Abstract
It is important to examine the mechanism of the metal–insulator transition (MIT) of vanadium dioxide (VO2), which is of great significance, to understand the considerably related properties required for developing smart window applications. Here, we investigated the effect of the MIT on the thermochromic performance of VO2. We prepared Si-doped VO2 thin films and then used the sol–gel approach to deposit these thin films on Al2O3. X-ray diffraction (XRD) results showed that there was a metastable phase M2 in the VO2 film, which is classified as a Mott insulator owing to electron correlation between the undimerized V ions. Additionally, we found that 3% Si-doped VO2 thin films exhibited good thermochromic performance with a luminous transmittance (ΔTlum) of 54.7% and a sol modulation efficiency (ΔTsol) of 13.9%, which is superior to that of undoped VO2 films with ΔTlum of 37.2% and ΔTsol of 7.3%. This can be attributed to simultaneous control of the transmittance in the visible and near-infrared regions of Si-doped VO2 thin films. Therefore, this study provides a novel method of tuning the optical and electrical characteristics of VO2 thin films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As a strongly correlated material, vanadium dioxide (VO2) can undergo a first-order phase transformation at ~ 340 K and has a reversible structural phase transformation from the M1 phase with low temperature to the R phase with high temperature [1,2,3]. For the M1 phase, the structural feature of VO2 is a zigzag-type chain comprising V-atom dimers twisting down the R structure’s c-axis (cR). This will cause abrupt decrease in optical transmittance and electrical resistivity of multiple orders of magnitude in the event of metal–insulator transition (MIT) [4, 5]. These unique characteristics make VO2 a potential material for various applications, such as smart windows [6], Mott transistors [2], sensors [7], and memory devices [8]. The mechanism of the MIT in VO2 is not conclusive; it has been suggested that there are three major mechanisms. The first mechanism is a structure-driven Peierls transition explained by the formation of V–V dimers along the rutile cR below the phase transition temperature (Tc). The second mechanism is an electron-correlation-driven Mott transition. The third mechanism is a combination of the Peierls and Mott transitions. Many studies have shown that there is another low temperature metastable M2 phase (C2/m) that is closely related to VO2 between the M1 and R phases [9, 10]. According to a previous study [11], the structure of the Mott insulation in the M2 phase is similar to that in the M1 phase, except that half of the V atoms continue to show dimerization in cR, while the remaining half form a zigzag-type chain with equal spacing and a local moment of Heisenberg 1/2 spin.
VO2 thin films have not been used in any recent practical applications of smart windows owing to three major challenges: low sol modulation efficiency (ΔTsol) (< 10%), low luminous transmittance (ΔTlum) (< 60%), and high Tc. Several attempts have been made to control Tc and improve the optical performance of VO2 thin films using doping elements [12,13,14,15], designing multilayer structures [16,17,18,19], and forming composite films [20]. For example, 2.93% F-doped VO2 nanoparticles can decrease the Tc to 35 °C and increase ΔTsol to 10.7%, which can meet the requirements of reducing Tc and improving the thermochromic performance [13]. Mg-doped VO2 leads to a wide bandgap, reducing Tc to 35 °C and improving ΔTsol and ΔTlum [16]. A buffer layer of TiO2 films can increase Tc to 64 °C, with Tlum and ΔTsol values of 53% and 5.2%, respectively [18]. Controlling the elemental doping ratio of VO2 thin films is an effective method to change Tc, ΔTsol and ΔTlum.
Si-doped VO2 films have been studied; however, the effect of Si dopants on the MIT is not clear and the optical properties of such films have not been greatly improved. For example, Wu et al. [21] observed that Tc was first adjusted to a low temperature and then irregularly transited to a high temperature in some Si-doped VO2 films. Krammer et al. [22] reported that Si-doping increases Tc (an increase of ~ 4.5 °C was reached). Meanwhile, Wu et al. [23] showed that Si-doped VO2 films increased Tlum to 36.1% and ΔTsol to 9.2% and decreased Tc to 46.1 °C. Therefore, it is necessary to experimentally explain the effects of Si dopants on the MIT and optical properties of VO2 thin films.
In this study, we grew undoped and Si-doped VO2 thin films on Al2O3 substrates using the sol–gel method. We systematically analyzed the crystal structure, electrical transport, and optical properties of these films. The results showed that a certain amount of Si-doping improves the optical properties, which considerably affects the practical applications of VO2 thin films in smart windows.
2 Experimental details
In this study, we successfully prepared Si-doped VO2 films at different Si concentrations on Al2O3 substrates using the sol–gel method. First, 0.67 g of VO(acac)2 and SiO2 powders of different concentrations were added to 20 mL of anhydrous methanol, and then, the solution was stirred for 5 min using a magnetic stirrer. Thereafter, we added 3.5 mL of hydrochloric acid (pH = 1) to the solution. Finally, 0.71 g of polyvinylpyrrolidone was added to the solution to form a sol. The concentration of SiO2 [SiO2/VO(acac)2 + SiO2] varied in the range of 0%, 1%, 3%, 5%, 8% and 10%, respectively. Before preparing the films, we conducted ultrasonic cleaning of the Al2O3 substrates two times for 15 min using acetone, deionized water, and anhydrous ethanol. The prepared sols were coated onto the Al2O3 substrates using a spinning coater at 3000 rpm for 15 s. The samples were first heated in an oven at 80 °C for 10 min and then annealed in a tubular furnace at 550 °C under Ar atmosphere.
X-ray diffraction (XRD, Rigaku Smartlab SE X-ray diffractometer) was conducted to analyze the crystal structures of VO2 thin films. X-ray photoelectron spectroscopy (XPS, Escalab 250Xi) was used to determine the elemental compositions of all the thin films in the experiment using an Mg Kα X-ray source with a resolution of 0.45 eV under ultrahigh vacuum conditions. We investigated each thin film for its electrical transport characteristics using four-point probe modules in the physical property measurement system (Quantum Design PPMS-9). In addition, an ultraviolet–visible spectrophotometer (UV–Vis, CARY5000, Varian) with an additional heating module was used to measure the optical characteristics of all of the VO2 films.
The wavelength ranges of Tlum and Tsol are between 380–780 nm and 300–2500 nm, respectively. The Tsol and Tlum values of all the VO2 thin films can be calculated using the following integral formula: \(T_{i} = \int {\Phi_{i} } \left( \lambda \right)T\left( \lambda \right){\text{d}}\lambda /\int {\Phi_{i} } \left( \lambda \right){\text{d}}\lambda\). Here, T(λ) is the film transmittance under the λ wavelength, i represents sol or lum, Φsol is the solar irradiance spectrum at 1.5 air mass, and Φlum is the standard luminous efficiency function (i.e., when the Sun is located 37° above the horizon). ΔTsol and the near-infrared switching efficiency (ΔT2000 nm) are obtained such that ΔT = T(T < Tc) − T(T < Tc). The formula of ΔTlum can be expressed as follows: ΔTlum = (Tlum (T < Tc) + Tlum (T < Tc))/2.
3 Results and discussion
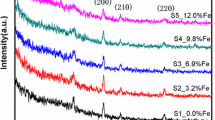
Figure 1 shows the typical XRD measurement of the Si-doped VO2 crystalline film structures deposited on the Al2O3 substrates. We used a simple substrate, Al2O3, to facilitate the testing of the optical properties. Only three major diffraction peaks were observed for the VO2 films, with ~ 27.8° corresponding to VO2 M1 (011) and the other two peaks at ~ 37.6° and 41.8° corresponding to Al2O3 (0001) Kβ and Al2O3 (0001) Kα, respectively. Figure 1b shows the magnified view of Fig. 1a, illustrating that the diffraction peaks migrated to larger diffraction angles as the Si-doping concentration increased. The average grain size of crystal faces of the thin films can be determined using the Scherer equation: D = Kλ/Bcosθ. Here λ, K, θ, and B represent the XRD wavelength (0.1541 nm), Scherrer constant (0.89), diffraction angle, and full width at half-maximum, respectively. Using this formula, the average particle size reduction of the Si-doped films can be calculated (Table 1). This phenomenon may be attributable to the disorder in the VO2 crystal lattice, indicating that Si-doping increases the number of nucleation centers [24]. The lattice parameter (d-spacing) was calculated using the Bragg equation: 2dsinθ = nλ. Here, θ represents the diffraction angle, n is an integer, and λ is the wavelength. According to the calculations, the d-spacing decreased from 0.3168 nm for an undoped film to 0.3205 nm for a film with 10% Si-doping. This phenomenon may occur because Si4+ (0.04 nm) has a slightly smaller radius than V4+ (0.058 nm). Additionally, the reduction in the d-spacing may indicate the presence of in-plane tensile stress [25].
Figure 2 shows the XRD patterns for the undoped and 3% Si-doped VO2 films at various temperatures. Examining the XRD patterns at various temperatures enables a further investigation of the structural changes associated with MIT, which is an effective way to observe accurate nanoscale alterations in the lattice structure. All samples were allowed to stabilize for 2 min at each temperature, followed by the measurement of the XRD patterns under the constant temperature in 0.1 °C. In Fig. 2a1, two diffraction peaks were observed at 27.8° and 27.7° associated with the M1 phase (011) and R phase (110) planes, respectively. Apart from the traditional major peak shift from M1(011) to R(110), another diffraction peak appeared at 28.25° associated with M2 (201) at the temperatures of 35–84 °C. When the temperature increased to 87 °C, the predominant R phase was observed and the M2 phase disappeared. Figure 2a2 shows the cooling XRD patterns for the undoped VO2 films. The XRD patterns in inset show Tc at 78.3 °C and 45.1 °C in the heating and cooling stages, respectively. Figure 2b1 shows that the M2 phase can be observed when the temperature increases to 60 °C after 3% Si-doping. As the temperature increases to 90 °C, the R phase begins to dominate and the M2 phase gradually disappears. Figure 2b2 shows that, after 3% Si-doping, the M2 phase can be clearly observed when the temperature drops to 70 °C in the cooling phase test. As the temperature drops to 30 °C, the M1 phase begins to dominate and the M2 phase gradually disappears. The XRD patterns in the inset of Figs. 2b1 and b2 show Tc at 79 °C and 45.9 °C in the heating and cooling stages, respectively.
To analyze the valence states of the V and O atoms on the surface of the Si-doped VO2 films, we acquired the V 2p and O 1s core-level XPS spectra (Fig. 3). The C 1s peak (284.6 eV) was used to correct the positive charge effect-induced shift. The core-level binding energy of the O 1s peak can be deconvoluted into two peaks located at ~ 530.2 and 531.5 eV assigned to the V–O [25] and Si–O bonds [26], respectively. The V 2p1/2 and V 2p3/2 peaks were observed at 523.5 and 515.9 eV, respectively, because of the V 2p spin–orbit splitting. The binding energy difference is 14.3 eV comparing O 1s with V 2p3/2, which conformed to the 14.0 eV value for VO2 nanoparticles, indicating that V4+ was the major valence state in the Si-doped VO2 films [27]. The V 2p3/2 peak can decompose into two peaks located near 515.9 and 517.3 eV, corresponding to V4+ and V5+ ions, respectively. In addition, the O/V ratio can be estimated from the integrated area of the empirical formula, (2IV4+ + 2.5IV5+)/(IV4+ + IV5+) [25], indicating the near stoichiometry O/V ratio inside the prepared VO2 films. It was inferred that the O/V ratios were 2, 2.23, 2.31, 2.40 and 2.41 when the Si concentration was increased to 1%, 3%, 5%, 8% and 10%, respectively. The XPS data analysis indicated that the chemical state of the Si-doped V changed from V4+ to V5+ and V4+, enhancing the electron–electron correlation and therefore increasing the peak area of the M2 phase [9, 28].
To investigate the electrical transport characteristics in VO2 samples doped with different Si concentrations, we measured the resistances of all the samples as the function of temperature using the PPMS (Fig. 4). The heating and cooling curves of all the samples were fitted using Gaussian functions to further determine the Tc, thermal hysteresis (ΔH), and transition sharpness (ΔT) of the Si-doped VO2 thin films. The minimum value of the Gaussian function was the transition temperatures of Theating and Tcooling, that is, the relationship curve between dR/dT and the temperature, as shown in the various illustrations in Fig. 4. Tc and ΔH were determined using the following equations: Tc = (Theating + Tcooling)/2 and ΔH = Theating − Tcooling, respectively. Similar to previous reports, Tc increased slightly with increasing Si concentration [22]. As shown in Table 2, the Tc values for the 0%, 1%, 3%, 5%, 8% and 10% Si-doped VO2 films are 333.9, 335.9, 336.1, 336.8, 337.0 and 341.1 K, respectively. In the Si-doped VO2 films, the small particle radius of Si4+ replaces the large particle radius of V4+, leading to lattice distortion. The lattice distortion affects the interactions between the V 3d and O 2p orbitals, especially the d// and π* electronic orbital occupancy [29]. With an increase in the Si-doping concentration, the π* band shifts upward and the π* orbital electron occupancy decreases, increasing Tc and the insulator resistance [30]. The 10% Si-doped VO2 films show a much wider ΔH of 36.0 K compared with the undoped VO2 films. The increase in ΔH with Si-doping may be due to the additional defects or scattered strain in the VO2 crystal lattice [31]. Finally, increasing the Si-doping concentration from0%, 1%, 3%, 5%, 8% and 10% increases ΔT from 20.7 K to 22.0, 33.9, 36.6, 37.7 and 38.5 K, respectively. The obvious phase transition hysteretic degradation may be due to the presence of Si ions in the VO2 lattice, which leads to the conjugate effect on the V–V chain and a V–O bond adjustment at the same time, limiting the MIT characteristics of the VO2 films. In addition, the increase in ΔT may be explained by the crystal showing a difference in Tc. The transition in the polycrystalline films is reflected by the distribution of different crystal Tc values. The change in Tc in a single crystal may be caused by strain. The lattice disorder, number of defects, and grain boundary density of doped films are better than those of undoped VO2 films.
Figure 5a shows the optical transmittance T(λ) at the wavelengths in the range of 300–2500 nm for the Si-doped VO2 thin films between 30 °C (solid curves) and 90 °C (dotted curves). The yellow shaded area indicates the standard solar spectrum. It can be seen that for each film, the transmittance decreased at 90 °C compared with its transmittance at 30 °C, suggesting that all the films have good thermochromic properties. Figure 5b shows ΔTlum, ΔTsol and ΔT2000nm for all the samples. When the Si-doping concentration increased to 3%, ΔTsol increased significantly from 7.3% to 13.9%. ΔTlum, ΔTsol and ΔT2000nm first increased and then decreased with increasing Si-doping content. When the Si-doping content reached 3%, the ΔTsol, ΔTlum and ΔT2000nm values were 13.9%, 54.7% and 45.4%, respectively. In addition, the bandgap values were 1.58, 1.93, 2.02, 1.86, 1.85 and 1.81 eV, corresponding to 0%, 1%, 3%, 5%, 8% and 10%, respectively. The bandgap increased with increasing Si-doping concentration, leading to higher barrier energy for the film and ultimately increasing Tc [32].
The VO2 phase transformation is a complicated process, and its driving mechanisms, in addition to temperature, could include electric current, electric field, and optical radiation. Therefore, the mechanism of the phase change has been debated and several different theories have been invoked, including lattice symmetry drive, the Peierls transition, and the Mott transition theories. In early 1968, Mott et al. [33] proposed that the phase transition of VO2 was caused by electronic interactions. However, they did not explicitly prove this theory. Kim et al. [34] conducted an XRD test of the VO2 MIT separation of phase and SPT transitions and confirmed the existence of the M2 phases, such as the monoclinic metallic phase or the strong metallic phase. Therefore, the M1 → M2 → R phase transition process has been confirmed for VO2 and the phase transition occurs prior to the transformation of the M phase into the R phase. However, the crystal structure does not change. From Figs. 2 and 4, the M2 phase generated in the MIT process of VO2 implies that SPT occurs later; therefore, the phase transition of VO2 belongs to the Mott transition. The electron transition Mott criterion is (nc)1/3aB≈0.25, where aB is the effective Bohr radius and nc is the critical density. From the formula, the nc values of the undoped and Si-doped VO2 films were nc≈3 × 1018 cm−3 and nc≈4.3 × 1018 cm−3, respectively. The nc value had the same order of magnitude as the VO2 electron density under the phase transformation point, nc≈1018 − 1019 cm−3 [35]. The results confirmed that in VO2, MIT was a pure electronic Mott–Hubbard transformation.
The following aspects of the change in Tc and the optical performances due to Si4+ doping have been resolved. First, Wu [36] reported that the modulated Tc and optical properties are attributable to lattice distortion and electronic effects. Second, Si-doped VO2 films have an increased bandgap because of the decrease in their π* orbital electron occupancy. The decrease in the shielding strength for the strong electron–electron correlations in the d// orbital increases Tc, which is consistent with previously reported results for Ti-doped VO2 films [30]. Sun et al. [37] reported that the change in Tc can be attributed to the grain size effect. Therefore, the XRD results in Fig. 1 indicate that Si4+ ions replace V4+ in the VO2 lattice, decrease the grain size, and increase the nucleation centers, resulting in a lattice distortion in the VO2 films. Therefore, the Si4+ ion-doping affects Tc and the optical properties. Finally, the change in Tc may be due to strain [27]. Doping with Si4+ ions resulted in a gradual decrease of the d-spacing in the VO2 films, indicating the existence of in-plane tensile stress and significantly improving VO2 films for use in smart windows.
4 Conclusions
In this study, Si-doped VO2 samples with different Si concentrations were synthesized using the sol–gel approach. The structure and thermochromic properties of all the samples were characterized using various techniques. The results showed that with an increase in the Si-doping content, the optical characteristics can be improved with a slight increase in Tc. The Tc values for the undoped and 3% Si-doped VO2 samples were 333.9 K and 336.1 K, ΔTsol were 7.3% and 13.9%, ΔTlum were 37.2% and 54.7%, and ΔT2000nm were 36.7% and 45.4%, respectively. Si-doping enhanced the strong electron–electron correlation of the d// orbitals, thus increasing Tc. In addition, the M2 phase was insulated by the Mott transition within the VO2 thin films at an intermediate temperature for the phase transition, verifying the role of potent electron–electron correlations in driving MIT. This study shows that Si-doped VO2 thin films can be prepared to slightly increase Tc, simultaneously improving ΔTlum, ΔT2000nm and ΔTsol.
References
S. Zhang, I.S. Kim, L.J. Lauhon, Nano Lett. 11, 1443–1447 (2011)
M.M. Qazilbash, M. Brehm, B.G. Chae, P.-C. Ho, G.O. Andreev, B.J. Kim, S.J. Yun, A.V. Balatsky, M.B. Maple, F. Keilmann, H.T. Kim, D.N. Basov, Science 318, 1750–1753 (2007)
Z. Shao, X. Cao, Q. Zhang, S. Long, T. Chang, F. Xu, Y. Yang, P. Jin, Sol. Energy Mater. Sol. Cells. 200, 110044 (2019)
R. Zhang, C. Yin, Q. Fu, C. Li, G. Qian, X. Chen, C. Lu, S. Yuan, X. Zhao, H. Tao, Ceram. Int. 44, 2809–2813 (2018)
I. Top, R. Binions, C. Sol, I. Papakonstantinou, M. Holdynski, S. Gaiaschi, I. Abrahams, J. Mater. Chem. C 6, 12555–12565 (2018)
J. Jin, D. Zhang, X. Qin, H.I.A. Imran, P. Fan, Energy Build. 202, 109345 (2019)
C.M. Leroy, M.F. Achard, O. Babot, N. Steunou, P. Masse, J.L. Binet, N. Brun, R. Backov, Chem. Mater. 19, 3988–3999 (2017)
T. Driscoll, H.T. Kim, B.G. Chae, B.J. Kim, Y.W. Lee, N.M. Jokerst, S. Palit, D.R. Smith, M.D. Ventra, D.N. Basov, Science 325, 1518 (2009)
D. Lee, T. Min, G. Lee, J. Kim, S. Song, J. Lee, J. Bae, H. Kang, J. Lee, S. Park, J. Phys. Chem. Lett. 11, 9680–9688 (2020)
A. Tselev, I.A. Luk’yanchuk, I.N. Ivanov, J.D. Budai, J.Z. Tischler, E. Strelcov, A. Kolmakov, V. Kalinin, Nano Lett. 10, 4409 (2010)
J.P. Pouget, H. Launois, J.P. D’Haenens, P. Merenda, T.M. Rice, Phys. Rev. Lett. 13, 873 (1975)
L. Dai, S. Chen, J. Liu, Y. Gao, J. Zhou, Z. Chen, Phys. Chem. Chem. Phys. 15(28), 11723–11729 (2013)
C. Chen, Y. Zhao, X. Pan, V. Kuryatkov, A. Bernussi, M. Holtz, Z. Fan, J. Appl. Phys. 110, 023707 (2011)
Y. Xu, W. Huang, Q. Shi, Y. Zhang, L. Song, Y. Zhang, J. Sol-Gel. Sci. Technol. 64(2), 493–499 (2012)
M. Panagopoulou, E. Gagaoudakis, N. Boukos, E. Aperathitis, G. Kiriakidis, D. Tsoukalas, Sol. Energy Mater Sol. Cells 157, 1004–1010 (2016)
J. Zheng, S. Bao, P. Jin, Nano Energy 11, 136–145 (2015)
N.R. Mlyuka, G.A. Niklasson, C.G. Granqvist, Sol. Energy Mater. Sol. Cells 93(9), 1685–1687 (2009)
C. Kang, C. Zhang, L. Zhang, S. Liang, C. Geng, G. Cao, H. Zong, M. Li, Appl. Surf. Sci. 463, 704–712 (2019)
V.R. Kolbunov, A.I. Ivon, Y.A. Kunitskiy, I.M. Chernenko, Ceram. Int. 39(4), 3613–3620 (2013)
F. Xu, X. Cao, J. Zhu, G. Sun, R. Li, S. Long, H. Luo, P. Jin, Mater. Lett. 222, 62–65 (2018)
X. Wu, Z. Wu, Z. Liu, C. Ji, Z. Huang, Y. Su, J. Gou, J. Wang, Y. Jiang, Appl. Phys. Lett. 109, 111903 (2016)
A. Krammer, O. Bouvard, A. Schüler, Energy Procedia 122, 745–750 (2017)
X. Wu, Z. Wu, H. Zhang, R. Niu, Q. He, C. Ji, J. Wang, Y. Jiang, Surf. Coat. Technol. 276, 248–253 (2015)
J. Du, Y. Gao, H. Luo, L. Kang, Z. Zhang, Z. Chen, C. Cao, Sol. Energy Mater. Sol. Cells 95(2), 469–475 (2011)
M. Wang, L. Fan, J. Bian, D. Zhang, H. Liu, H. Sun, Y. Luo, J. Mater. Sci. Mater. Electron. 28, 11046–11052 (2017)
M.M. Fadlelmula, E.C. Sürmeli, M. Ramezani, T.S. Kasırga, Nano Lett. 17, 1762–1767 (2017)
N. Shen, S. Chen, Z. Chen, X. Liu, C. Cao, B. Dong, H. Luo, J. Liu, Y. Gao, J. Mater. Chem. A 2, 15087 (2014)
S. Zhang, I.S. Kim, L.J. Lauhon, Nano Lett. 11(4), 1443–1447 (2011)
R. Zhang, H. Jin, D. Guo, J. Zhang, Z. Zhao, Y. Zhao, J. Li, Ceram. Int. 42, 18764–18770 (2016)
K. Huang, Y. Meng, X. Xu, P. Chen, A. Lu, H. Li, C. Wang, X. Chen, J. Phys. Condens. Matter 29, 355402 (2017)
J. Jian, A. Chen, Y. Chen, X. Zhang, H. Wang, Appl. Phys. Lett. 111, 153102 (2017)
Z. Zou, Z. Zhang, J. Xu, Z. Yu, M. Cheng, R. Xiong, Z. Lu, Y. Liu, J. Shi, J. Alloy. Comp. 806, 310–315 (2019)
N.F. Mott, Rev. Mod. Phys. 40(4), 677 (1968)
B.J. Kim, Y.W. Lee, S. Choi, J.W. Lim, S.J. Yun, H.T. Kim, Phys. Rev. B 77, 235401 (2008)
G. Stefanovich, A. Pergament, D. Stefanovich, J. Phys.: Condens. Matter. 12, 8837 (2000)
Y. Wu, Sci. Rep. 5, 9328 (2015)
Y. Sun, S. Jiang, W. Bi, R. Long, X. Tan, C. Wu, S. Wei, Y. Xie, Nanoscale 3, 4394 (2011)
Acknowledgements
This work was supported by the National Natural Science Foundation of China 12074291, 11804211, 11905119.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, Z., Zhang, Z., Xu, J. et al. Phase transition mechanism and application of silicon-doped VO2 thin films to smart windows. J Mater Sci: Mater Electron 32, 23825–23833 (2021). https://doi.org/10.1007/s10854-021-06752-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06752-2