Abstract
As one of the most promising electrode materials for supercapacitors, MnO2 has the problems of low voltage window and poor conductivity. It has been shown that Al-doped MnO2 can effectively improve the voltage window and specific capacitance, and polyaniline loading can further bring higher specific capacitance. Polyaniline-supported Al-doped MnO2 carbon cloth-based flexible electrode material (PANI@Al-MnO2@CC) was prepared by hydrothermal reaction and in-situ polymerization. Compared with MnO2 electrode material, the working voltage window of PANI@Al-MnO2@CC is increases to 1.25 V, the area specific capacitance can be as high as 1016 mF cm−2, and still maintain 80.1% of the original value at 20 mA cm−2 after 5000 cycles. Furthermore, the voltage window of the flexible symmetrical supercapacitor made of the material can reach 0–2.4 V, and has a super large area specific capacitance of 149.75 mF cm−2 and a high energy density of 1.60 mWh cm−3 at 6 mA cm−2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In order to better protect the environment, energy storage and conversion is very necessary. Therefore, the research on supercapacitors [1,2,3], lithium-ion batteries [4, 5], fuel cells [6] and other energy storage devices is more and more in-depth. As a new type of energy storage equipment, the supercapacitor has attracted the attention of various researchers in recent years because of its high theoretical specific capacity, excellent cycle stability and relatively low cost [7,8,9]. Compared with traditional capacitors, supercapacitors have greater power density and longer cycle life, which contribute to their broad application in renewable energy field [10], industrial field [11], rail transit field [12] and other fields. With the progress of technology, a variety of new supercapacitors emerge in endlessly. For example, supercapacitors with high-temperature NiO/ZrO2 separators can be used in oil drilling, military and other high-temperature applications [13], and note supercapacitors made from sticky CNT array electrodes can be used in wearable electronic products and flexible circuits [14]. With the rapid expansion of the application range of supercapacitor, enhancing energy density and expanding voltage window have become urgent problems to be solved [15, 16].
In addition, in order to meet the demands of mobile and wearable electronics, it requires better flexibility of supercapacitors. If the performance of the supercapacitor can be improved while becoming flexible and lightweight, it will provide more opportunities for the development of flexible electronic devices. Carbon cloth (CC) is considered as a desirable current collector material. Additionally, the porosity of CC allows to charge transfers rapidly, so CC has the potential to develop flexible portable energy storage devices. Nonetheless, pure CC has a very low specific capacitance, and its disadvantages as an electrode material are obvious. It is very common to use electrodeposition [17, 18], chemical deposition [5] and other methods to improve material properties. For example, Tishkevich et al. used electrochemical methods to deposit Ni nanowires on porous Al2O3 films [19] and deposit nanocrystalline bismuth films in perchloric acid electrolyte [20], Zeng et al. [5] deposited silver nanoparticles on porous silicon by chemical methods. Therefore, in order to solve a series of problems of pure CC, metal oxides are generally constructed to improve the electrochemical performance of CC-based supercapacitors. For example, Wang et al. [21] in-situ grew ultra-thin manganese dioxide grass on porous CC via simple wet-chemical method, resulting in MnO2/TCC composite electrode material with a specific capacitance of 178 F g−1 at 4 mA cm−2. Yang et al. [22] grew NiCo2O4@NiO core-shell nanowire hetero-nanostructure on CC and assembled into all-solid-state symmetrical supercapacitor. Huang et al. [23] and Shinde et al. [24] prepared nano fake MMoO4 (M = Ni, Co) and nanorod-like h-WO3 surface film on conductive CC by hydrothermal method, respectively. Gu et al. [25] attached Fe2O3 nanoplates to the surface of CC. Jia et al. [26] grew Nb2O5 nanotubes and nanowire/nanotube homo-junctions on CC through a hydrothermal process. The electrode material has a specific capacitance of 400 F g-1 at 1 mV s−1, and the fabricated tubular Nb2O5@CC//AC SICs has a high energy density of 195 W h kg−1 at 120 W kg−1. MnO2 has superior pseudocapacitance, a wide electrochemical potential window, low cost and environmental friendliness. The crystal size and morphology of MnO2 can be well controlled by simple hydrothermal reaction and variable control [27]. Therefore, it is considered to be a desired material for supercapacitors.
However, MnO2 has a low voltage window and poor conductivity, which limits its commercialization. Doping conductive materials is an effective way to increase MnO2 lattice defects, speed up charge transfer and enhance its conductivity. Supercapacitors using conductive polymers as electrode materials mainly store energy based on the principle of Faraday pseudocapacitance [28,29,30]. Rajesh et al. [31] successfully deposed poly(3,4-ethylenedioxythiophene) nanostructures on carbon fiber cloth and assembled into a symmetrical supercapacitor. After 12000 cycles at 10 A g−1, the specific capacitance of the supercapacitor reached 203 F g−1 at 5 mV s−1, and the capacitance retention rate reached 86%. Xiao et al. [32] improved the performance of symmetric supercapacitors by depositing polypyrrole on the activated CC. At the operating voltage of 1.4 V, it delivered the energy density of 3.63 mWh cm−3 at 29.17 mW cm−3. Zhou et al. [33] polymerized polyindole hollow nanospheres on CC and coated an ultrathin poly(3,4-ethylenedioxythiophene): poly (styrene sulfonate) films on the surface of polyindole hollow nanospheres. It showed a specific capacitance of 623 F g−1 at 0.5 A g−1 and a capacitance retention of 84.8% after 3000 cycles at 20 A g−1. The conductive polymers on the electrode have undergone rapid and reversible n-type or p-type redox reaction. This allowed the polymer to store high-density charges, resulting in high Faraday pseudocapacitance. The supercapacitor has the advantages of wide voltage window, high conductivity and designability. Polymerized polyaniline (PANI) has good conductivity [34, 35], which effectively enhances the specific capacitance of supercapacitors. Furthermore, PANI can be prepared in large quantities due to its low cost [36]. He et al. [37] PANI nanofibers on CC via an ice water bath. The obtained PANI/CC composite material has a specific capacitance of 439 F g−1 and the specific capacitance loss 6.8% after 1000 cycles at 1 A g−1. Wu et al. [38] prepared a manganese dioxide/graphene nanoribbons/PANI ternary composite material through a two-step polymerization process. Its specific capacitance could reach 472 F g−1, and the specific capacitance could still maintain 79.7% after 5000 cycles.
In current work, KMnO4 and CC are used as raw materials, Al-doped MnO2 nanomaterials are deposited on the CC firstly by hydrothermal method. The PANI is synthesized by in-situ polymerization, thereby obtaining PANI-supported Al-doped MnO2 carbon cloth-based flexible electrode material (PANI@Al-MnO2@CC). MnO2 is rich in resources, environment-friendly and has high theoretical specific capacitance [39, 40], while Al doping can effectively expand the voltage window of manganese dioxide [41]. In addition, the good conductivity of PANI can improve the inherent low conductivity of MnO2, improve the conductivity of the composite, and further expand the specific capacitance of the PANI@Al-MnO2@CC [42, 43]. Therefore, in this work, PANI was deposited on Al-doped MnO2 for the first time on a flexible carbon cloth substrate to prepare flexible supercapacitors with high voltage window, high specific capacitance and long cycle life.
According to the experimental results, the PANI composite and Al doping increase the conductivity and cycling stability of the MnO2 electrode material to a certain extent. In addition, the operating voltage window is successfully expanded to 1.25 V, and a high area specific capacitance of 1016 mF cm−2 is obtained at 6 mA cm−1. After 5000 cycles at the current density of 20 mA cm−2, the area specific capacitance retention reached 80.1%. The flexible symmetrical supercapacitor assembled by PANI@Al-MnO2@CC achieves a wide voltage window of 0-2.4 V. Furthermore, it not only has an area specific capacitance of up to 149.75 mF cm−2, but has an energy density of 1.60 mWh cm−3 at 6 mA cm−2.
2 Experiment
2.1 Preparation of Al-doped MnO2@CC
The CC (1 cm × 0.5 cm, Tianjin Aiweixin Chemical Technology Co., Ltd) was ultrasonically cleaned with propanol and isopropanol (Sinopharm Chemical Reagent Co., Ltd) for 15 min, then dried, acidified with concentrated nitric acid (Sinopharm Chemical Reagent Co., Ltd) for 5 min, and washed until neutral. The CC was then added to 100 mL deionized water, 5 mmol KMnO4 (Shanghai adamas Reagent Co., Ltd) and 0.45 mmol AlCl3 (Shanghai TITAN Technology Co., Ltd). After magnetic stirring for 0.5 h, the CC was poured into an autoclave with PTFE inner lining and reacted at 150 °C for 4 h. Thereafter, the carbon cloth was washed with deionized water and ethanol (Shanghai TITAN Technology Co., Ltd) for three times and dried at 60 °C.
2.2 Preparation of PANI@Al-MnO2@CC
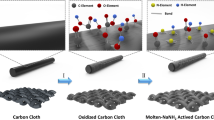
The PANI@Al-MnO2@CC was loaded with a layer of PANI on the surface by in-situ polymerization. The specific steps are shown in Fig. 1.
2.2.1 Preparation of PANI@Al-MnO2@CC under different reactant ratios
Flexible PANI@Al-MnO2@CC in different acidic conditions were prepared by a typical in-situ polymerization. Firstly, the prepared Al-MnO2@CC material was immersed in 30 mL H2SO4 (Sinopharm Chemical Reagent Co., Ltd) containing 38.5 µL of aniline (Shanghai TITAN Technology Co., Ltd). Then, 40 mL of H2SO4 aqueous solution containing ammonium persulfate (APS, Shanghai TITAN Technology Co., Ltd) was slowly added to the above mixture. The molar ratio of aniline and APS was changed from 2:1, 4:1 to 8:1 to achieve different reactant ratios, and then kept in the dark at about 0 °C for 2 h to achieve complete polymerization. Finally, it was taken out and washed for several times, dried overnight at 80 °C to obtain the flexible PANI@Al-MnO2@CC electrode material.
2.2.2 Preparation of PANI@Al-MnO2@CC under different reaction times
The PANI@Al-MnO2@CC flexible electrode materials was prepared in different acidic environments using a typical in-situ polymerization method. Firstly, the prepared Al-MnO2@CC material was immersed in 30 mL H2SO4 containing 38.5 µL of aniline. Then 40 mL of APS was slowly added to the above mixture (the molar ratio of aniline and APS is always 4:1). The reaction time varied from 1 h, 2 h, 4 h to 8 h to achieve different ice bath time. During the reaction, the solution was maintained in the dark at about 0 °C for 2 h to obtain complete polymerization. After washed for several times, and dried at 80 °C overnight, the final PANI@Al-MnO2@CC composite material was obtained.
2.3 Preparation of flexible solid PANI@Al-MnO2@CC || PANI@Al-MnO2@CC supercapacitor
6.0 g of PVA (Shanghai TITAN Technology Co., Ltd) was placed in a round-bottom flask and filled with 60 mL of deionized water. After stirring evenly at room temperature, the temperature was increased to 85 °C and stirred for 2 h, then slowly decreased the temperature to 45 °C while stirring. Next, when it became stable, 20 mL of Na2SO4 solution (1 M, Shanghai TITAN Technology Co., Ltd) was added. Finally, after stirring for 0.5 h, and cooling down, Na2SO4/PVA quasi-solid electrolyte was obtained.
The PANI@Al-MnO2@CC material was then placed under the optimal doping amount, optimal temperature, and optimal hydrothermal time to the above-mentioned Na2SO4/PVA quasi-solid electrolyte for 15 min. After PANI@Al-MnO2@CC was taken out, it was cut into cuboids (1 cm × 0.5 cm × 0.033 cm) and stacked two of them together, and a non-woven membrane was used to separate them. The mass of the active materials in this work is about 1.0 mg cm-2.
2.4 Characterization
The surface morphology and microstructure of the sample were observed by Nova Nano-SEM 450 field emission scanning electron microscope (SEM, 0.02-30 kV, 3 pA-20 nA) and FEI TALOS F200S transmission electron microscope (TEM, 200 kV). The particle size of the sample was observed by Bruker Dimension ICON atomic force scanning microscope (AFM, 3×3 μm, 2 Hz). The X-ray diffraction (XRD, Cu-Kα, λ = 1.54 Å, 5° min-1) patterns were obtained by crystal structure characterization using D/MAX 2550VB3+/PC, X-ray photoelectron spectroscopy (XPS, AXIS Ultra DLD apparatus, Al source, 15 kV), Fourier transform infrared spectra (FTIR, EQUINOX 55, DTGS KBr, 1000-3000 cm-1) measurements and TG-DTA synchronous thermal analyzer (STAZ 449C from NETZSCH, 10°C min-1, O2 atmosphere) were also used.
The electrochemical performance tests, such as cyclic voltammetry (CV, 30 mV s-1), galvanostatic charge-discharge (GCD, 6 mA cm-2) and electrochemical impedance spectroscopy (EIS, 105 to 10-2 Hz) were tested in a CHI 760E electrochemical workstation.
3 Results and discussion
3.1 Optimization of reactant ratio
The SEM images of PANI@Al-MnO2@CC electrode material having different molar ratios of aniline to APS are shown in Fig. S1. It could be found that the electrode materials with a charging ratio of 2:1 and 4:1 all presented perfect nanosphere shape, and the electrode material of 8:1 was relatively weak, indicating that the overall feed ratio has little effect on the material morphology.
A three electrode system was used to test the electrochemical performance of PANI@Al-MnO2@CC with different feeding ratios using 1 M Na2SO4 as electrolyte. The CV curve of PANI@Al-MnO2@CC is shown in Fig. 2a, which demonstrated that the CV curve of PANI@Al-MnO2@CC with a charging ratio of 4:1 showed a better quasi-rectangular shape. Fig. 2b indicated their GCD curves were in a window of 0-1.2 V. All three materials had good triangular symmetry, but PANI@Al-MnO2@CC with a feeding ratio of 4:1 had the longest charge and discharge time. The area specific capacitance measured by calculation was 945 mF cm-2. Therefore, we could determine that the optimal reactant ratio is 4:1.
3.2 Optimization of reaction time
SEM images of PANI@Al-MnO2@CC electrode materials with different reaction times are shown in Fig. S2. It was observed that the shape of the PANI@Al-MnO2@CC electrode material had not been formed within 1 h. When it reached 2 h, the material exhibited a relatively perfect nanosphere shape. When the reaction time continued to extend, the material become thinner.
We also used three electrode systems to further test the electrochemical properties of PANI@Al-MnO2@CC electrode materials with different reaction time, in which 1 M Na2SO4 served as electrolyte. Firstly, the capacitance performance of PANI@Al-MnO2@CC with different reaction time was tested (Fig. 2c). It could be seen that the CV curve of PANI@Al-MnO2@CC for 2 h showed the closest approximation of the quasi-rectangular shape, indicating that it had the best pseudo-capacitance performance. And the GCD curves of PANI@Al-MnO2@CC for determining the capacitance characteristics are shown in Fig. 2d. The curves presented by the three materials were similar to triangles, and all had good symmetry, which proved that PANI@Al-MnO2@CC had good charging and discharging performance. By calculating, the area specific capacitances of the three electrode materials reached 677 mF cm-2, 945 mF cm-2, 648 mF cm-2 and 102 mF cm-2, respectively. Therefore, we could determine that the optimal reaction time is 4 h.
3.3 Structural characterization of the optimal flexible PANI@Al-MnO2@CC
Figure 3a–c and Fig. S3a–c showed the TEM, HRTEM and SAED images of Al-MnO2@CC and optimal PANI@Al-MnO2@CC. Al-MnO2@CC and the optimal PANI@Al-MnO2@CC presented a nanosheet morphology at the 20 nm scale, which was consistent with the SEM observation results. The HRTEM image showed a lattice spacing of 0.27-0.29 nm, which corresponded to (-110) plane of δ-MnO2 [44], further demonstrating the nanocrystalline structure. Furthermore, the selected area electron diffraction (SAED) also proved that the electrode material we prepared had a polycrystalline structure [45]. Fig. S4 showed that the composition elements mainly included C, Mn, O, N, Al, where C mainly come from CC, Al come from Al-MnO2, and the presence of N and O demonstrated that PANI had been successfully loaded on the surface of the material [46]. The elements measured by STEM mappings in Fig. 3d were also in good agreement with energy dispersive X-ray spectroscopy (EDX). In addition, the AFM image obtained in Fig. S5 can show the particle size of PANI@Al-MnO2 deposited on the carbon cloth [21], and its particle size is between 0-1.3 μ m, with an average size of about 890 nm.
Figure 4a shows the XRD patterns of PANI@Al-MnO2@CC and Al-MnO2@CC nanocomposites. For Al-MnO2@CC, it mainly included the diffraction peaks of CC and the related peaks corresponding to potassium manganese oxide, indicating that it was δ-MnO2 (JCPDS No.80-1098) [45]. Compared with Al-MnO2@CC, the full width at half maximum of one peak at 25.25° of PANI@Al-MnO2@CC was increased. This was mainly due to the XRD peak of PANI [47].
Applying spectrum analysis was an important technique for studying the interaction between molecules [48]. The FTIR spectra of PANI@Al-MnO2@CC nanocomposite is shown in Fig. 4b. The vibration peaks centered at 1565 cm-1 and 1468 cm-1 were attributed to the stretching vibration peak of C=N in PANI quinonoid structure and the stretching vibration peak of C=C in benzene structure [49]. We also observed C-N stretching vibration peaks from 1163 to 1300 cm-1, which represented the secondary amine on the benzene ring of PANI chain, and the bending vibration peaks of protonated PANI in the C-H plane at 1124 cm-1 [50, 51]. The peaks of MnO2 were not observed by low loading, but it still showed that PANI@Al-MnO2@CC nanocomposite was composed of PANI [52].
Figure 4c shows the TG curves of the optimal PANI@Al-MnO2@CC and Al-MnO2@CC. It could be found that PANI@Al-MnO2@CC degraded from the beginning, while Al-MnO2@CC degraded at about 500 °C, which corresponded to the decomposition temperature of MnO2. It indicated that due to PANI initially, PANI@Al-MnO2@CC degraded, and continued to degrade due to the presence of MnO2 at 500 °C [53].
3.4 Electrochemical performance of the optimal PANI@Al-MnO2@CC
In order to investigate the electrochemical performance of the optimal PANI@Al-MnO2@CC, different tests were performed. Figure 5a shows that as the voltage window increased, the CV curves of the material were orderly magnified and highly equaled, which indicated that the material had good capacitance performance. Figure 5b indicated that the PANI@Al-MnO2@CC electrode material still maintained great symmetry, and had the longest discharge time when it was extended to 1.25 V. In addition, its specific capacitance even reached 1016 mF cm-2, which was much higher than 945 mF cm-2 under 1.2 V. It revealed the enlargement of the voltage window was beneficial to increase storage capacity.
The CV curve of the optimal PANI@Al-MnO2@CC at different scan rates was shown for verifying the rate performance (Fig. 5c), which always maintained a good quasi-rectangular shape, and the material exhibited excellent rate performance and reversibility. As the scan rate increased, it tended to the spindle shape. The increase of the scan rate caused the ions in electrolyte to enter PANI@Al-MnO2@CC not completely, which increased the internal resistance, thereby reduced the performance [54]. Fig. 5d show the voltage drop of GCD curves of electrode materials increased as the current density increased. This may be because ions could not enter the material to increase the internal resistance.
Nyquist plots of the optimal PANI@Al-MnO2@CC were studied to analyze conductivity (Fig. 5e, f). The Nyquist chart consisted of semicircles and straight lines, corresponding to equivalent series resistance (RS), electric double layer capacitance (C) and Faraday capacitance (CPE). The CPE was divided into charge transfer and material transfer, i.e., charge transfer resistance (R1) and Warburg resistance (W) [55]. As the resistance of the electrode material and the electrolyte, RS was located at the intercept of the real axis. From the illustration in Fig. 5e, the resistance value of the PANI@Al-MnO2@CC was 0.98 Ω, and the profile was steep, indicating that it had a higher capacitance behavior.
Through Fig. S6, we compared the GCD curve of PANI@Al-MnO2@CC and Al-MnO2@CC we prepared before [45]. It could be found that PANI@Al-MnO2@CC had better symmetry, i.e., better charge and discharge performance and stability. The specific capacitances of PANI@Al-MnO2@CC are shown in Fig. S7. The specific capacitance reached 1016 mF cm-2 at 6 mA cm-2, which was 12.1% higher than 906 mF cm-2 of Al-MnO2@CC. Obviously, Al doping expands the voltage window of MnO2, loading a small amount of PANI on the surface of Al-MnO2 could further improved the electrochemical properties of the material, such as conductivity. Therefore, PANI@Al-MnO2@CC prepared in this work has obvious advantages over other types of electrode materials, as shown in Table 1.
3.5 Electrochemical performance of PANI@Al-MnO2@CC quasi-solid-state flexible supercapacitor (PANI-FSSC)
The optimal PANI@Al-MnO2@CC electrode material was assembled into a quasi-solid-state flexible symmetric supercapacitor. Then Fig. 6a shows the CV curves of the electrode material under different voltage windows. It could be found that as the voltage window increased, the CV of the material showed an orderly amplification, and the degree of overlap was extremely high, indicating that the material had good capacitance performance. Fig. 6b shows the CV curves of the assembled device, which reflected that there was no obvious polarization when its voltage window reached 2.4 V. Regardless of the scan rate, the device still had an ideal shape, further indicating that it had excellent charge-discharge and rate performance. The GCD curves of the assembled devices are shown in Fig. 6c. It revealed that a large area specific capacitance of 149.75 mF cm-2 at 6 mA cm-2 could be achieved. Furthermore, the thickness of assembled capacitor was measured to be 0.075 cm. It was found that when the power density was 96.16 mW cm-3, the device had an energy density of 1.60 mWh cm-3.
The mechanical property of supercapacitor materials was an essential factor in practical applications [56, 57]. To test the flexibility of the equipment, the single electrode material was bent at 60 °, 120 ° and 180 °. After 100 repetitions, the GCD curves shown in Fig. 6d were essentially coincident, which indicated that the area specific capacitance generally remained the same and only had a small loss. Therefore PANI-FSSC had great flexibility.
The cycle stability of PANI-FSSC was another indispensable part. After 5000 times charge-discharge at 20 mA cm-2 (Fig. 6e), the loss of PANI-FSSC was 19.9%, and the GCD curves were basically unchanged (Fig. 6f). To further study the practical application of PANI-FSSC, it was charged with GCD, and then the LED bulb shown in Fig. 6e could successfully light, which proved that it had wide potential prospects.
4 Conclusion
In conclusion, carbon cloth was used as a flexible substrate, PANI@Al-MnO2@CC flexible electrode material was successfully prepared through simple hydrothermal reaction and in-situ polymerization. The polyaniline formed on Al-MnO2@CC can effectively improve the conductivity of the electrode material while maintaining good cycle stability. The result indicates that good flexibility was shown in PANI@Al-MnO2@CC loaded with nano-spherical polyaniline. Its voltage window can increase to 1.25 V, with an area specific capacitance of up to 1016 mF m-2, and can maintain 80.1 % after 5000 cycles at 20 mA cm-2. The voltage window of the flexible symmetrical supercapacitor assembled from the electrode material can be increased to 0-2.4 V, and the highest area specific capacitance at 6 mA cm-2 can reach 149.75 mF cm-2, exhibiting excellent electrochemical performance. The high-performance, quasi-solid state flexible supercapacitor prepared in this research provides a new method for the development of multi-functional flexible electronic devices such as nano robots, wearable electronic devices, rolling curtain displays and so on.
References
L. Miao, Z. Song, D. Zhu, L. Li, L. Gan, M. Liu, Ionic Liquids for supercapacitive energy storage: A mini-review. Energ. Fuels 35, 8443–8455 (2021)
M. Mansuer, L. Miao, D. Zhu, H. Duan, Y. Lv, L. Li, M. Liu, L. Gan, Facile construction of highly redox active carbons with regular micropores and rod-like morphology towards high-energy supercapacitors. Mater. Chem. Front. 5, 3061–3072 (2021)
J. Yan, L. Miao, H. Duan, D. Zhu, Y. Lv, W. Xiong, L. Li, L. Gan, M. Liu, Core-shell hierarchical porous carbon spheres with N/O doping for efficient energy storage. Electrochim. Acta 358, 136899 (2020)
J. Chen, J. Wu, X. Wang, A. Zhou, Z. Yang, Research progress and application prospect of solid-state electrolytes in commercial lithium-ion power batteries. Energy Storage Mater. 35, 70–87 (2021)
J. Zeng, N. Fu, X. Wang, A. Zhou, Z. Yang, Multipath conduction and large capacity silicon-based anodes for high stabilizing lithium-ion batteries. Appl. Surf. Sci. 557, 149860 (2021)
O.S. Powar, L. Bhatta, R. Prasad, K. Venkatesh, A.V. Raghu, Influence of carbon based electrodes on the performance of the microbial fuel cell. Int. J. Res. Granthaalayah 5, 7–16 (2017)
N.F. Hamed, B.P. Ghobad, N.S. Mehdi, E. Parisa, PVA-based supercapacitors. Ionics 25, 2951–2963 (2019)
Q. Zhu, D. Zhao, M. Cheng, J. Zhou, K.A. Owusu, L. Mai, Y. Yu, A new view of supercapacitors: Integrated supercapacitors. Adv. Energy Mater. 9, 1901081 (2019)
J. Yu, M. Li, X. Wang, Z. Yang, Promising high-performance supercapacitor electrode materials from MnO2 nanosheets@bamboo leaf carbon. ACS Omega 5, 16299–16306 (2020)
Z. Zhang, X. Zhang, W. Chen, Y. Rasim, W. Salman, H. Pan, Y. Yuan, C. Wang, A high-efficiency energy regenerative shock absorber using supercapacitors for renewable energy applications in range extended electric vehicle. Appl. Energ. 178, 177–188 (2016)
J.H. Peter, M. Mojtaba, S.I. Fletcher, F.B. Sillars, A.J.R. Rennie, G.O. Shitta-Bey, G. Wilson, A. Cruden, R. Carter, Energy storage in electrochemical capacitors: Designing functional materials to improve performance. Energ. Environ. Sci. 9, 1236–1251 (2010)
C. Peter, M. Tariq, C. Kevin, Cutting vehicle emissions with regenerative braking. Transport. Res. D-Tr. E. 3, 160–167 (2010)
Z. Pang, J. Duan, Y. Zhao, Q. Tang, B. He, L. Yu, A ceramic NiO/ZrO2 separator for high-temperature supercapacitor up to 140 °C. J. Power Sources 400, 126–134 (2018)
J. Cao, Y. Zhao, Y. Xu, Y. Zhang, B. Zhang, H. Peng, Sticky-note supercapacitors. J. Mater. Chem. A 6, 3355–3360 (2018)
L. Li, Z.A. Hu, N. An, Y.Y. Yang, Z.M. Li, H.Y. Wu, Facile synthesis of MnO2 /CNTs composite for supercapacitor electrodes with long cycle stability. J. Phys. Chem. C 118, 22865–22872 (2014)
M. Li, J. Yu, X. Wang, Z. Yang, 3D porous MnO2@carbon nanosheet synthesized from rambutan peel for high-performing supercapacitor electrodes materials. Appl. Surf. Sci. 530, 147230 (2020)
D.I. Tishkevich, A.I. Vorobjova, D.A. Vinnik, Template assisted Ni nanowires fabrication. Mater. Sci. Forum 946, 235–241 (2019)
A.I. Vorobjova, D.L. Shimanovich, O.A. Sycheva, T.I. Ezovitova, D.I. Tishkevich, A.V. Trykhanov, Studying the thermodynamic properties of composite magnetic material based on anodic alumina. Russ. Microelectron. 48, 107–118 (2019)
D.I. Tishkevich, A.I. Vorobjova, A.V. Trukhanov, Thermal stability of nano-crystalline nickel electrodeposited into porous alumina. Solid St. Pheno. 299, 281–286 (2020)
D. Tishkevich, S. Grabchikov, T. Zubar, D. Vasin, S. Trukhanov, A. Vorobjova, D. Yakimchuk, A. Kozlovskiy, M. Zdorovets, S. Giniyatova, D. Shimanovich, D. Lyakhov, D. Michels, M. Dong, S. Gudkova, A. Trukhanov, Early-stage growth mechanism and synthesis conditions-dependent morphology of nanocrystalline Bi films electrodeposited from perchlorate electrolyte. Nanomaterials 10, 1245 (2020)
H. Wang, C. Xu, Y. Chen, Y. Wang, MnO2 nanograsses on porous carbon cloth for flexible solid-state asymmetric supercapacitors with high energy density. Energy Storage Mater. 8, 127–133 (2017)
W. Yang, Z. Gao, J. Ma, X. Zhang, J. Wang, J. Liu, Hierarchical NiCo2O4@NiO core-shell hetero-structured nanowire arrays on carbon cloth for a high-performance flexible all-solid-state electrochemical capacitor. J. Mater. Chem. A 2, 1448–1457 (2014)
Z. Huang, Z. Zhang, X. Qi, X. Ren, G. Xu, P. Wan, X. Sun, H. Zhang, Wall-like hierarchical metal oxide nanosheet arrays grown on carbon cloth for excellent supercapacitor electrodes. Nanoscale 8, 13273–13279 (2016)
P.A. Shinde, A.C. Lokhande, N.R. Chodankar, A.M. Patil, J.H. Kim, C.D. Lokhande, Temperature dependent surface morphological modifications of hexagonal WO3 thin films for high performance supercapacitor application. Electrochim. Acta 224, 397–404 (2017)
Y.J. Gu, W. Wen, S. Zheng, J.M. Wu, Rapid synthesis of high-areal-capacitance ultrathin hexagon Fe2O3 nanoplates on carbon cloth via a versatile molten salt method. Mater. Chem. Front. 4, 2744–2753 (2020)
R. Jia, Y. Jiang, R. Li, R. Chai, Z. Lou, G. Shen, D. Chen, Nb2O5 nanotubes on carbon cloth for high performance sodium-ion capacitors. Sci. China Mater. 63, 1171–1181 (2020)
I. Inamuddin, A.M. Asiri, E. Lichtfouse, Nanophotocatalysis and Environmental Applications. Environ. Chem. Sust. World (2020). https://doi.org/10.1007/978-3-030-12619-3
P. Tang, L. Han, L. Zhang, Facile synthesis of graphite/PEDOT/MnO2 composites on commercial supercapacitor separator membranes as flexible and high-performance supercapacitor electrodes. ACS Appl. Mater. Inter. 6, 10506–10515 (2014)
J. Han, L. Li, P. Fang, R. Guo, Ultrathin MnO2 nanorods on conducting polymer nanofibers as a new class of hierarchical nanostructures for high-performance supercapacitors. J. Phys. Chem. C 116, 15900–15907 (2012)
R.P. Mahore, S.B. Kondawar, D.K. Burghate, B.H. Meshram, Electrochemical performance of polyaniline/CNT/MnO2 and polypyrrole/CNT/MnO2 ternary nanocomposites as electrode materials for supercapacitor. J. Chin. Adv. Mater. Soc. 3, 45–56 (2015)
M. Rajesh, C.J. Raj, R. Manikandan, B.C. Kim, S.Y. Park, K.H. Yu, A high performance PEDOT/PEDOT symmetric supercapacitor by facile in-situ hydrothermal polymerization of PEDOT nanostructures on flexible carbon fibre cloth electrodes. Mater. Today Energy 6, 96–104 (2017)
T. Xiao, P. Chen, S. Wang, W. Zhou, F. Chen, F. Tao, X. Chen, X. Tan, P. Xiang, L. Jiang, X. Chen, A novel two-prong strategy to boost the capacitive performance of commercial carbon cloth. J. Alloy. Compd. 831, 154615 (2020)
Q. Zhou, D. Zhu, X. Ma, D. Mo, F. Jiang, J. Xu, W. Zhou, PEDOT: PSS-assisted polyindole hollow nanospheres modified carbon cloth as high performance electrochemical capacitor electrodes. Electrochim. Acta 212, 662–670 (2016)
K.R. Reddy, B. Hemavathi, G.R. Balakrishna, A.V. Raghu, S. Naveen, M.V. Shankar, Polymer Composites with Functionalized Nanoparticles (Elsevier, Amsterdam, 2019), pp. 357–379
R. Prabhu, B. Roopashree, T. Jeevananda, S. Rao, K.R. Reddy, A.V. Raghu, Synthesis and corrosion resistance properties of novel conjugated polymer-Cu2Cl4L3 composites. Mater. Sci. Energy Techn. 4, 92–99 (2021)
B. Joyita, D. Kingshu, K.M. Abdul, K.N. Sanjay, An overview on the recent developments in polyaniline-based supercapacitors. Polym. Adv. Technol. 30, 1902–1921 (2019)
X. He, B. Gao, G. Wang, J. Wei, C. Zhao, A new nanocomposite: Carbon clothbased polyaniline for an electrochemical supercapacitor. Electrochim. Acta 111, 210–215 (2013)
T. Wu, C. Wang, Y. Mo, X. Wang, J. Fan, Q. Xu, Y. Min, A ternary composite with manganese dioxide nanorods and graphene nanoribbons embedded in a polyaniline matrix for high-performance supercapacitors. RSC Adv. 53, 33591–33599 (2017)
L.-H. Tseng, C.-H. Hsiao, D.D. Nguyen, P.-Y. Hsieh, C.-Y. Lee, N.-H. Tai, Activated carbon sandwiched manganese dioxide/graphene ternary composites for supercapacitor electrodes. Electrochim. Acta 266, 284–292 (2018)
H. Li, J. Liang, H. Li, X. Zheng, Y. Tao, Z.-H. Huang, Q.-H. Yang, Activated carbon fibers with manganese dioxide coating for flexible fiber supercapacitors with high capacitive performance. J. Energy Chem. 31, 95–100 (2019)
M. Xu, Y. Cai, T. Wang, X. Wang, A. Zhou, Z. Yang, Two-dimensional Mg-doped MnO2@carbon cloth nanosheets for high performance typical flexible solid supercapacitor. J. Alloy. Compd. 877, 160243 (2021)
J. Zhu, Q. Zhang, H. Chen, R. Zhang, L. Liu, J. Yu, Setaria viridis-inspired electrode with polyaniline decorated on porous heteroatom-doped carbon nanofibers for flexible supercapacitors. ACS Appl. Mater. Interfaces 12, 43634–43645 (2020)
A. Eftekhari, L. Li, Y. Yang, Polyaniline supercapacitors. J. Power Sources 347, 86–107 (2017)
S. Jadhav, R.S. Kalubarme, C. Terashima, B.B. Kale, V. Godbole, A. Fujishima, S.W. Gosavi, Manganese dioxide/reduced graphene oxide composite an electrode material for high-performance solid state supercapacitor. Electrochim. Acta 299, 34–44 (2019)
M. Xu, N. Fu, X. Wang, Z. Yang, A high energy density flexible symmetric supercapacitor based on Al-doped MnO2 nanosheets@carbon cloth electrode materials. J. Mater. Sci-Mater. EI. 31, 16027–16036 (2020)
R.S. Kalubarme, M.-S. Cho, K.-S. Yun, T.-S. Kim, C.-J. Park, Catalytic characteristics of MnO2 nanostructures for the O2 reduction process. Nanotechnology 22, 395402 (2011)
A. Shirmardi, M.A.M. Teridi, H.R. Azimi, W.J. Basirun, F. Jamali-Sheini, R. Yousefi, Enhanced photocatalytic performance of ZnSe/PANI nanocomposites for degradation of organic and inorganic pollutants. Appl. Surf. Sci. 462, 730–738 (2018)
M.M. Mezgebe, Z. Yan, G. Wei, S. Gong, F. Zhang, S. Guang, H. Xu, 3D graphene-Fe3O4-polyaniline, a novel ternary composite for supercapacitor electrodes with improved electrochemical properties. Mater. Today Energy 5, 164–172 (2017)
L. Yu, M. Gan, L. Ma, H. Huang, H. Hu, Y. Li, Y. Tu, C. Ge, F. Yang, J. Yan, Facile synthesis of MnO2/polyaniline nanorod arrays based on graphene and its electrochemical performance. Synthetic Met. 198, 167–174 (2014)
R. Prabhu, T. Jeevananda, K.R. Reddy, A.V. Raghu, Polyaniline-fly ash nanocomposites synthesized via emulsion polymerization: Physicochemical, thermal and dielectric properties. Mater. Sci. Energy Techn. 4, 107–112 (2021)
K.R. Reddy, K.V. Karthik, S.B.B. Prasad, S.K. Soni, H.M. Jeong, A.V. Raghu, Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 120, 169–174 (2016)
S.X. Wang, L.X. Sun, Z.C. Tan, F. Xu, Y.S. Li, Synthesis, characterization and thermal analysis of polyaniline (PANI)/Co3O4 composites. J. Therm. Anal. Cal. 89, 609–612 (2007)
J. Zhang, D. Shu, T. Zhang, H. Chen, H. Zhao, Y. Wang, Z. Sun, S. Tang, X. Fang, X. Cao, Capacitive properties of PANI/MnO2 synthesized via simultaneous-oxidation route. J. Alloy. Compd. 532, 1–9 (2012)
J.H. Jang, K. Machida, Y. Kim, K. Naoi, Electrophoretic deposition (EPD) of hydrous ruthenium oxides with PTFE and their supercapacitor performances. Electrochim. Acta 52, 1733–1741 (2006)
Y. Wang, Z. Shi, Y. Huang, Y. Ma, C. Wang, M. Chen, Y. Chen, Supercapacitor devices based on graphene materials. J. Phys. Chem. C 113, 13103–13107 (2009)
X. Liang, G. Long, C. Fu, M. Pang, Y. Xi, J. Li, W. Han, G. Wei, Y. Ji, High performance all-solid-state flexible supercapacitor for wearable storage device application. Chemi. Eng. J. 345, 186–195 (2018)
X. Li, J. Shao, S.-K. Kim, C. Yao, J. Wang, Y.-R. Miao, Q. Zheng, P. Sun, R. Zhang, P.V. Braun, High energy flexible supercapacitors formed via bottom-up infilling of gel electrolytes into thick porous electrodes. Nat. Commun. 9, 2578 (2018)
S. Xi, Y. Zhu, Y. Yang, Y. Liu, Direct synthesis of MnO2 nanorods on carbon cloth as flexible supercapacitor electrode. J. Nanomater. (2017). https://doi.org/10.1155/2017/7340961
P. Sivaraman, R. Kushwaha, K. Shashidhara, V. Hande, A. Thakur, A. Samui, M. Khandpekar, All solid supercapacitor based on polyaniline and crosslinked sulfonated poly [ether ether ketone]. Electrochim. Acta 55, 2451–2456 (2010)
H. Jiang, J. Ma, C. Li, Polyaniline-MnO2 coaxial nanofiber with hierarchical structure for high-performance supercapacitors. J. Mater. Chem. 22, 16939 (2012)
X. Wang, H. Wei, W. Du, X. Sun, L. Kang, Y. Zhang, X. Zhao, F. Jiang, Recycled carbon fiber-supported polyaniline/manganese dioxide prepared via one-step electrodeposition for flexible supercapacitor integrated electrodes. Polymers-Basel 10, 1152 (2018)
S.M. Jadhav, R.S. Kalubarme, N. Suzuki, C. Terashima, J. Mun, B.B. Kale, S.W. Gosavi, A. Fujishima, Cobalt-doped manganese dioxide hierarchical nanostructures for enhancing pseudocapacitive properties. ACS Omega 6, 5717–5729 (2021)
J. Kim, H. Ju, A.I. Inamdar, Y. Jo, J. Han, H. Kim, H. Im, Synthesis and enhanced electrochemical supercapacitor properties of Ag-MnO2-polyaniline nanocomposite electrodes. Energy 70, 473–477 (2014)
C. Xia, Y. Xie, H. Du, W. Wang, Ternary nanocomposite of polyaniline/manganese dioxide/titanium nitride nanowire array for supercapacitor electrode. J. Nanopart. Res. 17, 12–30 (2015)
Acknowledgments
This work was supported by National Key Research and Development Program of China (2017YFA0204600) and the Fundamental Research Funds for the Central Universities (No. 22120210170).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, J., Wang, R., Xu, M. et al. Polyaniline-supported Al-doped MnO2@carbon cloth-based electrode material for quasi-solid-state flexible supercapacitor. J Mater Sci: Mater Electron 32, 19820–19831 (2021). https://doi.org/10.1007/s10854-021-06505-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06505-1