Abstract
In the present work, the physical, UV–Vis–NIR spectroscopy, and nuclear shielding properties of novel borate glasses with nominal compositions 50B2O3–25BaO–25PbO–xSrO2: x = 0–30 mol% (BBPS0–BBPS30) have been investigated. The non-crystalline nature of the fabricated glasses was verified utilizing X-ray diffraction (XRD) measurements. Direct and indirect (\(E_{{{\text{gap}}}}^{{{\text{Direct}}}}\), \(E_{{{\text{gap}}}}^{{{\text{Indirect}}}}\)) optical energy gaps, average of refractive index (n), Urbach’s energy for (BBPS0–BBPS30) glasses were determined. MCNPX simulation code and WinXCOM program were employed to evaluate the mass attenuation coefficient, half value layer, tenth value layer, mean free path, and effective atomic number (μm, HVL, TVL, MFP, and Zeff) of gamma for the proposed glasses. Neutron shielding survey was examined by determining neutron removal cross section (NRCS, ΣR) of glasses. Results reveal that \(E_{{{\text{gap}}}}^{{{\text{Direct}}}}\) varied from 3.15 to 2.38 eV, while \(E_{{{\text{gap}}}}^{{{\text{Indirect}}}}\) from 3.07 to 2.28 and n from 2.268 to 2.606 for BBPS0 and BBPS30 glasses, respectively. The HVL, MFP, and TVL values corresponding to the materials were in a descending order BBPS0 > BBPS5 > BBPS10 > BBPS15 > BBPS20 > BBPS30. Therefore, the BBPS30 has superior shielding features against gamma-rays than the other material in the studied sample of glasses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In last decades, heavy metal oxide (HMO) based glasses have gained significant attention due to their unique interesting electrical, optical, and magnetic properties. Additionally, these glasses have large exciton binding energy (BE) and wide bandgap. These properties make HMO based glasses potential candidates in several applications such as UV-emitting lasers, optoelectronic devices, gas sensors, and solar energy converters and gas sensors [1]. Boric oxide (B2O3) is an attractive oxide among the HMOs since it is one of the most common glass formers. Borate glasses exhibit more desirable physical and chemical properties, including a low melting point, strong clarity, chemical resistance, and thermal stability [1, 2]. Additionally, owing to their motivating nonlinear and linear optical properties, borate glasses of various compositions play a significant role in optical devices [3,4,5,6,7,8,9,10,11,12]. Through incorporating lead oxide into borate glass structures, such properties such as optical nonlinearity may be enhanced due to the strong polarizability of Pb2 + ions in glass networks [13]. Environments of Pb cations in oxide glasses have been also probed by X-ray studies [14]. On the other side, the interaction of PbO and B2O3 in the glass matrix has a dual impact on the glass network, acting as a glass former in the presence of high levels of boron oxide or lead oxide and as a network modifier at low concentrations. Thus, lead borate glasses exhibit a wide glass forming range, which is advantageous for the fabrication of structurally and optically diverse structures [15,16,17]. On the other hand, Lead borate glasses are known to be excellent candidate materials for optoelectronic, photoelectric, and optical switches for the reasons mentioned previously. The additive of alkaline earth oxides namely BaO or/and SrO into borate glass structure allows to increase the glass refractive index and thermal stability. Previously, Manikandan et al. [18] investigated effect of BaO on the optical and thermal properties of some tellurite glasses. The authors confirmed that optical properties of glasses were enhanced with increasing BaO content in their compositions. In addition, they reported that thermal stability of glasses improved with increasing BaO concentration making these glasses with BaO attractive candidates for amplifiers in the mid-IR and fiber lasers [9]. In other study, Elkhoshkhany et al. [19] evaluated structural properties, UV–Vis–NIR spectroscopy and thermal characteristics of oxyhalide–tellurite glasses with SrCl2. They reported that glass systems with Sr have high thermal stability and high index of refraction that makes them suitable as attractive candidates for optical fiber production. Recent research has shown that glasses are suitable for use as radiation filters owing to their unique properties, including their transparency to visible light and their capacity to absorb X-rays and neutrons [20]. By incorporating oxides into the glass matrix, the properties of the glasses against nuclear radiation shielding may be improved [21, 22]. Numerous scholars asserted that borate glasses continue to be the best option for radiation protection [10, 23,24,25,26,27,28,29]. The mass attenuation coefficient is the most useful gamma-ray attenuation parameter for evaluating the radiation shielding capability of materials. Numerous techniques, including Monte Carlo simulations and theoretical estimates using the XCOM and Phy-X/PSD systems, can be used to approximate the mass attenuation coefficients (MAC) [10, 26,27,28,29,30,31].
The main objective of this study is to determine potential synergistic effects of increasing SrO2 additive on B2O3–BaO–PbO–SrO2 glass system. To observe the direct effects of utilized chemical substitutions (i.e., B2O3/SrO2), MAC of glass samples were determined in this analysis using an experimental transmission setup. The obtained findings were compared to those obtained by Monte Carlo simulations (MCNPX v.2.6.0) and theoretical calculations (WinXcom program). The obtained MAC values were used to quantify other critical shielding parameters, including linear attenuation coefficients (LAC), half value layer (HVL), mean free path (MFP), tenth value layer (TVL), and effective atomic numbers (Zeff), as well as fast neutron removal cross sections values for novel lead–borate glasses. Along with their protecting properties against nuclear radiation, the physical and optical properties of the prepared glasses were studied.
2 Experimental and methods
2.1 Materials and measurements
By utilization of traditional and well-known method namely melt-quenching technique, commercial powders of high reagent grade of B2O3 of purity 99.96% Sigma-Aldrich, BaCO3 of purity 99.99% Sigma-Aldrich as a source of BaO, PbO of purity 99.9% Sigma-Aldrich, and SrO2 of purity 99.99% Sigma-Aldrich were utilized to synthesis samples of lead borate glasses series with the compositions:
-
50B2O3–25BaO–25PbO–0SrO2 coded as BBPS0,
-
45B2O3–25BaO–25PbO–5SrO2 coded as BBPS5,
-
40B2O3–25BaO–25PbO–10SrO2 coded as BBPS10,
-
35B2O3–25BaO–25PbO–15SrO2 coded as BBPS15,
-
30B2O3–25BaO–25PbO–20SrO2 coded BBPS20, and
-
20B2O3–25BaO–25PbO–30SrO2 coded as BBPS30.
For each glass sample, the required weights of chemical powers were determined by an electric digital balance with accuracy of ± 0.0001. Next, they mixed well and melted in a porcelain-crucible at 950 °C for 25 min. Next, the melt-liquid was poured into preheated molds. The produced glass samples were annealed for 5 h at 300 °C to erase thermal strains and then left to cool down gradually. The produced glasses were polished well to make the two opposite faces parallel and flat to be ready for the optical measurements. The details of manufactured glasses with their sample codes and compositions are presented in Table 1. On the other hand, manufactured samples with their general appearance are shown in Fig. 1. Shimadzu 7000 diffractometer device with Cu a radiation source (λ = 1.54060 Å) in the 2θ range of 10°–100° was used to perform the XRD of the glass samples to examine the state of the prepared glasses. Densities of the prepared samples measured by Archimedes’ technique using toluene (density, ρ = 0.867 g/cm3) as liquid for immersion with accuracy of ± 0.01 g/cm3 through the following Eq. (1):
Here,
W1 = glass’s weight in air.
W2 = glass’s weight in liquid.
ρtoluene = toluene liquid’s density.
Molar volume (Vm) of each glass was calculated using Eq. (2):
The optical absorption measurements for the (BBPS0–BBPS30) were carried out and drawn in wavelength from 190 to 1100 nm range by utilizing UV–Vis–NIR spectrophotometer of JASCO model V-570.
2.2 Methods
2.2.1 Monte Carlo simulations
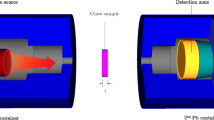
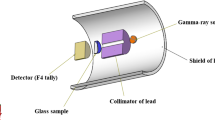
The value of mathematical simulation methods in studies of nuclear radiation shielding remains a hot topic in the literature. Numerous scenarios are possible during numerical simulation, based on the characteristics and features of the running code. The section on Monte Carlo simulations will explore the technological aspects of Monte Carlo simulations utilizing the MCNPX [32] code. Each glass sample was modeled in this study using elemental mass fractions and densities (Table 1). Material definitions for fabricated composites encoded as BBPS0, BBPS5, BBPS10, BBPS15, BBPS20, and BBPS30 were entered into the MCNPX code's input register. The MCNPX’s substance description (Mn) entails many critical definition measures, including elemental mass fractions, atomic numbers, and mass numbers of components. Figure 2a–b illustrates the overall presence of the modeled simulation setup with the simulation package used to calculate mass attenuation and transmission variables. Finally, one of the detection tallies encoded in MCNPX code (F4 tally mesh) was implemented within the virtual detection area to record the attenuated rays from the glass samples. It should be remembered that the MCNPX simulations were run for each glass sample at various photon energies. As a physics library, the D00205ALLCP03 MCNPXDATA kit was introduced, which consisted of DLC-200/MCNPDATA.
2.2.2 µ/ρ, HVL, and MFP, and Z eff
The total mass attenuation coefficient (μm = μ/ρ, usually in cm2/gram) is a fundamental concept used to describe the potential of a substance (in this case, Goethite glasses) to attenuate gamma radiation. Numerous methods, like XCOM, MCNP, Xmudat, and Geant4, may be used to obtain the _m factor. Fortunately, both of these techniques are based on the mixture law, as described in [33]:
wi denotes the weight fraction of the ith material contained in the glass sample. The μm values for Goethite glasses were obtained in our work using the XCOM software. Other shielding terms such as transmitting factors (e.g., MFP and HVL) and Zeff were then calculated using the following equations [34, 35]:
3 Results and discussion
3.1 XRD and physical parameters
XRD spectra for fabricated glasses BBPS0–BBPS30 don’t illustrate any crystalline peaks as presented in Fig. 3, which confirm that glasses are in the amorphous nature.
Equation 1 and Eq. 2 were used to evaluate ρglass and the corresponding Vm. The obtained data are collected in Table 1 and depicted in Fig. 3. From Table 1 and Fig. 4, one can observe that ρ value raises from 4.685 to 5.315 g/cm3 with increasing SrO2 content in the proposed glasses. This increasing in ρglass may be due to the partial replacement between the light B2O3 with molar mass (69.63 g/mol) and heavy SrO2 with molar mass of (119.619 g/mol). The Vm decrease with increasing SrO2 content in the proposed glasses as in Table 1 and Fig. 3. This decreasing may be attributed to (i) the amount of B2O3 with shorter atomic radii (1.589 Å) was larger than that of SrO2 with longer atomic radii (4.303 Å) and (ii) the number of NBO decreases with decrease B2O3 making glasses in less inter-atomic spacing and disordered formation.
3.2 UV–Vis spectroscopy and optical properties
The UV–vis spectra for BBPS0–BBPS30 glasses are illustrated in Fig. 5. It is clear that the spectra haven’t color center and the absorption spectra are slightly shifter to longer wavelength with increasing SrO2 content in the glasses. With help of UV–vis spectra in Fig. 4, the cut-off wavelength (λcut-off, nm) which necessary to calculate the optical absorption coefficient α(λ) was evaluated for each spectrum and written in Table 2. It seen that the value of λcut-off increases with enhancement of SrO2 content in the glasses, λcut-off = 387, 404, 424, 445, 471, and 503 nm for BBPS0, BBPS5, BBPS10, BBPS15, BBPS20, and BBPS30 glasses, respectively.
In the present work, the optical absorption coefficients α(ν) for each glass sample was estimated using the next relation via the absorbance (A) and sample thickness (d) [36]:
Tauc’s model [37] depends on the optical absorption coefficient α(hν) was used to determine the allowed gaps for optical energy bands of the proposed glasses. This model modified by Mott and Davis as [38]:
where the transition probability depends on the constant C. The power m points to the type of electronic transition (m = 2 and 0.5 for indirect and direct allowed transitions) [38]. The variation of (αhν)2 and (αhν)1/2 with photon energy (hν) for BBPS0–BBPS30 glasses are shown in Fig. 6a and b, respectively. The optical energy gaps in direct transition (\(E_{{{\text{gap}}}}^{{{\text{Direct}}}}\)) and for indirect transition (\(E_{{{\text{gap}}}}^{{{\text{Indirect}}}}\)) were determined with help of the linear region of the graphs at the points (αhν)1/2 or (αhν)2 = 0. All obtained values of \(E_{{{\text{gap}}}}^{{{\text{Direct}}}}\) and \(E_{{{\text{gap}}}}^{{{\text{Indirect}}}}\) are collected in Table 2. Results reveal that the optical gap in both direct and indirect allowed shifts decrease with raising SrO2 content in the prepared samples as shown in the inset figures of Fig. 6a and b, respectively. The values in case of direct allowed transition were changes between 3.15 eV for BBPS0 glass and 2.38 eV for BBPS30 sample, while 3.07 eV and 2.28 eV for BBPS30, respectively in case of direct allowable transition. Utilizing the values of \(E_{{{\text{gap}}}}^{{{\text{Direct}}}}\) and \(E_{{{\text{gap}}}}^{{{\text{Indirect}}}}\), the average linear refractive on index (n) for all proposed glass samples was determined using the next relation [39, 40]:
The obtained n values are listed Table 2. The n changes from 2.368 for BBPS0 sample to 2.606 for BBPS30. Generally, n for the proposed glasses increase with enhancing SrO2. Additionally, n is high, thus, the investigated glasses can be used as latent candidates in optical applications.
Urbach’s energy (EU) for all proposed glasses was calculated via the following relation [41]:
Here, \(\bar{\alpha }\left( \nu \right)\) is a constant. By taking logarithm for both sides of Eq. (10) and drawing ln(α) versus (hν), the values of EU can be obtained. The variation of ln(α) with hν is shown in Fig. 7, from the linear part of the curves, EU values can be evaluated. EU values recorded in Table 2 and plotted against SrO2 content in the inset figure of Fig. 7. The increasing of EU with increasing the amount of SrO2 in the prepared glasses due to increase of localized state concentration in the bandgap.
3.3 Nuclear shielding competence
The mass attenuation coefficients-MAC values (μm) of the prepared glasses at the energy ranges are between 0.02 and 20 MeV were derived from MCNPX simulation code and WinXCOM. Table 3 shows these obtained MAC data. From Table 3, it can be seen that MCNPX and WinXCOM, data are in well agreement to each other. Therefore, obtained outcomes verifies that MCNPX simulation is accurate to compute the μm values [42, 43]. Figure 8 compares the results of MAC. It can be noted that MAC values of the studied glass materials (encoded BBPS0, BBPS5, BBPS10, BBPS15, BBPS20, BBPS30) are reflective to the variation in the energy levels. If photon energies approach 20 MeV, the MAC values begin to decrease. Among the glass samples examined, BBPS30 had the highest MAC values. In the other hand, HVL-MFP and TVL are critical parameters to consider when predicting the performance of investigated materials in shielding applications. It should be remembered that these parameters represent the materials' thickness. Figures 9, 10, and 11 illustrate the variations of HVL, TVL, and MFP values as a function of incident hν, respectively. Given the inverse relationship between HVL and μ (HVL = ln2/μ), it is reasonable to assume that the sample with the greatest values of µ can offer the lowest HVL values [44, 45]. This situation has been confirmed by our findings that BBPS30 sample was reported with the minimum HVL values among the investigated glasses. On the other hand, TVL has similar inverse relationship with µ values (TVL = ln10/μ) [46, 47]. As a result, a similar pattern was seen for the BBPS30 sample, with the lowest TVL values among the examined glasses. The term MFP refers to the average distance traveled by an electron throughout the scattering process. When all target particles are at rest except one, the MFP may be calculated using the average distance between the particles as MFP = 1/µ. The smallest MFP can be interpreted as an indication of gamma-ray attenuation supremacy, since it suggests that the incident photon's average moving distance is also the smallest. The results indicate that BBPS30 has the lowest MFP values at the photon energies studied. Overall, the findings list the HVL, MFP, and TVL values associated with each material in ascending order as BBPS0 > BBPS5 > BBPS10 > BBPS15 > BBPS20 > BBPS30. The various photons with B, C, O, Sr, Ba, Pb that derived from high Z dependency have a reducing impact on the HVL, MFP and TVL values. For example, while SrO2 concentration in glass rise, HVLs for hν fall. The effective atomic numbers (Zeff) values for the investigated glass sample with various SrO2 concentration with respect to varying energy levels are reported in Fig. 12. From Fig. 12, possessing higher the Zeff values, BBPS30 serves better in terms of radiation shielding [48,49,50,51]. The fast neutron effective removal cross-section values (ΣR) for BBPS0, BBPS5, BBPS10, BBPS15, BBPS20, BBPS30 samples are given in Fig. 13. It should be noted that ΣR decline with increasing SrO2 concentration in the glass structure. The results show that the BBPS0 glass sample is better in terms of fast neutron shielding [19,20,21,22].
4 Conclusion
The major aims of this work were to investigate the physical, UV–Vis–NIR spectroscopy and nuclear shielding properties of novel borate glasses with nominal compositions 50B2O3–25BaO–25PbO–0SrO2: x = 0–30 mol% (BBPS0–BBPS30). From the obtained results, one can concludes the following points:
-
1-
Densities of the fabricated glasses increased from 4.685 to 5.315 g/cm3, while molar volumes decreased from 29.869 to 29.152 cm3/mol.
-
2-
The non-crystalline nature of the fabricated glasses is confirmed by utilization of X-ray diffraction (XRD) measurements.
-
3-
Direct and indirect (\(E_{{{\text{gap}}}}^{{{\text{Direct}}}}\), \(E_{{{\text{gap}}}}^{{{\text{Indirect}}}}\))optical energy gaps, average of refractive index (n), and Urbach’s energy for (BBPS0–BBPS30) glasses were varied from varied from 3.15 to 2.38 eV, 3.07–2.28 eV, 2.268–2.606 eV, and 0.239–0.296 eV, respectively.
-
4-
BBPS30 has maximum mass attenuation coefficient (μ/ρ) value which varies from 0.0420 to 60.259 cm2/g for hν 0.015 and 15 MeV.
-
5-
The HVL, MFP, and TVL values corresponding to the materials were in a descending order BBPS0 > BBPS5 > BBPS10 > BBPS15 > BBPS20 > BBPS30.
-
6-
The ∑R decrease with increasing the density of glass samples.
Therefore, results confirm that BBPS30 glass has superior shielding features against gamma-rays than the other material in the studied sample of glasses.
References
A. Pan, A. Ghosh, Structural and optical properties of lithium bismuthate glasses. J. Mater. Res. 17, 1941–1944 (2002)
P. Yasaka, N. Pattnaboonmee, H.J. Kim, P. Limkitjaroenporn, J. Kaewkhao, Gamma radiation shielding and optical properties measurements of zinc bismuth borate glasses. Ann. Nucl. Energy 68, 4–9 (2014)
P. Becker, Borate materials in nonlinear optics. Adv. Mater. 10, 979–992 (1998)
A.S. Abouhaswa, Y.S. Rammah, S.E. Ibrahim, A.A. El-Hamalawy, Structural, optical, and electrical characterization of borate glasses doped with SnO2. J. Non-Cryst. Solids 494, 59–65 (2018)
G. Jagannath, B. Eraiah, K. NagaKrishnakanth, S. Venugopal Rao, Linear and nonlinear optical properties of gold nanoparticles doped borate glasses. J. Non-Cryst. Solids 482, 160–169 (2018)
S. Barbi, C. Mugoni, M. Montorsi, M. Affatigato, C. Gatto, C. Siligardi, Structural and optical properties of rare-earths doped barium bismuth borate glasses. J. Non-Cryst. Solids 481, 239–247 (2018)
A.A. Ali, Y.S. Rammah, R. El-Mallawany, D. Souri, FTIR and UV spectra of pentaternary borate glasses. Measurement 105, 72–77 (2017)
Y.S. Rammah, A.A. Ali, R. El-Mallawany, A.M. Abdelghany, Optical properties of bismuth borotellurite glasses doped with NdCl3. J. Mol. Struct. 1175, 504–511 (2019)
Y.S. Rammah, A.S. Abouhaswa, M.I. Sayyed, H.O. Tekin, R. El-Mallawany, Structural, UV and shielding properties of ZBPC glasses. J. Non-Cryst. Solids 509, 99–105 (2019)
Y.S. Rammah, M.I. Sayyed, A.S. Abohaswa, H.O. Tekin, FTIR, electronic polarizability and shielding parameters of B2O3 glasses doped with SnO2. Appl. Phys. A 124, 650 (2018)
Y.S. Rammah, M.I. Sayyed, A.A. Ali, H.O. Tekin, R. El-Mallawany, Optical properties and gamma-shielding features of bismuth borate glasses. Appl. Phys. A 124, 832 (2018)
Y.S. Rammah, A.S. Abouhaswa, A.H. Salama, R. El-Mallawany, Optical, magnetic characterization, and gamma-ray interactions for borate glasses using XCOM program. J. Theor. Appl. Phys. 13, 155–164 (2019)
A. Terczynska-Madej, K. Cholewa-Kowalska, M. Łaczka, J. Opt. Mater. 33, 12 (2011)
U. Hoppe, R. Kranold, A. Ghosh, C. Landron, J. Neuefeind, P. Jovari, Environments of lead cations in oxide glasses probed by X-ray diffraction. J. Non-Cryst. Solids 328, 146 (2003)
V. Martin, B. Wood, U. Werner-Zwanziger, J. Zwanziger, Structural aspects of the photoelastic response in lead borate glasses. J. Non-Cryst. Solids 357, 2120 (2011)
Z. Pan, S. Morgan, B. Long, Raman scattering cross-section and non-linear optical response of lead borate glasses. J. Non-Cryst. Solids 185, 127 (1995)
T. Takaishi, J. Jin, T. Uchino, T. Yoko, Structural study of PbO–B2O3 glasses by X-ray diffraction and 11B MAS NMR techniques. J. Am. Ceram. Soc. 83, 2543 (2000)
N. Manikandan, A. Ryasnyanskiy, J. Toulouse, Thermal and optical properties of TeO2–ZnO–BaO glasses. J. Non-Cryst. Solids 358, 947–951 (2012)
N. Elkhoshkhany, H.M. Mohamed, E. Yousef, UV–Vis-NIR spectroscopy, structural and thermal properties of novel oxyhalide tellurite glasses with composition TeO2–B2O3–SrCl2–LiF–Bi2O3 for optical application. Results in Physics 13, 102222 (2019)
N. Chantima, J. Kaewkhao, P. Limsuwan, Study of photon interactions and shielding properties of silicate glasses containing Bi2O3, BaO and PbO in the energy region of 1 keV to 100 GeV. Ann. Nucl. Energy 41, 119–124 (2012)
S. Kaewjaeng, U. Maghanemi, S. Kothan, H.J. Kim, P. Limkitjaroenporn, J. Kaewkhao, New gadolinium based glasses for gamma-rays shielding materials. Nucl. Eng. Design 280, 21–26 (2014)
S. Kaewjaeng, J. Kaewkhao, P. Limsuwan, U. Maghanemi, Effect of BaO on optical, physical and radiation shielding properties of SiO2–B2O3–Al2O3–Na2O glass system. Proced. Eng. 32, 1080–1086 (2012)
M.S. Al-Buriahi, A.S. Abouhaswa, H.O. Tekin, C. Sriwunkum, F.I. El-Agawany, T. Nutaro, E. Kavaz, Y.S. Rammah, Structure, optical, gamma-ray and neutron shielding properties of NiO doped B2O3–BaCO3–Li2O3 glass systems. Ceram. Int. 46, 1711–1721 (2020)
M.S. Al-Buriahi, Y.S. Rammah, Investigation of the physical properties and gamma-ray shielding capability of borate glasses containing PbO, Al2O3 and Na2O. Appl. Phys. A 125, 717 (2019)
M.S. Al-Buriahi, B.T. Tonguc, Study on gamma-ray buildup factors of bismuth borate glasses. Appl. Phys. A 125, 482 (2019)
Y.S. Rammah, M.S. Al-Buriahi, A.S. Abouhaswa, B2O3–BaCO3–Li2O3 glass system doped with CO3O4: structure, optical, and radiation shielding properties. Phys. B 576, 411717 (2019)
Y.S. Rammah, A. Askin, A.S. Abouhaswa, F.I. El-Agawany, M.I. Sayyed, Synthesis, physical, structural and shielding properties of newly developed B2O3–ZnO–PbO–Fe2O3 glasses using Geant4 code and WinXCOM program. Appl. Phys. A 125, 523 (2019)
M.I. Sayyed, I.A. El-Mesady, A.S. Abouhaswa, A. Askin, Y.S. Rammah, Comprehensive study on the structural, optical, physical and gamma photon shielding features of B2O3–Bi2O3–PbO–TiO2 glasses using WinXCOM and Geant4 code. J. Mol. Struct. 1197, 656–665 (2019)
Y. Al-Hadeethi, M.I. Sayyed, Y.S. Rammah, Investigations of the physical, structural, optical and gamma-rays shielding features of B2O3–Bi2O3–ZnO–CaO glasses. Ceram. Int. 45, 20724–20732 (2019)
E. Şakar, Ö.F. Özpolat, B. Alım, M.I. Sayyed, M. Kurudirek, Phy-X/PSD: development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 166, 108496 (2020)
Ö.F. Özpolat, B. Alım, E. Şakar, M. Büyükyıldız, M. Kurudirek, Phy-X/ZeXTRa: a software for robust calculation of effective atomic numbers for photon, electron, proton, alpha particle, and carbon ion interactions. Radiat. Environ. Biophys. 59, 321–329 (2020). https://doi.org/10.1007/s00411-019-00829-7
MCNPX 240, Monte Carlo N-Particle transport code system for multiparticle and high energy applications (Sep 2004). Available on-line: http://www.nea.fr/abs/html/ccc-0715.html
V. Mosorov, The Lambert–Beer law in time domain form and its application. Appl. Radiat. Isot. 128, 1–5 (2017)
L. Gerward, N. Guilbert, K.B. Jensen, H. Levring, WinXCom—a program for calculating X-ray attenuation coefficients. Radiat. Phys. Chem. 71, 653–654 (2004)
F. Akman, R. Durak, M.F. Turhan, M.R. Kaçal, Studies on effective atomic numbers, electron densities from mass attenuation coefficients near the K edge in some samarium compounds. Appl. Rad. Iso. 101, 107–113 (2015)
D.A. Rayan, Y.H. Elbashar, M.M. Rashad, A. El-Korashy, Spectroscopic analysis of phosphate barium glass doped cupric oxide for bandpass absorption filter. J. Non Cryst. Solids 382, 52–56 (2013)
J. Tauc (ed.), Amorphous and Liquid Semiconductors (Plenum Press, New York, 1974)
N.F. Mott, E.A. Davies, Electronic Processes in Non-Crystalline Materials (Clarendon Press, Oxford, 1979)
V. Dimitrov, S. Sakka, Electronic oxide polarizability and optical basicity of simple oxides. J. Appl. Phys. 79, 1736–1740 (1996)
R. El-Mallawany, M.D. Abdalla, I.A. Ahmed, New tellurite glasses, optical properties. Mater. Chem. Phys 109, 291–296 (2008)
F. Urbach, Phys. Rev. 92, 1324 (1953)
H.O. Tekin, T.T. Erguzel, M. Karahan, U. Kara, O. Kilicoglu, A. Mesbahi, M.I. Sayyed, Investıgatıon of water equivalance and shielding properties of different solid phantoms using MCNPX code. Dig. J. Nanomater. Biostruct. 13, 9 (2018)
H.O. Tekin, M.I. Sayyed, O. Kilicoglu, M. Karahan, T.T. Erguzel, U. Kara, M. Konuk, Calculation of gamma-ray attenuation properties of some antioxidants using Monte Carlo simulation method. Biomed. Phys. Eng. Express. 4, 057001 (2018)
O. Agar, E. Kavaz, E.E. Altunsoy, O. Kilicoglu, H.O. Tekin, M.I. Sayyed, T.T. Erguzel, N. Tarhan, Er2O3 effects on photon and neutron shielding properties of TeO2–Li2O–ZnO–Nb2O5 glass system. Res. Phys. 13, 102277 (2019). https://doi.org/10.1016/j.rinp.2019.102277
M.G. Dong, O. Agar, H.O. Tekin, O. Kilicoglu, K.M. Kaky, M.I. Sayyed, A comparative study on gamma photon shielding features of various germanate glass systems. Compos. B 165, 636–647 (2019)
M.I. Sayyed, H.O. Tekin, O. Kılıcoglu, O. Agar, M.H.M. Zaid, Shielding features of concrete types containing sepiolite mineral: comprehensive study on experimental XCOM and MCNPX results. Res. Phys. 11, 40–45 (2018). https://doi.org/10.1016/j.rinp.2018.08.029
S.A.M. Issa, Y.B. Saddeek, M.I. Sayyed, H.O. Tekin, O. Kilicoglu, Radiation shielding features using MCNPX code and mechanical properties of the PbONa2OB2O3CaOAl2O3SiO2 glass systems. Compos. B 167, 231–240 (2019). https://doi.org/10.1016/j.compositesb.2018.12.029
H.O. Tekin, O. Kilicoglu, E. Kavaz, E.E. Altunsoy, M. Almatari, O. Agar, M.I. Sayyed, The investigation of gamma-ray and neutron shielding parameters of Na2O–CaO–P2O5–SiO2 bioactive glasses using MCNPX code. Res. Phys. 12, 1797–1804 (2019). https://doi.org/10.1016/j.rinp.2019.02.017
S.A. Issa, H.O. Tekin, R. Elsaman, O. Kilicoglu, Y.B. Saddeek, M.I. Sayyed, Radiation shielding and mechanical properties of Al2O3–Na2O–B2O3–Bi2O3 glasses using MCNPX Monte Carlo code. Mater. Chem. Phys. 223, 209–219 (2019). https://doi.org/10.1016/j.matchemphys.2018.10.064
I.S. Mahmoud, S.A. Issa, Y.B. Saddeek, H.O. Tekin, O. Kilicoglu, T. Alharbi, M.I. Sayyed, T.T. Erguzel, R. Elsaman, Gamma, neutron shielding and mechanical parameters for lead vanadate glasses. Ceram. Int. 45, 14058–14072 (2019). https://doi.org/10.1016/j.ceramint.2019.04.105
O. Kilicoglu, Characterization of copper oxide and cobalt oxide substituted bioactive glasses for gamma and neutron shielding applications. Ceram. Int. 45, 23619–23631 (2019). https://doi.org/10.1016/j.ceramint.2019.08.073
Acknowledgements
Taif University Researchers Supporting Project number (Grant No. TURSP-2020/84), Taif University, Taif, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abouhaswa, A.S., Tekin, H.O., Ahmed, E.M. et al. Synthesis, physical, linear optical and nuclear radiation shielding characteristics of B2O3–BaO–PbO–SrO2 glasses. J Mater Sci: Mater Electron 32, 18163–18177 (2021). https://doi.org/10.1007/s10854-021-06359-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06359-7