Abstract
The drawback of traditional capacitors with low energy density forces people to develop high-performance supercapacitor materials. Herein, the Al-doped MnO2 @ carbon cloth is selected as the ideal electrode material. A typical hydrothermal reaction can be used to synthesize Al-doped MnO2 @ carbon cloth at 150 °C for 4 h. Its operating window can be successfully expanded from 0–0.8 to 0–1.2 V, and the areal-specific capacitance reaches 1043 mF cm−2, keeping 91.1% of the original after cycling 5000 times at a current density of 20 mA cm−2. Using the electrode materials designed above, the flexible symmetrical supercapacitor is successfully assembled. This device shows a greater operating window of 0–2.3 V, a superior large areal-specific capacitance of 521.91 mF cm−2, and the energy density remarkably reaches 4.72 mWh cm−3. After 100 bending cycles, there is also basically no loss in performance. This study highlights the promising prospects of the improvement of MnO2 @ CC materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Energy depletion is becoming more and more serious, and the development of energy storage devices is particularly necessary. As a kind of energy storage device, the supercapacitor (SC) has attracted great attention in the field of electronics, transportation, and aerospace with its high energy density and good cycle stability [1,2,3]. The supercapacitors could be divided into electrostatic double-layer capacitors (EDLCs) and faradaic pseudocapacitors [4]. The EDLCs are mainly physically adsorbed on the electrode surface by electrolyte ions, such as graphene, carbon fiber, and carbon nanotubes [5,6,7,8]. The faradaic pseudocapacitors refer to the rapid reversible chemical reaction of transition metal oxides or conductive polymers during charge and discharge of electrode materials [9, 10]. Among them, oxides of metal materials such as Ru, Zn, Mn, Co, and Ni are recognized for their superior performance [11,12,13,14]. RuO2 is identified as the most suitable material owing to its high specific capacity and good electrical conductivity [15], but its unreasonable price limits its development. Ni and Co are only suitable for the alkaline environment, whose working window couldn’t meet the requirements of high voltage devices [16, 17]. Among them, MnO2 is affordable, abundant and environmentally friendly, it seems as one of the best choices. Traditional studies have shown that MnO2 could be synthesized by the hydrothermal reaction of potassium permanganate (KMnO4) and C [18,19,20,21]:
Unfortunately, pure MnO2 is poorly conductive as an electrode material, seriously affecting the capacity of the device. According to the energy density E = 1/2CV2 [22], we may infer the effective methods to enhance the energy density (E) of SCs by raising the operating voltage window (V) and areal-specific capacitance (C). Doping has been considered an alternative way. Poonguzhali et al. [23] studied the influence on the capacitance of Fe doped MnO2 nanoparticles synthesized by chemical precipitation. Ahmed M. Hashem discussed the crystallization of Al, Cu, and Mg-doped MnO2 [24]. In addition, V [25], Ni [26], and Co [27] have also been extensively studied due to their special properties. Wherein the radius of Al [28] is really close to Mn, which is considered to be the most ideal material to insert the gap of MnO2. In the conventional capacitors, the metal oxide needs to be deposited or bonded to the current collector (i.e., metal foam), which is not easily damaged, but increases the weight of the electrode material, resulting in poor electrical conductivity [29,30,31]. Moreover, with the prevalence of flexible and wearable devices [22], collapsible energy storage devices occupy a more important position, requiring excellent flexibility of the electrode material. Therefore, carbon cloth (CC) is selected as the proper substrate [32]. The material is directly grown on the carbon cloth material to achieve uninterrupted charge transfer with the pore structure of CC, and the electrochemical performance of the material is significantly improved.
Based on the above view, herein, a simple hydrothermal synthesis reaction was used to synthesize Al-intercalated MnO2 nanosheets on carbon cloth (Al–MnO2 @ CC). The operating voltage was successfully expanded to 1.2 V. Its areal--specific capacitance reached 1043 mF cm−2, increasing by 18.5% compared to pure MnO2 electrode material. Moreover, under the current density of 20 mA cm−2, the Al–MnO2 @ CC was cycled 5000 times, whose areal-specific capacitance kept 91.1% of the original. Furthermore, the electrode materials prepared above consist of a flexible symmetrical supercapacitor (FSSC) that realized a broad voltage window of 2.3 V, an ultra-large areal-specific capacitance of 521.91 mF cm−2 and an excellent energy density of 4.72 mWh cm−3.
2 Materials and methods
2.1 Pretreatment of carbon cloth
For a typical carbon cloth (1 cm × 0.5 cm, Fig. S1), it was ultrasonically washed for 15 min with acetone and isopropyl alcohol, then immersed in concentrated HNO3 for 10 min. After being finally washed thoroughly with deionized water, it was dried overnight.
2.2 Preparation of Al–MnO2 @ carbon cloth
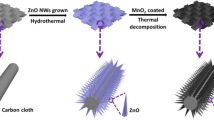
Al-doped MnO2 @ carbon cloth (Al–MnO2 @ CC) was prepared by a typical hydrothermal reaction. The pretreated carbon cloth was placed in 100 mL deionized water, adding 5 mM KMnO4 and 0.22 mM (4.5%), 0.45 mM (9.0%), and 0.9 mM (18.0%) AlCl3, respectively. The stirring was magnetically kept for 30 min to obtain a homogeneous solution, then solution (40 mL) prepared above was kept at 150 °C for 4 h in an autoclave, as shown in Scheme 1. After the reaction, the Al–MnO2 @ CC was cooled to room temperature and washed several times.
2.3 Assembly of the flexible symmetric supercapacitor
Na2SO4 (1 M) was used as the electrolyte. The specific preparation of the flexible symmetric supercapacitor (FSSC) was as follows: two pieces of Al–MnO2 @ CC (1 cm × 0.5 cm × 0.033 cm) were stacked together, which were separated by a non-woven membrane (1 cm × 1 cm × 0.5 cm). After the assembly, the cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) were used to measure their electrochemical properties.
2.4 Characterization and electrochemical measurements
The surface morphology of Al–MnO2 @ CC and MnO2 @ CC was observed by the scanning electron microscope (SEM). Transmission electron microscopy (TEM), selected area electronic diffraction (SAED), and STEM mapping (JEM-2100F) were also utilized to observe the nanomorphology, crystal structure, and composition. The X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and TG–DTA/DSC were used for comprehensive analysis of materials, as well.
The Al–MnO2 @ CC and MnO2 @ CC’s electrochemical performance tests were completed in a CHI 760E workstation, including cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS). The scanning rate of the EIS was set to 100 kHz to 0.01 Hz.
3 Results and discussion
3.1 Characterization of Al–MnO2 @ CC
The SEM images of Al–MnO2 @ CC which have the doping amount of 4.5%, 9.0%, and 18.0% at 1 μm and 10 μm scales are shown in Figs. 1a–c and S2, respectively. It can be seen that the nanoneedle morphology (Fig. S3) of pure MnO2 is changed. Figure 1a shows a still visible nanoneedle morphology of 4.5% Al–MnO2 @ CC, but 9.0% Al–MnO2 @ CC shows an ideal nanosheet morphology. When the doping amount was continuously increased to 18.0%, the nanosheets of Al–MnO2 accumulated to form nanospheres, resulting in poor conductivity. Figure 1d–f shows TEM images, a HRTEM image, and a SAED pattern of 9.0% Al–MnO2 @ CC. It can be seen that 9.0% Al–MnO2 @ CC exhibits nanosheet morphology at a 100 nm scale compared to the nanoneedle morphology of MnO2 @ CC (Fig. S4), consistent with the observations of SEM. The HRTEM shows the lattice spacing about 0.27 nm, equivalent to the (− 110) of δ-MnO2 [22], confirming that the obtained nanosheet is a crystalline structure. In addition, it is also discernible that the material is of a polycrystalline structure from the SAED [33, 34]. Using STEM mapping and EDX spectrometry (Fig. S5) to analyze the composition of the material, the elements of C, Mn, O, and Al can be seen in Fig. 1g–j in the area as indicated in Fig. S6, where C may probably come from the CC, and the presence of Al, Mn, and O indicates that Al is successfully inserted into the MnO2 nanosheets [22].
Furthermore, the XRD of 9.0% Al–MnO2 @ CC and MnO2 @ CC is shown in Fig. 2a. It mainly includes a diffraction peak of carbon cloth and the correlation peak corresponding to potassium manganese oxide hydrate birnessite (JCPDS No. 80-1098), demonstrating that the material is δ-MnO2 and the intercalation of Al does not affect the crystal structure of the material [33]. The XPS of 9.0% Al–MnO2 @ CC and MnO2 @ CC can be seen in Figs. 2b–e and S7 [35]. It can be seen clearly from the XPS full spectrum in Fig. 2b; there are K and Cl besides Mn, O, and Al, which are derived from KMnO4 and AlCl3, respectively. As shown in Fig. 2b, 653.78 and 642.08 eV correspond to 1/2 and 3/2 of the Mn 2p spin–orbit bimodal, respectively [36, 37], wherein the main peaks (641.91 and 653.74 eV) are derived from MnO2, and the secondary peak (640.75 and 652.68 eV) is derived from KxMnO2 [38] that has not been completely converted. In addition to the main peak and the secondary peak, there are accompanying peaks. We can conclude the Mn electrons move from the 3d orbit to 2p spectrum during the ejection of the core 2p photoelectron to empty 1s track [39]. Figure 2c shows the peaks of O at different positions, where the main peak at 529–530 eV corresponds to a lattice bond with Mn [40], and 531.34 eV can be attributed to oxygen species adsorbed on the surface of the material, such as H–O, C–O [41]. Figure 2d shows a peak consistent with the 2p peak position of Al (73.45 eV), indicating that Al has been doped into the MnO2 lattice perfectly. Mn (35.5%), O (61.7%) and Al (2.8%) of atoms were represented by the high-resolution XPS spectra (Table S1). Compared with the composition of the sample MnO2, O accounts for a smaller proportion, which is related to the intercalation of Al. In addition, we found that the binding energies of Mn and O increased by 0.1 eV in contrast to the XPS peaks of pure MnO2. We can conclude that the doping of Al increases the lattice defects of MnO2, but the atomic valence states of Mn and O haven’t changed, which caused the crystal binding energy a small change and have little effect on the stability of the material. The TG curves (Fig. S8) of 9.0% Al–MnO2 @ CC and MnO2 @ CC show that they all degrade at about 500 °C, corresponding to the decomposition temperature of MnO2, and the residue of 9.0% Al–MnO2 @ CC is more than MnO2 @ CC, which is considered to be related to Al and H2O.
3.2 Electrochemical performance of Al–MnO2 @ CC
For analyzing the electrochemical properties of Al–MnO2 @ CC, a three-electrode system was used for electrochemical measurement, wherein 1 M Na2SO4 aq. acted as the electrolyte. Firstly, the capacitance performance of Al–MnO2 @ CC was tested by CV. The obtained CV curves of 4.5%, 9.0%, and 18.0% Al–MnO2 @ CC in 0–1.2 V operating window are shown in Fig. 3a, and the scanning rate is 20 mV s−1. As can be seen, compared with 4.5% Al–MnO2 @ CC and 18.0% Al–MnO2 @ CC, the CV curve of 9.0% Al–MnO2 @ CC exhibits a better quasi-rectangular shape, also indicating that the electrode material has a better pseudocapacitance behavior. The GCD can also be used to judge the capacitive properties of the electrode materials. Figure 3b shows the GCD curves of 4.5%, 9.0%, and 18.0% Al–MnO2 @ CC in the window of 0–1.2 V, owning a current density of 4 mA cm−2. All three materials exhibit good triangular symmetry, proving that the electrode materials have good charge and discharge behavior. The areal-specific capacitances of these three electrode materials could be calculated to be 992.33 mF cm−2, 1043 mF cm−2, and 988.33 mF cm−2, respectively. The EIS was used to analyze their conductivity. The Nyquist diagram of 4.5%, 9.0% and 18.0% Al–MnO2 @ CC were shown in Fig. 3c, where the inset is the local part of Nyquist magnified views. All three materials’ Nyquist diagrams consist of semicircles and straight lines. From the equivalent circuit diagram in Fig. 3d, the electrode material is not only affected by the system resistance (RS), but also by the electric double-layer capacitor and the Faraday capacitor. Faraday capacitors can further be divided into the charge transfer and mass transfer (i.e., charge transfer resistance (R1) and the Warburg impedance (W)) [22, 42, 43].
Generally, compared to the other two electrode materials, the 9.0% Al–MnO2 @ CC not only performs the superior nanosheet morphology, but has smaller resistance and a steeper curve, which indicates the highest capacitance behavior together. Therefore, 9.0% Al–MnO2 @ CC is chosen as the ideal electrode material.
So as to investigate the electrochemical performance of 9.0% Al–MnO2 @ CC, Fig. 4a has shown us the CV curves of the electrode material under different voltage windows (0–0.8 V, 0–1.0 V, 0–1.2 V) at 20 mV s−1. Regardless of the voltage window, there is always a pair of redox peaks (0.38 V/0.65 V), which can be ascribed to a reversible redox reaction of Mn3+/Mn4+ and the insertion and extraction of Al3+ [44]. Furthermore, when the operating window is increased from 0.8 to 1.0 V, a single peak of 0.65 V can be seen; when further expanded to 1.2 V, the curve appears a pair of redox peaks of 1.12 V and 0.65 V. This is due to the limitation of the voltage window of 0.8 V, the insertion of Al is not complete, and a new redox pair related to the insertion of Al is generated until the voltage window is expanded to 1.2 V [33]. Figure 4b shows the GCD curves of this electrode material under different operating windows (0–0.8 V, 0–1.0 V, 0–1.2 V) with a current density of 4 mA cm−2. As can be seen, even if the voltage window is expanded to 1.2 V, the 9.0% Al–MnO2 @ CC electrode material also maintains good symmetry, which can be attributed that the intercalation of Al reduced the gap of δ-MnO2 and offered more electrons to δ-MnO2. Observing the discharge time of materials under different windows, it’s the longest under the voltage window of 1.2 V, even the areal-specific capacitance reaches 1043 mF cm−2, which is much higher than 730 mF cm−2 at 0.8 V and 871.6 mF cm−2 at 1.0 V. It fully demonstrates that the expansion of the voltage window can improve the storage capacity of the electrode material. In order to verify the rate performance of the material, Fig. 4c shows the CV curves of 9.0% Al–MnO2 @ CC under different scanning rates. The scanning rate is increased from 5 to 20 mV s−1, and the material always maintains a good quasi-rectangular shape, showing excellent rate performance and reversibility. Until the scanning rate is increased to 25 mV s−1, the electrode material tends to have a shape, often spindle-shaped, because the increment of scanning rate leads to the ions in the aqueous electrolyte fail to fully enter into the electrode material, resulting in the addition of internal resistance, thereby reducing the properties of the material [45, 46]. Figure 4d shows the GCD curves of electrode material at different current densities. As can be seen, the electrode material exhibits increasing pressure drop with increasing current density, which may be attributed to the increase in current density, resulting in the ions couldn’t get inside the material and increases in internal resistance. We calculated the areal-specific capacitance of 9.0% Al–MnO2 @ CC at different current densities (Fig. 4e) [47, 48]. When the current density is 4 mA cm−2, the areal-specific capacitance is 18.5% higher than the specific capacitance 880 mF cm−2 of pure MnO2 @ CC (Figs. S9, S10). With current density increases, the specific capacitance at a current density of 10 mA cm−2 is still 8.58% higher than that of MnO2 @ CC (Fig. S11). Obviously, after doping a small amount of Al into the lattice of MnO2, the lattice defects increase, and the number of oxygen vacancies is also increased, which reduces the internal resistance of MnO2, thereby improving the electronic conductivity and other electrochemical properties of the electrode material. Moreover, at a current density of 20 mA cm−2, 9.0% Al–MnO2 @ CC was cycled 5000 times, whose areal-specific capacitance can keep 91.1% of the original. We can also see from Fig. 4f inset that the electrode material maintains good cycle stability.
a Electrochemical characterization of 9.0% Al–MnO2 @ CC. CV curves under different voltage windows (0–0.8 V, 0–1.0 V, 0–1.2 V) at 20 mV s−1, b GCD curves under different voltage windows (0–0.8 V, 0–1.0 V, 0–1.2 V) at 4 mA cm−2, c CV curves under several different scanning rates, d GCD curves under several different current densities, e areal-specific capacitance related to current density, and f charge–discharge cycling stability, current density: 20 mA cm−2 (inset: charge–discharge profiles)
3.3 Electrochemical performance of the FSSC
The 9.0% Al–MnO2 @ CC electrode materials were assembled into the FSSC and its electrochemical performance was tested by CV and GCD. Figure 5a shows the CV curves of the FSSC at scanning rates of 10, 20, 30, and 40 mV s−1. The voltage window does not show significant polarization when it reaches 2.3 V. Regardless of the scanning rates, the FSSC still has an ideal quasi-rectangular shape. It shows that the FSSC has good charge–discharge performance and rate performance. The GCD curves of FSSC at different current densities and their specific capacitances under different current densities of 4, 6, 8, and 10 mA cm−2 are shown in Fig. 5b and c, respectively. An ultra-large areal-specific capacitance of 521.91 mF cm−2 can be achieved at 4 mA cm−2, which is much higher than the value of the previously reported MnO2-based supercapacitors (SCs). We measured the thickness of the assembled capacitor to be 0.08 cm and calculated the energy density of the supercapacitor material under different power densities shown in Fig. 5d. The device was found to have a good energy density of 4.72 mWh cm−3 when its power density reached 56.67 mW cm−3, higher than some of previously reported energy density of SCs [33, 49,50,51,52,53,54,55,56,57].
a Analysis of the FSSC CV curves at different scanning rates, b GCD curves at different current densities, c areal-specific capacitance related to current density, d energy density and power density of FSSC compared to previous SCs, e areal-specific capacitance under different angles for 100 times (inset: bending effect diagram of FSSC), and f charge–discharge cycling stability, current density: 20 mA cm−2 (inset: an optical image of FSSCs 2.2 V LED lighting)
The mechanical properties of the supercapacitor material are indispensable factors in practical application. In order to verify the flexibility of the device, the 90° and 180° bending operations were performed. After repeating 100 times, the areal-specific capacitance of the material after bending was reduced by 4.74% and 5.23% in Fig. 5e, respectively, confirming that the FSSC has good flexibility.
The FSSC’s cycle stability is also an integral part, so it was charged and discharged 5000 times at 20 mA cm−2 (Fig. 5f), resulting in a loss of 11.5% shown in Fig. 5f, The CV and GCD curves of the device do not change substantially (Figs. S12, S13). In order to study the practical application of the FSSC, charge the device with GCD, then it can light the 2.2 V LED up successfully shown in Fig. 5f inset, proving a great potential in practical applications.
4 Conclusion
In summary, we can get Al-doped MnO2 @ carbon cloth utilizing a typical hydrothermal reaction. The voltage window was successfully expanded to 1.2 V, and the areal-specific capacitance reached 1043 mF cm−2. Compared with pure MnO2 @ CC, the areal-specific capacitance of 9.0% Al–MnO2 @ CC increased by 18.5%, and it kept 91.1% of the original after 5000 cycling times at 20 mA cm−2. The prepared Al–MnO2 @ CC materials were further assembled into a flexible symmetric supercapacitor (FSSC), it can reach a large voltage window of 2.3 V. This device can achieve an ultra-high areal-specific capacitance of 521.91 mF cm−2 and an energy density of 4.72 mWh cm−3 at 4 mA cm−2. After 100 bending cycles at 90° and 180°, there is only 4.74% and 5.23% loss in performance. Therefore, this paper not only provides an improvement direction for MnO2 @ CC materials, but also lays a further application in real life.
References
X. He, X. Mao, C. Zhang, W. Yang, Y. Zhou, Y. Yang, J. Xu, Flexible binder-free hierarchical copper sulfide/carbon cloth hybrid supercapacitor electrodes and the application as negative electrodes in asymmetric supercapacitor. J. Mater. Sci. Mater. Electron. 31, 2145–2152 (2019)
Y. Lv, A. Liu, Z. Shi, J. Mu, Z. Guo, X. Zhang, H. Che, Hierarchical MnCo2O4/NiMn layered double hydroxide composite nanosheet arrays on nickel foam for enhanced electrochemical storage in supercapacitors. ChemElectroChem 5, 3968–3979 (2018)
H. Wang, C. Xu, Y. Chen, Y. Wang, MnO2 nanograsses on porous carbon cloth for flexible solid-state asymmetric supercapacitors with high energy density. Energy Storage Mater. 8, 127–133 (2017)
A. Muzaffar, M. Basheer Ahamed, K. Deshmukh, Hydrothermal synthesis of ZnWO4–MnO2 nanopowder doped with carbon black nanoparticles for high-performance supercapacitor applications. J. Mater. Sci. Mater. Electron. 30, 21250–21258 (2019)
A.G. Pandolfo, A.F. Hollenkamp, Carbon properties and their role in supercapacitors. J. Power Sources 157, 11–27 (2006)
G. Moreno-Fernandez, M. Kunowsky, M.Á. Lillo-Ródenas, J. Ibañez, J.M. Rojo, New carbon monoliths for supercapacitor electrodes. Looking at the double layer. ChemElectroChem 4, 1016–1025 (2017)
Z. Song, H. Duan, D. Zhu, Y. Lv, W. Xiong, T. Cao, L. Li, M. Liu, L. Gan, Ternary-doped carbon electrodes for advanced aqueous solid-state supercapacitors based on a “water-in-salt” gel electrolyte. J. Mater. Chem. A 7, 15801–15811 (2019)
J. Chen, H. Wei, H. Chen, W. Yao, H. Lin, S. Han, N/P co-doped hierarchical porous carbon materials for superior performance supercapacitors. Electrochim. Acta 271, 49–57 (2018)
J. Zhang, Y. Wang, C. Yu, T. Zhu, Y. Li, J. Cui, J. Wu, X. Shu, Y. Qin, J. Sun, J. Yan, Y. Zhang, Y. Wu, Hierarchical NiCo2O4/MnO2 core–shell nanosheets arrays for flexible asymmetric supercapacitor. J. Mater. Sci. 55, 688–700 (2019)
H. Chen, J. Li, C. Long, T. Wei, G. Ning, J. Yan, Z. Fan, Nickel sulfide/graphene/carbon nanotube composites as electrode material for the supercapacitor application in the sea flashing signal system. J. Mar. Sci. Appl. 13, 462–466 (2014)
A. Bello, K. Makgopa, M. Fabiane, D. Dodoo-Ahrin, K.I. Ozoemena, N. Manyala, Chemical adsorption of NiO nanostructures on nickel foam-graphene for supercapacitor applications. J. Mater. Sci. 48, 6707–6712 (2013)
H. Jiang, T. Zhao, C. Yan, J. Ma, C. Li, Hydrothermal synthesis of novel Mn3O4 nano-octahedrons with enhanced supercapacitors performances. Nanoscale 2, 2195–2198 (2010)
R. Wang, X. Yan, J. Lang, Z. Zheng, P. Zhang, A hybrid supercapacitor based on flower-like Co(OH)2 and urchin-like VN electrode materials. J. Mater. Chem. A 2, 12724–12732 (2014)
J. Li, W. Zhao, F. Huang, A. Manivannan, N. Wu, Single-crystalline Ni(OH)2 and NiO nanoplatelet arrays as supercapacitor electrodes. Nanoscale 3, 5103–5109 (2011)
V. Subramanian, Mesoporous anhydrous RuO2 as a supercapacitor electrode material. Solid State Ion. 175, 511–515 (2004)
T. Zhu, J.S. Chen, X.W. Lou, Shape-controlled synthesis of porous Co3O4 nanostructures for application in supercapacitors. J. Mater. Chem. 20, 7015–7020 (2010)
Z. Tang, C.-H. Tang, H. Gong, A high energy density asymmetric supercapacitor from nano-architectured Ni(OH)2/carbon nanotube electrodes. Adv. Funct. Mater. 22, 1272–1278 (2012)
S. He, W. Chen, Application of biomass-derived flexible carbon cloth coated with MnO2 nanosheets in supercapacitors. J. Power Sources 294, 150–158 (2015)
P.A. Shinde, V.C. Lokhande, T. Ji, C.D. Lokhande, Facile synthesis of hierarchical mesoporous weirds-like morphological MnO2 thin films on carbon cloth for high performance supercapacitor application. J. Colloid Interface Sci. 498, 202–209 (2017)
D. Guo, X. Yu, W. Shi, Y. Luo, Q. Li, T. Wang, Facile synthesis of well-ordered manganese oxide nanosheet arrays on carbon cloth for high-performance supercapacitors. J. Mater. Chem. A 2, 8833–8838 (2014)
Z. Xu, S. Sun, W. Cui, J. Lv, Y. Geng, H. Li, J. Deng, Interconnected network of ultrafine MnO2 nanowires on carbon cloth with weed-like morphology for high-performance supercapacitor electrodes. Electrochim. Acta 268, 340–346 (2018)
Q. Zong, Q. Zhang, X. Mei, Q. Li, Z. Zhou, D. Li, M. Chen, F. Shi, J. Sun, Y. Yao, Z. Zhang, Facile synthesis of Na-doped MnO2 nanosheets on carbon nanotube fibers for ultrahigh-energy-density all-solid-state wearable asymmetric supercapacitors. ACS Appl. Mater. Interfaces 10, 37233–37241 (2018)
R. Poonguzhali, N. Shanmugam, R. Gobi, A. Senthilkumar, G. Viruthagiri, N. Kannadasan, Effect of Fe doping on the electrochemical capacitor behavior of MnO2 nanocrystals. J. Power Sources 293, 790–798 (2015)
A.M. Hashem, H.M. Abuzeid, N. Narayanan, H. Ehrenberg, C.M. Julien, Synthesis, structure, magnetic, electrical and electrochemical properties of Al, Cu and Mg doped MnO2. Mater. Chem. Phys. 130, 33–38 (2011)
Z. Hu, X. Xiao, L. Huang, C. Chen, T. Li, T. Su, X. Cheng, L. Miao, Y. Zhang, J. Zhou, 2D vanadium doped manganese dioxides nanosheets for pseudocapacitive energy storage. Nanoscale 7, 16094–160949 (2015)
J. Li, Y. Ren, S. Wang, Z. Ren, J. Yu, Transition metal doped MnO2 nanosheets grown on internal surface of macroporous carbon for supercapacitors and oxygen reduction reaction electrocatalysts. Appl. Mater. Today 3, 63–72 (2016)
C. Tang, X. Wei, Y. Jiang, X. Wu, L. Han, K. Wang, J. Chen, Cobalt-doped MnO2 hierarchical yolk-shell spheres with improved supercapacitive performance. J. Phys. Chem. C 119, 8465–8471 (2015)
Z. Yang, X. Wang, Y. Huang, First-principles study on the doping effects of Al in α-MnO2. Curr. Appl. Phys. 15, 1556–1561 (2015)
C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen, X.W.D. Lou, Ultrathin mesoporous NiCo2O4 nanosheets supported on Ni foam as advanced electrodes for supercapacitors. Adv. Funct. Mater. 2, 4592–4597 (2012)
J. Yang, L. Lian, H. Ruan, F. Xie, M. Wei, Nanostructured porous MnO2 on Ni foam substrate with a high mass loading via a CV electrodeposition route for supercapacitor application. Electrochim. Acta 136, 189–194 (2014)
L. Yu, G. Zhang, C. Yuan, X.W. Lou, Hierarchical NiCo2O4@MnO2 core–shell heterostructured nanowire arrays on Ni foam as high-performance supercapacitor electrodes. Chem. Commun. 49, 137–139 (2013)
J. Yan, Z. Fan, T. Wei, W. Qian, M. Zhang, F. Wei, Fast and reversible surface redox reaction of graphene–MnO2 composites as supercapacitor electrodes. Carbon 48, 3825–3833 (2010)
Y. Liu, L. Guo, X. Teng, J. Wang, T. Hao, X. He, Z. Chen, High-performance 2.5 V flexible aqueous asymmetric supercapacitors based on K+/Na+-inserted MnO2 nanosheets. Electrochim. Acta 300, 9–17 (2019)
H. Wei, J. Wang, S. Yang, W. Wang, D. Hou, T. Li, Synthesis of 3D mesoporous wall-like MnO2 with improved electrochemical performance. J. Electron. Mater. 46, 1539–1545 (2016)
N. Fu, J. Yu, J. Zhao, R. Liu, F. Li, Y. Du, Z. Yang, In-situ preparation of nitrogen-doped unimodal ultramicropore carbon nanosheets with ultrahigh gas selectivity. Carbon 149, 538–545 (2019)
N.C. Maile, S.K. Shinde, R.T. Patil, A.V. Fulari, R.R. Koli, D.Y. Kim, D.S. Lee, V.J. Fulari, Structural and morphological changes in binder-free MnCo2O4 electrodes for supercapacitor applications: effect of deposition parameters. J. Mater. Sci. Mater. Electron. 30, 3729–3743 (2019)
J. Wang, Y. Yang, Z. Huang, F. Kang, Synthesis and electrochemical performance of MnO2/CNTs-embedded carbon nanofibers nanocomposites for supercapacitors. Electrochim. Acta 75, 213–219 (2012)
W. Yao, J. Wang, H. Li, Y. Lu, Flexible α-MnO2 paper formed by millimeter-long nanowires for supercapacitor electrodes. J. Power Sources 247, 824–830 (2014)
L. Yin, I. Adler, T. Tsang, L.J. Matienzo, S.O. Grim, Paramagnetism and shake-up satellites in X-ray photoelectron spectra. Chem. Phys. Lett. 24, 81–84 (1974)
J.P. Holgado, G. Munuera, J.P. Espinós, A.R. González-Elipe, XPS study of oxidation processes of CeOx defective layers. Appl. Surf. Sci. 158, 164–171 (2000)
Z. Song, H. Duan, L. Li, D. Zhu, T. Cao, Y. Lv, W. Xiong, Z. Wang, M. Liu, L. Gan, High-energy flexible solid-state supercapacitors based on O, N, S-tridoped carbon electrodes and a 3.5 V gel-type electrolyte. Chem. Eng. J. 372, 1216–1225 (2019)
J. Yu, N. Fu, J. Zhao, R. Liu, F. Li, Y. Du, Z. Yang, High specific capacitance electrode material for supercapacitors based on resin-derived nitrogen-doped porous carbons. ACS Omega 4, 15904–15911 (2019)
K.M. Nam, D.H. Shin, N. Jung, M.G. Joo, S. Jeon, S.M. Park, B.Y. Chang, Development of galvanostatic Fourier transform electrochemical impedance spectroscopy. Anal. Chem. 85, 2246–2252 (2013)
L.-D. Chen, Y.-Q. Zheng, H.-L. Zhu, Manganese oxides derived from Mn(II)-based metal–organic framework as supercapacitor electrode materials. J. Mater. Sci. 53, 1346–1355 (2017)
D. Xue, D. Zhu, H. Duan, Z. Wang, Y. Lv, W. Xiong, L. Li, M. Liu, L. Gan, Deep-eutectic-solvent synthesis of N/O self-doped hollow carbon nanorods for efficient energy storage. Chem. Commun. 55, 11219–11222 (2019)
S. Chen, J. Zhu, Q. Han, Z. Zheng, Y. Yang, X. Wang, Shape-controlled synthesis of one-dimensional MnO2 via a facile quick-precipitation procedure and its electrochemical properties. Cryst. Growth Des. 9, 4356–4361 (2009)
J.P. Alper, M.S. Kim, M. Vincent, B. Hsia, V. Radmilovic, C. Carraro, R. Maboudian, Silicon carbide nanowires as highly robust electrodes for micro-supercapacitors. J. Power Sources 230, 298–302 (2013)
T. Qin, H. Chen, Y. Zhang, X. Chen, L. Liu, D. Yan, S. Ma, J. Hou, F. Yu, S. Peng, Modulating surface chemistry of heteroatom-rich micropore carbon cloth electrode for aqueous 2.1 V high-voltage window all-carbon supercapacitor. J. Power Sources 431, 232–238 (2019)
L. Wang, H. Yang, X. Liu, R. Zeng, M. Li, Y. Huang, X. Hu, Constructing hierarchical tectorum-like α-Fe2O3/PPy nanoarrays on carbon cloth for solid-state asymmetric supercapacitors. Angew. Chem. Int. Ed. 56, 1125–1130 (2017)
W. Zilong, Z. Zhu, J. Qiu, S. Yang, High performance flexible solid-state asymmetric supercapacitors from MnO2/ZnO core–shell nanorods//specially reduced graphene oxide. J. Mater. Chem. C 2, 1331–1336 (2014)
S. Zhai, H.E. Karahan, L. Wei, X. Chen, Z. Zhou, X. Wang, Y. Chen, Hydrothermal assembly of micro-nano-integrated core-sheath carbon fibers for high-performance all-carbon micro-supercapacitors. Energy Storage Mater. 9, 221–228 (2017)
C. Choi, H.J. Sim, G.M. Spinks, X. Lepró, R.H. Baughman, S.J. Kim, Elastomeric and dynamic MnO2/CNT core–shell structure coiled yarn supercapacitor. Adv. Energy Mater. 6, 1502119 (2016)
X. Li, X. Deng, Q. Li, S. Huang, K. Xiao, Z. Liu, Y. Tong, Hierarchical double-shelled poly(3,4-ethylenedioxythiophene) and MnO2 decorated Ni nanotube arrays for durable and enhanced energy storage in supercapacitors. Electrochim. Acta 264, 46–52 (2018)
P. Yang, Y. Ding, Z. Lin, Z. Chen, Y. Li, P. Qiang, M. Ebrahimi, W. Mai, C.P. Wong, Z.L. Wang, Low-cost high-performance solid-state asymmetric supercapacitors based on MnO2 nanowires and Fe2O3 nanotubes. Nano Lett. 14, 731–736 (2014)
H. Zhang, D. Xiao, Q. Li, Y. Ma, S. Yuan, L. Xie, C. Chen, C. Lu, Porous NiCo2O4 nanowires supported on carbon cloth for flexible asymmetric supercapacitor with high energy density. J. Energy Chem. 27, 195–202 (2018)
W. Liu, K. Feng, Y. Zhang, T. Yu, L. Han, G. Lui, M. Li, G. Chiu, P. Fung, A. Yu, Hair-based flexible knittable supercapacitor with wide operating voltage and ultra-high rate capability. Nano Energy 34, 491–499 (2017)
Y. Liu, X. Miao, J. Fang, X. Zhang, S. Chen, W. Li, W. Feng, Y. Chen, W. Wang, Y. Zhang, Layered-MnO2 nanosheet grown on nitrogen-doped graphene template as a composite cathode for flexible solid-state asymmetric supercapacitor. ACS Appl. Mater. Interfaces 8, 5251–5260 (2016)
Acknowledgements
This work was supported by National Key Research and Development Program of China (No. 2017YFA0204600) and the Fundamental Research Funds for the Central Universities (No. 22120200228).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, M., Fu, N., Wang, X. et al. A high energy density flexible symmetric supercapacitor based on Al-doped MnO2 nanosheets @ carbon cloth electrode materials. J Mater Sci: Mater Electron 31, 16027–16036 (2020). https://doi.org/10.1007/s10854-020-04165-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04165-1