diffraction peak is sharp, which proves that the pure-phase KNN powder has good crystallinity.
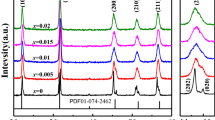
Figure 2 shows the XRD pattern of BT-KNN-based ceramics sintered at 1350 °C for 4 h. In order to show the effect of KNN doping on the phase structure of BaTiO3 ceramics more clearly, the diffraction peaks near the (200) crystal plane are magnified. It can be seen from Fig. 2a that the BaTiO3 doped with KNN still maintains the perovskite structure, which is similar to the PDF card (NO.05-0626) spectrum of BaTiO3 powder and there is no miscellaneous phase. The (101) crystal plane diffraction peak is sharp and the crystallinity is very good, but its peak height is different. Doping KNN less than 0.5% can enhance the main peak height and increase the crystallinity. From Fig. 2b, it can be seen that the BaTiO3 ceramic (200) doped with KNN is offset in the high-angle direction. This may be because KNN is incorporated into the BaTiO3 lattice in the form of K+, Na+, and Nb5+ ions. Their ionic radius are K+(0.138 nm), Na+(0.102 nm), Nb5+(0.064 nm), and Ba2+(0.135 nm). According to Bragg Eq. (2dsinθ = n), the crystal plane spacing d decreases and then the diffraction angle θ will increase large. So the ions with a smaller radius are incorporated into the BaTiO3 lattice and the diffraction peaks shift to the high-angle direction [23, 24].
Figure 3a–f is the SEM spectra of BT-KNN-based ceramic samples, where a–f is the amount of KNN doped, which is 0%, 0.1%, 0.3%, 0.5%, 0.7%, and 1%. Figure 4 is the EDS energy spectrum analysis diagram of the BT-0.5% KNN-based ceramic sample. It can be seen from Fig. 3 that with the increase of KNN doping, the grain size of BaTiO3 ceramics first increases and then decreases. Pure-phase BaTiO3 ceramics have a grain size of about 3 µm. When 0.1% KNN is incorporated, the grain size is about 10 µm. When 0.3% KNN is incorporated, the grain size is about 5 µm. When 0.5% KNN is doped, combined with the analysis of EDS energy spectrum when 0.5% KNN is doped in Fig. 4, two crystal grains appear. The small crystal grain size is similar to that of pure-phase BaTiO3, and the crystal grain size is about 3 µm. The large grain size is about 50 ~ 70 µm. When doped with 0.7% KNN, the grain size is about 3 µm. When doped with 1% KNN, it can be clearly seen that the grain size is already less than 1 µm.
Table 1 shows the density change of BaTiO3 ceramics with different amounts of KNN, and Fig. 5 shows the density and shrinkage changes of BT-xKNN ceramics. It can be seen from Fig. 5 that as the amount of KNN added increases, the density of BaTiO3 ceramics first increases and then decreases. When the doping amount of KNN is 0.1%, the density is 5.8028 g/cm3, which is the highest, and the incorporation of a large amount of KNN inhibits the crystal growth and reduces the density. This corresponds to the appearance of the ceramic in Fig. 3.
Table 2 shows the Curie temperature and dielectric constant of BT-xKNN ceramics. It can be seen from Table 2 that the Curie temperature of pure- phase BaTiO3 is 123.8 °C and the dielectric constant is 2441.5. With the incorporation of KNN, the Curie temperature decreases while the dielectric constant increases. The reason for the increase of the dielectric constant is that the donor element is doped to release the charge and the dipole increases, which leads to the increase of the dielectric constant [25]. The actual Curie temperature of the KNN ceramic material is 420 °C. In theory, the high Curie temperature should increase the Curie temperature of BaTiO3, but actually lowers the Curie temperature. This may be because K2O and Na2O volatilize at around 900 °C and the temperature is too high, which leads to the imbalance of the KNN stoichiometric ratio. Figure 6 shows the KNN phase diagram. From the KNN phase diagram, it can be seen that the phase structure is very complex and the stoichiometric ratio is very complicated. The imbalance caused by cooling and crystallization may not be the material with high Curie point. The decrease in Curie temperature may also be due to the fact that KNN is incorporated into BaTiO3 in the form of K+, Na+, and Nb5+ into the BaTiO3 lattice. The ionic radius of these elements is smaller than that of Ba2+, and the decrease in c/a reduces the Curie temperature.
Figure 7 is a graph showing the change in dielectric constant versus temperature and the change in dielectric loss versus temperature of BaTiO3 ceramics with different KNN doping levels. It can be seen from Fig. 7a that the dielectric constant of pure-phase BaTiO3 will increase stepwise near the Curie temperature and a dielectric peak appears. This is because BaTiO3 undergoes a ferroelectric–paraelectric transition near the Curie temperature. The BaTiO3 crystal form changes from a tetragonal phase to a square phase and the polarization direction changes and spontaneous polarization increases [25]. When the amount of KNN incorporated is less than 0.5%, it can be found that the dielectric peak is enhanced relative to the pure-phase BaTiO3, which may be beneficial to the increase in the lift-to-drag ratio of PTC ceramics. As KNN continues to increase, the Curie peak appears to have a voltage peak, the dielectric peak is broadened, and the stability of the dielectric constant is improved. It can be seen from Fig. 7b that the dielectric loss after doping KNN is greater than the dielectric loss of pure-phase BaTiO3 ceramics, which may be caused by the increase of conductivity loss when KNN is doped.
Figure 8 is the change of dielectric constant with frequency and the change of dielectric loss with frequency of BaTiO3 ceramics with different KNN doping levels. It can be seen from Fig. 8a that when the amount of KNN incorporated is 0.5%, the dielectric constant is much larger than that of other groups and the dielectric constant decreases with the increase of frequency. This may be because the samples with 0.5% KNN are mainly space charge polarization. This corresponds to Fig. 8b, which is dominated by conductance loss and large dielectric loss. The other component samples are mainly relaxed polarization.
4 Conclusion
BT-xKNN (x = 0, 0.1, 0.3, 0.5, 0.7, 1wt%) ceramics were prepared by solid-phase method. The results show that the diffraction peaks of KNN-doped BaTiO3 ceramics shifted to high angles and the grain size first increased and then decreased with the increase of KNN doping. The Curie temperature of the BaTiO3 ceramic doped with KNN decreases and the dielectric constant increases. But the dielectric loss also increases. When the amount of KNN incorporated is 0.5wt%, the minimum Curie temperature is 110.2 °C and the maximum dielectric constant is 19359.4. With the increase of KNN doping amount, a pressure peak phenomenon appears, the dielectric peak is broadened, and the temperature stability of the dielectric constant is improved.
References
Y.C. Han, Research on the application and prospect of electronic functional materials. Electron. Compon. Inf. Technol. 2020(6), 4–5 (2020)
N. Teng, H. Wang, Research on management improvement of electronic component data information database. Electron. World 603(21), 84–86 (2020)
X.Y. Li, Study on modification of ST semiconductor ceramic materials. University of Electronic Science and Technology of China, 2019.
X.L. Gao, Study on dielectric properties and defect chemistry of BaTiO3 ceramics co-doped with R/Ca(R=Tb,Ho) and Pr/Ce. Jilin Institute of Chemical Technology, 2020.
S.L. Jin, G.F. Fan, W.Z. Lu et al., Research progress of piezoelectric ceramics based on BiScO3-PbTiO3with high Curie temperature. Electron. Compon. Mater. 2017(1), 8–13 (2017)
R.Q. Zhu, Dielectric composite materials and applications. Inf. Rec. Mater. 21(10), 15–16 (2020)
M. Wei, J. Zhang, K. Wu et al., Effect of BiMO3 (M=Al, In, Y, Sm, Nd, and La) doping on the dielectric properties of BaTiO3 ceramics. Ceram. Int. 43(13), 9593–9599 (2017)
H. Yu, Z.G. Ye, Dielectric properties and relaxor behavior of a new (1x)BaTiO3-xBiAlO3 solid solution. J. Appl. Phys. 103(3), 241 (2008)
M. Liu, H. Hua, Y. Zhen et al., Temperature stability of dielectric properties for xBiAlO3-(1–x)BaTiO3 ceramics. J. Eur. Ceram. Soc. 35(8), 2303–2311 (2015)
A. Khare, S.S. Yadava, K.D. Mandal et al., Effect of sintering on the dielectric properties of 0.5BaTiO3–0.5Bi2/3Cu3Ti4O12 nanocomposite synthesized by solid state route. J. Mater. Sci. 164, 1–6 (2016)
X.F. Jiang, S.H. Ding, T.X. Song et al., Dielectric properties of BaTiO3 ceramics doped with Al3+ and Nb5+. Ferroelectrics 488(1), 178–185 (2015)
J.J. Zhao, Y.P. Pu, P.P. Zhang et al., Effect of CuO Doping on sintering behavior and dielectric properties of Ba0.96(Bi0.5K0.5)0.04TiO3 ceramics. J. Synth. Cryst. 43(10), 2620–2624 (2014)
Z. Zhang, J.L. Zhang, S.F. Shao et al., Influence of CuO additive on physical properties of BaTiO3 ceramics. Mater. Sci. Forum 687, 287–291 (2011)
T. Zheng, J. Wu, D. Xiao et al., Recent development in lead-free perovskite piezoelectric bulk materials. Prog. Mater Sci. 98, 552–624 (2018)
R.J. Zhao, Study on doping modification and phase structure of KNN-based piezoelectric ceramics. North China University of Science and Technology, 2020
W.L. Ma, Dielectric properties and impedance study on doped barium titanate. Xi’an University of Science and Technology, 2019
W.S. Kang, Study on the modification of lead-free piezoelectric ceramics of bismuth sodium titanate. North China University of Science and Technology, 2020.
H. Hao, Study on processing and properties of bismuth layered structure SrBi4Ti4O15 is piezoelectric ceramies. Wuhan University of Technology, 2005.
X. Lai, Preparation of Mg-doped BaTiO3-based dielectric ceramics and study on its dielectric properties. Wuhan University of Technology, 2020.
W.L. Ma, Dielectric and impedance properties study on doped barium titanate. Xi’an University of Science and Technology, 2019.
Y. Liu, Preparation and properties of potassium sodium niobate-based ceramic materials. Qilu University of Technology, 2020.
Y.Y. Liu, Phase structure and electrical properties of BKH modified KNN-based lead-free piezoelectric ceramics. Southwest University, 2020.
N.R. Yang, Inorganic non-metallic materials test methods. Wuhan University of Technology Press, 1993.
P.W. Lu, Science foundation of inorganic materials. Wuhan University of Technology Press, 1996.
Q.J. Ning, G.Q. Tan, Y.S. Shi, Physical properties of inorganic materials. Chemical Industry Press, 2006.
Acknowledgements
This research was supported by National Defense Science and Technology Key Laboratory Fund (Grant No. 61420050301) and WuXi Noble Electronics Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, S., Dong, G., Guan, R. et al. Effect of KNN doping on the dielectric properties of BaTiO3 lead-free ceramics. J Mater Sci: Mater Electron 33, 810–816 (2022). https://doi.org/10.1007/s10854-021-07351-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07351-x