Abstract
The element diffusion behavior, the interfacial evolution and mechanical properties of the joining of gallium arsenide (GaAs) with Sn3.5Ag4Ti(Ce,Ga) alloy filler at 250 °C in air were investigated. The interfacial microstructure, elemental diffusion and absorption, interface reaction and evolution were analyzed in detail. Titanium (Ti) and gallium (Ga) element were found to obviously take part in the active bonding between GaAs substrate and Sn3.5Ag4Ti(Ce,Ga) alloy filler. According to the transmission electron microscopy analysis, the titanium elements were observed to successively segregate at the interface, while some of the element Ga included in GaAs to dissolve into the molten alloy. In addition, there is a resultant formed discontiniously along the interface which was identified as Ga4Ti5, but no arsenic compounds were observed. The joining mechanisms related to the adsorption and reaction were discussed based on the thermodynamics theories, the molecular dynamic (MD) model and the reaction product controlled (RPC) model. The analysis results show that the RPC model was a special form of MD model, both the chemical reaction and the adsorption of active elements may control the reactive wetting of Sn3.5Ag4Ti(Ce,Ga) filler alloy on GaAs substrate together. In order to better understand the interfacial evolution of bonding, a simple interfacial evolution model between GaAs substrate and Sn3.5Ag4Ti(Ce, Ga) active solder was established. Finally, the effect of holding time on shear strength was investigated and the maximum shear strength of 23.32 MPa was obtained when soldered at 250 °C for 1 h. The interface separation could be caused by the mixed fracture mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the field of integrated circuit manufacturing, gallium arsenide (GaAs) is one of the most commonly used substrate materials [1,2,3]. Owing to its superior electrical performance and optical properties, it presents in a wide range of applications from optical devices to high speed digital circuits and microwave devices [4,5,6].
Nowadays, active welding has great advantages over traditional welding. One of the biggest advantages is that the substrate does not need to be pre-metallized. And the key factor to realize active welding is the addition of active elements, such as Ti, Cr, Zr, etc. [7]. However, there are also some shortcomings in active welding, especially welding in high temperature. For example, during the cooling process after high temperature welding, the interface thermal stress caused by the difference of thermal expansion coefficient between ceramic and metal will make the joint worse. In addition, high temperature welding process will reduce both the performance and reliability of the chip. So it is not suitable for integrated circuit manufacturing. In order to reduce the problems caused by high welding temperature, tin based active solders with low welding temperature have been developed.
Summarizing the previous research, it states clearly that adding active Ti can make the best improvement of wettability and mechanical properties of active solder. However, most research on active solder added Ti was mainly focused on the field of high temperature active welding [8,9,10], and the research on welding at low temperature is less. Especially for SnAgTi, only Chang and Tsao etc. had carried out some researches on it in the early days. Chang et al. used Sn3.5Ag4Ti (Ce, Ga) active solder to realize the joining between ZnS–SiO2 and Cu, and indium tin oxide ceramic (ITO) and Cu at 250 °C [11, 12]. Tsao adopted Sn3.5Ag4Ti (Ce) active solder to realize the welding of porous TiO2 and Ti at 250 °C, and the welding interface was found to be formed by CeO2 and Ti6Sn5 [13, 14]. Besides, Tsao also used Sn3.5Ag4Ti (Ce) to realize the welding between Al and MAO–Al [15]. In last several years, Wu et al. used SnAgTi at melting point to bond SiC ceramics under the action of ultrasound, and found that some new products around 7.6 nm were formed at the welding interface [16]. In brief, although it has attracted some scholars to carry out research in low temperature active bonding in recent years, it is far from enough. There is no unified understanding of the surface wetting and interfacial bonding mechanism of active bonding in the low temperature. Meanwhile, GaAs is one of the most commonly used substrates in the field of integrated circuit; there is a certain engineering application value to study the low temperature active bonding of GaAs. So it is necessary to carry out the analysis of element diffusion behavior and the study of interfacial reaction mechanism of low temperature active bonding between SnAgTi and GaAs. In addition, the bonding temperature is the key parameter of wetting on substrate with active solder. At low temperature, whether or not the bonding mechanism of the low temperature active solders follows the high temperature welding mechanism needs to be further studied and analyzed. Besides, during low temperature bonding, the environmental temperature of the interface reaction between active solder and substrate is also different from high temperature. So it may affect the behavior of active elements at the interface, and the welding dynamic process will also have some differences. All of these need to be analyzed and discussed in detail experimentally and theoretically for the industry application.
In this work, the element diffusion and adsorption behavior, the interfacial reaction and evolution, and mechanical properties of the joining of GaAs substrate with Sn3.5Ag4Ti(Ce,Ga) alloy filler at 250 °C in air were investigated. The interfacial microstructures, element distributions and interface reactions were analyzed in detail. Underlying wetting and reaction mechanism were elucidated based on the molecular dynamic (MD) model, reaction product controlled (RPC) model and thermodynamics theories. GaAs substrate were bonded by using Sn3.5Ag4Ti(Ce,Ga) alloy with soldering times of 1 min, 30 min, and 60 min, respectively. The effect of holding time on shear strength was investigated, and the interface fracture mechanism was discussed simultaneously.
2 Experimental
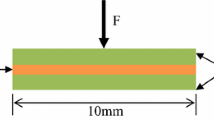
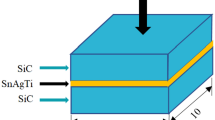
In this study, the Sn3.5Ag4Ti(Ce,Ga) alloy filler is provided by S-BOND Technologies, and its composition is listed in Table 1. The GaAs substrate is supplied by Harbin Tebo Technology Co., Ltd. The GaAs substrate for joining used in this study was sliced into 10 mm × 10 mm and 5 mm × 5 mm as the bonding substrates. The making method of GaAS/SnAgTi/GaAs samples are the same as our preliminary studies[17]. Prior to soldering, the GaAs specimens were preheated on a heating plate at 250 °C for 5 min. The active solder foil was then placed on the bond surface. Once the solder was molten, the molten solder was agitated for about 30 s for wetting on the bond surface, and then another GaAs specimen was place on the molten solder to be joined by being rubbed together for 30 s. The joint was held firmly in place and cooled, leading to the solidification of the molten solder. The schematic representation of soldering is demonstrated in Fig. 1. The bonding temperature was set at 250 °C with holding time of 1 min, 30 min, and 60 min, respectively. When the samples making were completed, the traditional metallographic procedures were used to obtain a suitable cross section for metallographic observation, which was carried out on a scanning electron microscopy (SEM: HITACHI S-3700N). To further analyze the interfacial morphology, a field emission transmission electron microscopy (TEM: JEM-2100F) was used. Conventionally, the mechanical properties of the GaAs/SnAgTi joints were characterized by shear strength, which was performed with a bond tester (MFM1500) according to the test method of chip bonding shear strength in MIL-STD-883G-2006. The shear speed is set to be 300um/s. The geometry and dimension of the soldering specimens subjected to shear test are demonstrated in Fig. 2. Ten samples were tested in each group, and the average value was taken as the final test result. To analyze the fracture mechanism of the joint, the fracture surface after shear test was acquired by using a scanning electron microscopy (SEM: HITACHI S-3700N).
3 Experimental results
3.1 Microstructure
SEM backscatter electron (BSE) images of GaAs/Sn3.5Ag4Ti(Ce,Ga)/GaAs joints soldered at 250 °C with soldering time of 1 min are shown in Fig. 3. It can be seen that two pieces of GaAs substrate are bonded together well. It is obviously that there are some particles adhering to the surface of GaAs substrate irregularly. Besides, it can be seen that there seems to be some phases formed inside the active solder after soldering.
Backscatter images of SEM for the cross-sectional view of GaAs/Sn3.5Ag4Ti(Ce,Ga)/GaAs joints soldered at 250 °C with soldering time of 30 min are shown in Fig. 4. From the interface morphology, there is little difference from that of 1 min. EDS spectrum of the marked area in Fig. 4a is shown in Fig. 5. According to the atom ratio of tin to titanium, it can be inferred that the phase is Sn5Ti6. Moreover, the particles with long strip shape are Ag3Sn which had been confirmed by our previous study [18]. So it can be inferred that there are Ag3Sn and Sn5Ti6 phases formed inside the active solder after bonding. Besides, it is also obviously that there are some particles adhering to the surface of GaAs substrate irregularly. Maybe these particles are the interfacial chemical resultants between GaAs substrate and Sn3.5Ag4Ti(Ce,Ga) alloy to achieve good bonding. However, we can only get some rough data from the BSE images and it is not enough to judge whether the new phase formed at the interface. Hence, it is very necessary to get more experimental data.
EDS spectrum of the marked area in Fig. 4a
In order to preliminarily determine the phase of interfacial products, the energy dispersive spectrum (EDS) analysis was conducted at the marked point as denoted in Fig. 4b, and the result was shown in Fig. 6. It can be seen that the relative titanium concentration is about 13.2 wt%, which is obviously higher than 4wt.% in the active solder. Therefore, it may preliminarily infer that there is a Ti-rich region or substance formed at the interface. In addition, from the content of other elements in the EDS spectrum diagram, it can be found that the atomic weight of gallium is much higher than that of arsenic, which could be inferred that the gallium is diffused more strongly than arsenic into the active solder. Based on the comprehensive analysis of all elements in Fig. 6, it might be deduced that the area around the marked point perhaps consists of Sn, GaAs, Ga–Ti and Ag3Sn.
EDS spectrum of the marked point in Fig. 4b
SEM backscatter electron (BSE) images of GaAs/Sn3.5Ag4Ti(Ce,Ga)/GaAs joints soldered at 250 °C with soldering time of 60 min are shown in Fig. 7. The morphology of the interface is similar to that of soldering time of 1 min and 30 min, and there is no obvious difference. In order to deeply understand the structure of the interface of GaAs/Sn3.5Ag4Ti(Ce,Ga)/GaAs solder joints, and explore which mechanism makes bonding to come true at low temperature, that is, whether or not the mechanism is the interaction of titanium adsorption or interface chemical reaction. The samples with soldering time of 30 min were selected for TEM analysis and analyzed in the following sections.
3.2 The distribution of elements
To determine whether there is active element adsorption at the interface and to confirm the new interfacial reaction phase formed in soldered joint, interfacial microstructure and element distribution have been investigated by TEM. TEM micrographs and element maps for GaAs/Sn3.5Ag4Ti(Ce,Ga) joint soldered at 250 °C for 30 min are shown in Fig. 8. From the element mapping analysis, it shows that the titanium element obviously segregates at the GaAs/solder interface, as shown in Fig. 8f. Meanwhile, the gallium element is found to be diffused into the SnAgTi alloy and also formed a line shape region along the interface. In order to obtain a more obviously contrast among the contents of various elements, a line scan is also performed to detect the active element titanium and gallium across the interface, as shown in Fig. 9. It can be found that a peak of both relative titanium concentration and gallium concentration are appeared at the interface, which could further infers that active element titanium segregates at the gallium arsenic/solder interface and element gallium dissolves into SnAgTi molten alloy from GaAs substrate. The element maps of the silver, arsenic and tin are shown in Fig. 8b, c and e, respectively.
The EDS spectrum of the marked point at the interface in Fig. 9a is shown in Fig. 10. From the EDS spectrum data, it indicates clearly that the relative titanium concentration is about 31.86 at.%, and the relative gallium concentration is about 37.91 at.% which is much higher than arsenic’s concentration of 20.05 at.%. All of these are in good agreement with the above analysis of element mapping results. It infers once more that active element titanium segregates at the interface and element gallium dissolves into molten alloy.
EDS spectrum of the marked point in Fig. 9a
3.3 Reactants at the interface
TEM micrographs of cross-sectional view of GaAs/Sn3.5Ag4Ti(Ce,Ga) solder joints with soldering time of 30 min are shown in Fig. 11. Figure 11a is the micrograph of the interfacial reaction layer, together with Fig. 11c and d are the high resolution transmission electron microscopy (HRTEM) images of the corresponding reactants in Fig. 11a. Besides, Fig. 11b is the diffraction pattern of the reactants in the interface. To characterize the reactants at the interface precisely, the HRTEM image and diffraction pattern were analyzed together. The crystalline interplanar spacing measured in Fig. 11c is about 2.24 Å, 3.93 Å, and 3.4 Å respectively in three different lattice faces. Among all possible Ti–Ga and Ti–As substances, the crystalline interplanar spacing determined by HRTEM image has exactly matched with Ga4Ti5, which is in line with the lattice spacing of Ga4Ti5(112), Ga4Ti5(110), and Ga4Ti5(200), respectively. In addition, the diffraction pattern as shown in Fig. 11b was analyzed and the results show that the diffraction pattern is coincided with that of Ga4Ti5. Based on the above analysis, it could be conformed that there is Ga4Ti5 phase formed at the interface of gallium arsenide/SnAgTi active solder. However, no arsenic compounds were found from our experimental data.
4 Mechanism of bonding
4.1 Thermodynamics analysis
In the bonding material system, the chemical reaction between the active element Ti in Sn–Ag–Ti filler alloy and the GaAs substrate may promote the soldering successfully. The chemical reaction between Ti and GaAs substrate may generate Ga–Ti and As–Ti compounds in accordance with the law of element conservation. Based on the Gibbs–Helmholtz approximate equation, by calculating the change of free energy, thermodynamics can predict whether or not a reaction will occur at a specified temperature. So it is necessary to consider all possible reactions and predict these reactions at the bonding temperature of 250 °C. On the basis of Potzschke and Schubert’s study of Ga–Ti thermodynamics system [19], there are eight possible chemical reactions which may occur. These equations for the formation of substances have been listed in Fig. 12. The relative free energy change data with different bonding temperatures are calculated and described as curves shown in Fig. 12.
From Fig. 12, it can be seen that the standard reaction Gibbs free energy of different chemical equation at 250 °C are all below zero, which may indicate that all chemical equations listed in Fig. 12 could take place independently. However, the standard reaction Gibbs free energy of the Eq. 4 GaAs + 9Ti = Ga4Ti5 + 4AsTi at 250 °C is − 349.032 kJ/mol, which is the lowest in all possible chemical equations. This maybe show that the possibility of this reaction is the largest and spontaneous reaction will happen more easily [22]. This should be the thermodynamic factor for the formation of Ga4Ti5 at the bonding interface because of the relative chemical reaction happened when active bonding is taking between Sn3.5Ag4Ti(Ce,Ga) alloy filler and GaAs substrate. In addition, according to the above analysis, Ga–Ti and As–Ti compounds will coexist at the interface when the chemical reaction between Ti and GaAs happens. However, in our TEM test results there is no As–Ti phase found. Maybe because arsenic is easy to volatilize that will lead to volatilization in the process of TEM sample preparation [23,24,25]. As the triangle area is too small and the substance formed at the interface is quite brittle, it is quite hard to make a satisfactory sample. Another possible reason may be that the bonding surface of GaAs(111) is Ga layer[26], and the chemical reaction between Ti and Ga will happen firstly to form Ga4Ti5 layer which may resist the Ti atoms sequentially diffusing into the surface of GaAs substrate. Hence, the chemical reaction between Ti and As is prevented and no AsTi phase is formed. In brief, in order to further clarify the activity mechanism of arsenic, further efforts are still needed in the future research.
4.2 The relevance between adsorption and reaction to wetting behavior
The active Sn3.5Ag4Ti(Ce,Ga) solder filler and the GaAs substrate compose a typical reactive wetting system, in which either the chemical reaction at the bonding interface, or the diffusion of active element in the molten solder could control the spreading kinetics. As suggested by Cox, the relationship between wetting angle and spreading speed is given as follows [27], which is called molecular dynamic (MD) model.
where \({\theta }_{\text{e}}\) and \({\theta }_{\text{d}}\) are the final and dynamic contact angle, respectively. \({\sigma }_{lv}\) is the liquid surface tension. \(\lambda\) is the distance between two adsorption sites and \({k}_{\text{e}}\) is the quasi-equilibrium rate constant. \({k}_{\text{B}}\) is the Boltzman constant, and n represents the number of adsorption/desorption sites per unit area.
According to the characteristics of spreading dynamics for adsorption wetting by Qiaoli Lin [28], when the condition \({\sigma }_{lv}\left({\text{cos}}{\theta }_{\text{e}}-{\text{cos}}{\theta }_{\text{d}}\right)\gg 2nkT\) is satisfied, and considering that the chemical potential gradient along the wetting interface induces each molecule to produce a force as the driving force, the MD model could be deduced further as follows [29].
where \(\Delta {G}_{\text{r}}\) is Gibbs free energy of activation that happens, \({v}_{i}^{\text{p}}\) is the molar volume of the products, and \({K}_{2}\) is a constant. This expression is very close to the expression of the reaction product control (RPC) model, which has strict linear wetting behavior. The fitting results analyzed by Lin show that the RPC model overlaps with the MD model, and the RPC model can be regarded as a special form of MD model [28]. In addition, due to the adsorption characteristics of MD model, the contribution of adsorption to the diffusion of metal fluid in the reaction system cannot be ignored. Therefore, according to the above analysis and the experimental results, it can be derived that the Ti adsorption at the interface was responsible for the decrease of interfacial energy, which drove the molten bulk to move forward at low temperature. The contribution of adsorption for spreading in a reactive wetting system should not be neglected, and may be in dominant. Meanwhile, it could be inferred that both adsorption and reaction are important effects to promote the spreading behavior. Except the diffusion of active elements, both the chemical reaction and the adsorption of active elements may control the reactive wetting of filler alloy on gallium arsenide. The wetting behavior should be the result of the interaction of titanium adsorption and chemical reaction together. Besides, Chang et al. pointed out in their research papers that the role of the Ce and Ga owe to the high affinity of Ce for oxygen to protect Ti from oxidation. Thus, the activity of titanium was enhanced, and promoting the wetting and reaction of Ti with ZnS–SiO2, Al2O3, Cu and etc. A small amount of a rare earth element in solder can inhibit the intermetallic layer growth [11, 30]. So maybe the Ce and Ga also play an important role that should not be ignored. In order to further clarify the role of Ce and Ga, further efforts are still needed in the future research.
4.3 Interfacial evolution of bonding
Analyzing the interface evolution process between Sn3.5Ag4Ti and GaAs substrate during active bonding is an important part of elucidating the formation mechanism of joint. A comprehensive analysis of all the data we obtained in the early research, it had not found that the elements of the substrate materials such as alumina, silica and silicon are easy to dissolve into the molten alloy. However, in this study, the element Ga in gallium arsenide diffuses into the molten alloy strongly, whereas arsenic does not. Therefore, the diffusion behavior both of Ti and Ga should be considered comprehensively. Besides, chemical reaction can occur between GaAs and Sn3.5Ag4Ti(Ce,Ga) in terms of thermodynamics. A discontinuous and thin layer of resultant at interface observed from the experiment results could confirm that interfacial chemical reaction does take place.
Based on the above analysis, interfacial evolution processes between GaAs substrate and Sn3.5Ag4Ti(Ce, Ga) active solder could be discussed, and a simple model could be established, as shown in Fig. 13. It is reasonable to divide the interface evolution process of bonding into three steps. First, a part of active titanium elements move to the solid–liquid interface[17], and the Ga elements included in the GaAs substrate dissolves into the molten alloy, as shown in Fig. 13a; Second, when the active element Ti arrived at the GaAs/active solder interface, some of them segregate at the interface and some of them may diffuse back to the molten alloy because of atomic concentration gradient; while some of the Ga atoms dissolve in the opposite direction into the molten solder and much of them stay near the interface, as shown in Fig. 13b; Third, when titanium atoms meet Ga atoms, interfacial reaction is happening and a discontinuous reaction layer is gradually formed, as shown in Fig. 13c. Due to the spontaneous chemical reaction between Ti and Ga elements takes place, both Ti and Ga atoms at the interface are consumed to form new products. With the prolongation of bonding time, Ti atoms in the solder continue to diffuse to the interface and Ga atoms keep on dissolving as well, and the interfacial reaction continues.
5 Mechanical properties
In order to analyze the influence of holding time on the joint strength, shear strength tests were carried out on the specimens with soldering time of 1 min, 30 min and 60 min, respectively. The fracture morphologies of as soldered GaAs/Sn3.5Ag4Ti(Ce,Ga) /GaAs joints with different soldering time after shear test are shown in Fig. 14. Figure 14a and b are the fracture morphologies of the active solder side and the GaAs side of 60 min with low magnification, which shows clearly that the two fracture surfaces separated from a solder joint could match each other well. However, such fracture surfaces only show that the fracture occurs in the solder layer, it is not certain whether the fracture occurred in the solder, in the substrate or at the interface. Therefore, in order to clearly observe the residual particles in fracture surfaces, the fracture images of the active solder side and the GaAs side with high magnification were obtained, as shown in Fig. 14c, e, g and d, f, h respectively. Figure 15 is the EDS spectrum of material remained in the solder side marked in Figs. 14e and 16 is the EDS spectrum of material remained in the GaAs side marked in Fig. 14f. From the EDS analysis results, it shows that some of GaAs particles are still adhere to the active solder. Meanwhile, little solder and maybe Ga–Ti phase are adhere to the GaAs substrate as well. According to the analysis of fracture surface, it could indicate that all layers in the joint including the GaAs substrate, active solder layer and interface reactant layer experience fracture in the process of shear strength test. Shear test results show that fracture of all samples with different soldering time occurs at the solder/gallium arsenide interface, and that the fracture plane and the shear stress in the same direction. All of these show that the interface connection is the weakest place which causes the fracture occurred at the interface. Moreover, it could be found from Fig. 14c, e, g that there are obvious cleavage fracture patterns in the solder side. This may indicate that GaAs/Sn3.5Ag4Ti(Ce,Ga)/GaAs joints have experienced a mixed fracture in the process of shear strength test. The interface separation could be caused by the mixed fracture mechanism.
Fractographs of as soldered GaAs/Sn3.5Ag4Ti(Ce,Ga)/GaAs joints with different soldering time after shear testing a the active solder side of 60 min with low magnification; b the GaAs side of 60 min with low magnification c the active solder side of 1 min, d the GaAs side of 1 min, e the active solder side of 30 min, f the GaAs side of 30 min, g the active solder side of 60 min, h the GaAs side of 60 min
EDS spectrum of the marked point in Fig. 14e
EDS spectrum of the marked point in Fig. 14f
The test results show that the average shear strength of the samples for 1 min, 30 min and 60 min is 15.25 MPa, 17.43 MPa and 23.32 MPa, respectively. According to the die shear strength test methods and requirement in MIL-STD-883G-2006, the materials used to attach semiconductor die to substrates shall withstand a minimum strength of 11.68 MPa. The joint strength of GaAs/Sn3.5Ag4Ti(Ce,Ga)/GaAs exceeding the standard requirements of die bonding application in the MIL-STD-883G-2006 demonstrates that Sn3.5Ag4Ti(Ce,Ga) alloy filler could be used to attach semiconductor die or surface mounted passive elements to package headers or other substrates. It also shows that the low temperature active solder has a certain application prospect in the field of integrated circuit packaging. Test results reveal that the joint strength increases with the increase of soldering time. This is similar to that of Si/Sn3.5Ag4Ti(Ce,Ga)/Si joints and SiO2/Sn3.5Ag4Ti(Ce,Ga)/SiO2 joints in our previous studies, whose shear strength also increases with the increase of soldering time. The comparison chart of influence law of heating time is shown in Fig. 17.
6 Conclusions
In this study, the elemental adsorption and interface reaction of Sn3.5Ag4Ti(Ce,Ga) alloy filler on GaAs substrates were investigated and the mechanical properties of the joint were tested. Interfacial evolution of bonding between Sn3.5Ag4Ti(Ce,Ga) and GaAs substrate at low temperature was studied. The conclusions are summarized as follows:
-
1.
The active Ti and the Ga in GaAs substrate are found to obviously take part in the active bonding between GaAs substrate and Sn3.5Ag4Ti(Ce,Ga) alloy filler. The titanium elements are segregated successively at the interface and the element Ga diffuses into the molten alloy. The reactant Ga4Ti5 is formed discontinously at the interface of GaAs/Sn3.5Ag4Ti(Ce,Ga) soldered joints with soldering time of 30 min.
-
2.
The active bonding of Sn–Ag–Ti solder foil and GaAs substrate at 250 °C was well described by adsorption control model which is very close to the expression of the reaction product controlled model. The contribution of adsorption for spreading in a reactive wetting system should not be neglected, both the chemical reaction and the adsorption of active elements may control the reactive wetting of Sn3.5Ag4Ti(Ce,Ga) filler alloy on GaAs substrate. The wetting behavior should be the result of the titanium adsorption and chemical reaction together.
-
3.
Interfacial evolution of bonding is explored and it is reasonable to divide the interface evolution process of bonding into three steps. First, a part of active titanium elements move to the solid–liquid interface, and the Ga elements included in the GaAs substrate dissolves into the molten alloy; Second, when the active element Ti arrived at the GaAs/active solder interface, some of them segregate at the interface and some of them may diffuse back to the molten alloy, but much of them stay near the interface; Third, when titanium atoms meet Ga atoms, interfacial reaction happens and a discontinuous reaction layer is gradually formed.
-
4.
The average shear strength of the samples for 1 min, 30 min and 60 min was measured to be 15.25 MPa, 17.43 MPa and 23.32 MPa, respectively, which indicates that the joint strength increases with the increase of soldering time. The GaAs/GaAs interface separation is caused by the mixed fracture mechanism, and the interface connection is the weakest place which causes the fracture occurred at the interface.
Data availability
The data that support the finding of this study are available from the corresponding author upon reasonable request.
References
R.H. van Leest, P. Mulder, G.J. Bauhuis, H. Cheun, H. Lee, W. Yoon, R. van der Heijden, E. Bongers, E. Vlieg, J.J. Schermer, Phys. Chem. Chem. Phys. 19, 7607–7616 (2017)
P. Huo, I. Rey-Stolle, J. Electron. Mater. 45, 2769–2775 (2016)
Y. Ohno, J. Liang, N. Shigekawa, H. Yoshida, S. Takeda, R. Miyagawa, Y. Shimizu, Y. Nagai, Appl. Surf. Sci. 525, 146610 (2020)
E. Higurashi, T. Suga, Electr. Commun. Jpn. 99, 63–71 (2016)
E. Higurashi, K. Okumura, K. Nakasuji, T. Suga, Jpn. J. Appl. Phys. 54, 030207 (2015)
P. Karbownik, A. Trajnerowicz, A. Szerling, A. Wójcik-Jedlińska, M. Wasiak, E. Pruszyńska-Karbownik, K. Kosiel, I. Gronowska, R. Sarzała, M. Bugajski, Opt. Quant. Electron. 47, 893–899 (2015)
T. Li, D. Zhou, Y. Yan, S. Zhang, J. Liu, Mater. Sci. Eng. A 796, 139929 (2020)
L.X. Zhang, B. Zhang, Z. Sun, S.Y. Liu, M. Lei, J.C. Feng, Ceram. Int. 46, 10224–10232 (2020)
C. Xin, J. Yan, Q. Wang, W. Feng, C. Xin, J. Alloy. Compd. 820, 153189 (2020)
D. Liu, K. Zhao, Y. Song, L. Zhang, X. Song, W. Long, Mater. Charact. 157, 109890 (2019)
S.Y. Chang, T.H. Chuang, L.C. Tsao, C.L. Yang, Z.S. Yang, J. Mater. Process. Tech. 202, 22–26 (2008)
S.Y. Chang, L.C. Tsao, M.J. Chiang, T.H. Chuang, C.N. Tung, G.H. Pan, J. Mater. Eng. Perform. 12, 383–389 (2003)
L.C. Tsao, Mater. Sci. Eng. A 565, 63–71 (2013)
L.C. Tsao, J. Mater. Sci. 25, 233–243 (2014)
L.C. Tsao, Mater. Des. 56, 318–324 (2014)
B.Z. Wu, X.S. Leng, Z.Y. Xiu, J.C. Yan, Sci. Rep. 8, 16856 (2018)
L.X. Cheng, G.Y. Li, Z.L. Li, Z.Z. Wu, B. Zhou, J. Mater. Sci. 26, 6004–6012 (2015)
L.X. Cheng, G.Y. Li, X.Q. Wang, Z.L. Li, Z.Z. Wu, Mater. Sci. Eng. A 658, 42–49 (2016)
Y. Liu, J.C. Tedenac, X.P. Su, C. Colinet, J.H. Wang, Calphad 41, 140–149 (2013)
Y.J. Liang, Handbook of Inorganic Thermodynamic Data (Northeastern University Press, Shenyang, 1993), pp. 39–441
D.R. Journal of Physical and Chemical Reference Data (The American Chemical Society and The American Instutute of Physics, 1982), pp. 92–407
R.T. Li, W. Pan, J. Chen, J. Lian, Mater. Sci. Eng. A 335, 21–25 (2002)
T. Sebestyen, I. Mojzes, D. Szigethy, Electron. Lett. 16, 504 (1980)
R.P. Gupta, W.S. Khokle, J. Wuerfl, H.L. Hartnagel, Thin Solid Films 151, L121–L125 (1987)
A.F. Wright, N.A. Modine, J. Appl. Phys. 120, 215705 (2016)
Z.W. Li, J.H. Yin, Materials Fundamentals of Semiconductor Silicon, 2nd edn. (Beijing Chemical Industry Press, Beijing, 2012), pp. 125–136
T.D. Blake, Surfactant Sci. Ser. 49, 251–255 (1993)
Q. Lin, R. Cao, Comp. Mater. Sci. 99, 29–32 (2015)
M. Benhassine, E. Saiz, A.P. Tomsia, J. De Coninck, Acta Mater. 59, 1087–1094 (2011)
S.Y. Chang, T.H. Chuang, C.L. Yang, J. Electron. Mater. 36, 1193–1198 (2007)
Acknowledgements
This research is supported by the National Natural Science Foundation of China [Grant No. 61804057]; and the Science and Technology Planning Project of Guangdong [Grant No. 201803020022].
Funding
This research is supported by the National Natural Science Foundation of China [Grant No. 61804057]; and the Science and Technology Planning Project of Guangdong [Grant No. 201803020022].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LXC, XJY, ZZW, and JX. The first draft of the manuscript was written by LXC and GYL, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors and the responsible authorities at the institute for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, L.X., Yue, X.J., Xia, J. et al. Adsorption and interface reaction in direct active bonding of GaAs to GaAs using Sn–Ag–Ti solder filler. J Mater Sci: Mater Electron 32, 21248–21261 (2021). https://doi.org/10.1007/s10854-021-06627-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06627-6