Abstract
The dielectric and ferroelectric properties of Ba1−(3/2)xNdxBi4Ti4O15 (BNBT) ceramics prepared by using the solid-state reaction route have been studied. X-ray diffraction study revealed that all the samples are in the orthorhombic symmetry with space group A21am. The dielectric study as a function of temperature demonstrated a ferroelectric to paraelectric transition is of diffuse type. The transition temperature (Tc) was found to be increased from 420 °C (x = 0) for BNBT to 500 °C (x = 0.40) at 500 MHz, thus broadening the utilization temperature range of the ceramics. Sample x = 0.40 with the lowest TKε value, indicating the best dielectric temperature stability in all samples. The very high oxygen vacancy of the sample x = 0.40 partially contributes to its higher Tc. However, x = 0.10 sample shows the lowest tan δ at 300 °C, which shows the lowest electrical conductivity in all the samples. The effect of Nd additives on the degree of diffuseness of the dielectric constant curves of BNBT was discussed by using modified Curie–Weiss law. The ferroelectric nature of the samples has been confirmed from the measurement of the hysteresis loop at room temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bismuth layered structured ferroelectrics (BLSF) have been widely investigated for their high dielectric constant, diffuse phase transition, giant electrostriction, and relaxor ferroelectric properties that are useful for application in piezoelectric transducers, sensors, ferroelectric non-volatile memories (Fe-RAM), etc. [1]. Polycrystalline BaBi4Ti4O15 (BBT) lies under the four-layered perovskite structure having Ba2+ (Barium) and Bi3+ (bismuth) ions at site-A and Ti4+ (titanium) ions at the site-B of its each orthorhombic perovskite block. The crystal chemistry associated with the origin of the ferroelectric behavior and dielectric phase transition in MBi4Ti4O15 of BLSFs and they found that the size of M cation and the number of perovskites units in these compounds play a major role in the change in phase transition temperature (Tc) [2, 3]. This exhibits that the dielectric behaves like a relaxor with appreciable piezoelectric and ferroelectric characteristics. However, BBT ceramics are affected by problems linked with high dielectric loss, high conductivity, and increased dielectric dispersion with frequency [4, 5]. BLSFs attracted remarkable attention in the last years for application in non-volatile random access memory and high-temperature piezoelectric devices. The barium bismuth titanate (BBT) ceramics modified with La [6, 7], Nb [8], Ce [9], Sm [10], or with an excess of Bi2O3 [5, 11], have shown frequency-dependent relaxor behaviors. These materials present quite different dielectric constant values under the same measurement conditions. There have been few studies bent on improving the ferroelectric and/or dielectric properties with the addition of rare-earth (RE3+) ions at the Bi3+ site in BBT; however, the results are accompanied by the reduction in transition temperature (Tc) [7, 12,13,14,15]. Since the BLSFs are best known for their high transition temperature, they are suitable for application in high-temperature piezoelectric transducers. A small enhancement in the electrical properties at the expense of reducing the Tc is unacceptable. The compositional fluctuations due to A/B site substitution and oxygen/bismuth vacancies affecting the electrical properties demand further understanding and investigation. The motivation is to enhance the ferroelectric and dielectric properties of BBT without affecting its Tc. The oxygen vacancies can be controlled by substituting RE3+ ions at the appropriate lattice site and optimum concentration. The substitution of trivalent RE3+ for Ba2+ causes a charge imbalance which is compensated by the formation of A-site cationic vacancies [6]. Also the stronger Re–O bond than the Bi–O and Ba–O bonds would restrain the movement of oxygen vacancies.
With these considerations in mind, Nd-doped BBT ceramics are prepared and studied concerning their structure, dielectric, and ferroelectric properties for the first time.
2 Experimental procedure
Ba1−(3/2)xNdxBi4Ti4O15 (BNBT) ceramics with different Nd concentrations (x = 0, 0.10, 0.20, 0.25, 0.30, and 0.40) are prepared using the solid-state reaction process. Stoichiometric quantity of carbonate and oxides with high purity, Nd2O3 (99.9%, Himedia Lab., India), Bi2O3 (99%, CDH, India Ltd.), and BaCO3 & TiO2 (purity ≥ 99%, Merck, India Ltd.), is hand grounded in an agate mortar to procure a uniform mixture by adding acetone and distilled water. Further 3 wt% of Bi2O3 was added to the initial stoichiometric ratio to balance the bismuth loss due to high-temperature processing. The obtained mixtures were calcined for 6 h at 900 °C. The calcined samples were ball milled for 4 h in the planetary ball mill (FRITSCH “Pulverisette 5”). The final milled powders were re-calcined for 12 h at 950 °C. The resultant powders were mixed with PVA and then pelletized at \(60\;{\text{kg/cm}}^{2}\) employing a die of 5 mm radius. All pellets were sintered at 1000 °C for 12 h in a box furnace. The X-ray diffraction (XRD) technique was employed for structural investigation by using Cu-Kα radiation (Bruker D-8 Advanced, Germany). The XRD profile of each ceramics was recorded at 1°/min of scanning rate from 20° to 70° with a step size of 0.02°. The surface morphology and microstructural study of the sintered pellets were analyzed via a scanning electron microscope (SEM, JSM-6390, Japan). The dielectric behaviors as a function of the temperature of the pellets were measured by an LCR meter (IM3536, Hioki, Japan) from 30 to 500 °C. The Ferroelectric behavior (P–E loops) of BNBT samples was investigated by using a ferroelectric loop tracer at room temperature (Precision premier-II, Radiant, USA).
3 Results and discussion
3.1 XRD and microstructural analysis
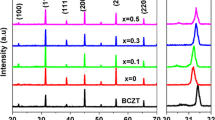
X-ray diffraction spectra of BNBT powders show an orthorhombic structure with the space group A21am as presented in Fig. 1 [4, 16]. The diffraction peak indexed as (1 1 9) plane shows the maximum intensity, indicating that all the compositions have pure bismuth layer perovskite with n = 4 (where n is the no. of perovskite layers) and well in accord with the available literature [17]. All samples show a single-phase character with no impurity peaks associated with the reactant oxides as observed in Fig. 1. The lattice parameters derived by using a standard CCP-14 program chekcell are listed in Table 1. There is no appreciable change in the lattice parameters observed for the samples with different Nd3+ concentrations. Although it is expected that the lattice constant could decrease due to the substitution of low radii Nd3+ (1.27 Å) in place of larger radii Ba2+ (1.61 Å) ion, the Bi2O2 interlayer would act as a structural restriction that may not allow the unit cell volume/lattice constants to vary significantly [12]. Figure 2 shows the surface morphology of the pellets for all the compositions under study characterized by SEM. The pure BBT composition consists of mixed plate-like and spike-like grains that are irregularly shaped. However, for x = 0.10 composition, the grain morphology shows circular disk-shaped grains of size ~ 2 µm which is unique among all the samples. The disk-shaped plate-like grains are a characteristic component of bismuth layered compounds [18] that deformed with the increase in doping concentration. A very dense morphology is seen up to x = 0.25 after which porosity increases due to the lower diffusivity of Nd3+ ions that restrain the grain growth [19, 20]. The grain size (average) of the perovskite decreases with increasing the doping concentration.
3.2 Dielectric study
Figure 3 displays the temperature-dependent dielectric constant at different frequencies for the compositions with x = 0–0.40. The dielectric constant (\(\varepsilon^{\prime}\)) exhibits a broad inconsistency around 417 °C for the parent composition which is in agreement with the earlier studies [21, 22]. Small frequency dependence of dielectric dispersion around the temperature of dielectric maximum (Tm) is noticed for the BNBT (x = 0 and 0.10), along with the reduction in the peak dielectric constant \(\left( {\varepsilon_{{\text{m}}} } \right)\) with rise in frequency. This behavior is attributed to the diffused phase transition (ferroelectric–paraelectric) due to the change in structural symmetry at this temperature. However, Tm is frequency independent for higher Nd3+ concentration (x > 0.10). The diffuseness or broadness of the phase transition is found higher for the Nd3+ substituted samples as compared to pure BBT. The structural inhomogeneity or random disorderliness induced in the lattice due to smaller Nd3+ ion substitution on the Ba2+ site is the possible reason for the increase in diffuseness of the dielectric peaks. In comparison to reports suggesting typical relaxor behavior of BBT ceramics where the dielectric spectra merge completely (10 kHz to 1 MHz) for temperatures (T > Tm) [7, 23], the dielectric peaks for x ≤ 0.20 samples almost merged at frequencies (> 100 kHz), indicating a signature to relaxor characteristics. The dielectric permittivity for a relaxor type ferroelectric obeys the modified Curie–Weiss law that defines the diffuseness of phase transition at elevated temperatures which is given by
where γ and C′ (= \(2\varepsilon_{{\text{m}}} \delta^{2}\)) are constants [24]. The phase transition nature determines by γ; for a normal ferroelectric, the equation reduces to the Curie–Weiss law for γ = 1, while γ = 2 represents a relaxor material. The values of γ (diffusivity) and δ (diffuseness) are obtained by slope and intercept of the linear fit of \(\ln \left( {1/\varepsilon - 1/\varepsilon_{{\text{m}}} } \right)\) vs. \(\ln \left( {T - T_{{\text{m}}} } \right)\) for 500 kHz given in Fig. 4. The value of γ = 1.71 and δ = 15.63 for composition x = 0 enhanced to γ = 1.98 and δ = 16.95 for x = 0.25 composition, suggesting an increase in diffuseness or broadness of the dielectric peaks with the addition of neodymium [25]. Further, for x = 0.40 composition the value of γ and δ decreases to 1.40 and 13.65, respectively, due to the lower diffusivity of Nd3+ ions or an increase in porosity. It can be assumed that the increase in amount of Nd3+ concentration (x > 0.25) leads to compositional fluctuation [26] that helps stabilize perovskite phase in BBT [27].
Figure 5a shows the dielectric constant (temperature-dependent) and Fig. 5b dielectric loss for all the compositions at 500 kHz, respectively. A considerable increase in Tc is observed with an increase in Nd3+ ion concentration. Thus, extending the utilization temperature range of the ceramics. The structural inhomogeneity (distortion) arising due to the substitution of Nd3+ (1.27 Å) in place of higher ionic radii Ba2+ (1.61 Å) site results in a rise in Tm. However, the dielectric constant (\(\varepsilon^{\prime}\)) decreases with the rise in doping concentration, which may be attributed to the cationic vacancies created in the lattice that weakens the coupling of octahedral in the perovskites units. The substitution of trivalent Nd3+ for Ba2+ causes a charge imbalance which is compensated by the formation of A-site cationic vacancies. The oxygen vacancy will become the released centers of the internal stresses induced by ferroelectric phase transition with cooling temperature and then weakens the effect of internal stress on Tc and hence weakens the coupling of octahedral in the perovskites units [28]. Again, it is clear from Fig. 5b that the dielectric loss for Nd3+ composition is less than the undoped BBT, especially at high temperatures. The dielectric loss (\(\varepsilon^{\prime\prime}\)) in Aurivillius phases is affected by oxide ion conductivity or oxygen vacancies at elevated temperatures [29]. Here, the substitution of Nd3+ ion replacing Ba2+ compensates the oxygen vacancies in the lattice as per the defect chemistry reaction given in Eq. (1), i.e.,
where \({\text{O}}_{{\text{o}}}^{x}\) corresponds to an oxygen ion with a neutral charge at the oxygen site. Hence, the dielectric loss reduces with the stabilization of oxygen vacancies due to Nd3+ doping.
In order to further explore the dielectric temperature dependence of Nd-doped BBT ceramics, the temperature coefficient of dielectric constant (TKε) as a function of temperature is shown in Fig. 5c. All the ceramics show good temperature stability up to 300 °C. Especially sample x = 0.40 has the lowest TKε value, indicating its best dielectric temperature stability among all the samples [28].
Figure 5d shows the dielectric loss (tan δ) of ceramics as a function of temperature (at 500 Hz). It is obvious that the tan δ of all the ceramics is less than 1 % below 300 °C. But the tan δ of the sample x = 0.40 begins to increase sharply at ~ 300 °C. It is known that the oxygen vacancy generated by the volatilization of Bi2O2 in BLSF will begin to move at a higher temperature which leads to the electric conduction of ceramics with sharp increasing tan δ. Therefore, x = 0.40 sample may involve much oxygen vacancy. So the much oxygen vacancy of x = 0.40 sample partly contributes to its higher Tc. However, x = 0.10 sample shows the lowest tan δ at 300 °C, indicating its lowest electric conductivity among all the samples [28, 30].
3.3 Ferroelectric study
Figure 6 illustrates the hysteresis (P–E) loops of the BNBT ceramics sample obtained by applying an electric field of about 60 kV/cm (maximum). The coercive field (Ec) and remanent polarization (Pr) values for different compositions are mentioned in Table 1. There is a decrease in Pr value from 0.71 to 0.11 µC/cm2 (up to x = 0.20) and further increases to 0.58 µC/cm2 (up to x = 0.40) composition. Similar behavior in Ec is also observed where it first reduces for composition with x = 0–0.20 and then increases beyond x = 0.20. There are several effects on remanent polarization. One of the possibilities here is the grain size of Nd-doped BBT samples that decreases with the increase in the doping level. The small grain size prevents the creation of large domains (ferroelectric) that decreases the efficient contribution to total polarization [31]. On the other hand, reports suggest that the perovskites with diffused dielectric phase transition character show less remanent polarization because of the coexistence of ferroelectric and paraelectric phases from room temperature to Tc [32]. As it is mentioned above that the diffuseness of dielectric peaks increases with Nd3+ substitution, this character may decrease the Pr values. Secondly, the quantity Ec reduces with the rise in neodymium concentration in doped BBT ceramics. The result may be ascribed to the reduction in the concentration of oxygen vacancy (\(V_{{{\ddot{\text{O}}}}}\)) as per the defect chemistry reaction given in Eq. 2. By screening/neutralizing the polarization charge, \(V_{{{\ddot{\text{O}}}}}\) may influence the kinetics of the domain wall, or in other words, domain wall pinning (head-to-head polarization) might also stabilize the domain arrangement or configuration. Here the domain wall pinning reduces due to the stabilization of \(V_{{{\ddot{\text{O}}}}}\) with the substitution of Nd3+ ion in place of Ba2+ ion. This indicates that the domain wall moves freely (disappear), which leads to the lowering of the Ec. Again the Pr and Ec values increase simultaneously for x ≥ 0.20 BNBT series. The trivalent Nd3+ (1.27 Å) ions are prone to be substituted at the Bi3+ site (1.17 Å) since the difference in their ionic radii is smaller than Ba2+ (1.61 Å) ion. Moreover, the structure determination studies of BBT [4, 33] claimed the Ba ions randomly occupying Bi sites and/or entering the Bi2O2 layer. So there is a high chance of Nd ions being partially substituted at the Bi site with a higher doping level. This weakens defect mobility that contributes to domain pinning, thereby increasing polarization effects [34]. The bismuth vacancies formed due to partial substitution of Nd3+ ions may contribute to the accumulation of \(V_{{{\ddot{\text{O}}}}}\) at domain walls, leading to an increase in the coercive field.
4 Conclusion
In summary, the ferroelectric Ba1−(3/2)xNdxBi4Ti4O15 ceramic samples have been prepared successfully via the solid-state reaction process. X-ray diffraction analysis confirmed the orthorhombic structure of the samples with the A21am space group at room temperature. Ferroelectric to paraelectric transition has been observed from the dielectric properties as a function of temperature. The Nd3+ substitution on the Ba2+ site resulted in pronounced structural distortion and lattice mismatch, which leads to higher Curie temperature (Tc). It can be concluded that Nd3+ substitution enhances the utilization temperature range of the BBT ceramics. The TKε value of the sample enables us to find the best dielectric temperature stability in all samples, whereas the value of tan δ allows us to know the electrical conductivity in all the samples. The room-temperature hysteresis profile (P–E loop) validates the ferroelectric nature of the ceramics. A simultaneous change in Pr and Ec values were observed with the doping concentration. Although the low coercive field and high remanent polarization of material are best suitable for ferroelectric applications, there is no significant improvement in the Pr values as compared to pure BBT. However, a relatively low Ec is observed for x = 0.20 composition which can be processed further with better sintering conditions to control the grain growth for enhancing the Pr values.
Data availability
Yes.
References
B.H. Park, B.S. Kang, S.D. Bu, T.W. Noh, J. Lee, W. Jo, Nature 401, 682 (1999). https://doi.org/10.1038/44352
E.C. Subbarao, Integr. Ferroelectr. 12, 33 (1996). https://doi.org/10.1080/10584589608225746
R.E. Newnham, R.W. Wolfe, J.F. Dorrian, Mater. Res. Bull. 6, 1029 (1971). https://doi.org/10.1016/0025-5408(71)90082-1
A. Khokhar, M.L.V. Mahesh, A.R. James, P.K. Goyal, K. Sreenivas, J. Alloys Compd. 581, 150 (2013). https://doi.org/10.1016/j.jallcom.2013.07.040
A. Khokhar, P.K. Goyal, O.P. Thakur, K. Sreenivas, Ceram. Int. 41, 4189 (2015). https://doi.org/10.1016/j.ceramint.2014.12.103
A. Khokhar, P.K. Goyal, O.P. Thakur, A.K. Shukla, K. Sreenivas, Mater. Chem. Phys. 152, 13 (2015). https://doi.org/10.1016/j.matchemphys.2014.11.074
A. Chakrabarti, J. Bera, J. Alloys Compd. 505, 668 (2010). https://doi.org/10.1016/j.jallcom.2010.06.105
J.D. Bobić, M.M. Vijatović Petrović, J. Banys, B.D. Stojanović, Mater. Res. Bull. 47, 1874 (2012). https://doi.org/10.1016/j.materresbull.2012.04.069
C.L. Diao, J.B. Xu, H.W. Zheng, L. Fang, Y.Z. Gu, W.F. Zhang, Ceram. Int. 39, 6991 (2013). https://doi.org/10.1016/j.ceramint.2013.02.036
P. Fang, P. Liu, Z. Xi, Phys. B Condens. Matter 468–469, 34 (2015). https://doi.org/10.1016/j.physb.2015.03.033
P.M.V. Almeida, C.B. Gozzo, E.H.N.S. Thaines et al., Mater. Chem. Phys. 205, 72 (2018). https://doi.org/10.1016/j.matchemphys.2017.10.069
S. Kumar, K.B.R. Varma, J. Phys. D Appl. Phys. (2009). https://doi.org/10.1088/0022-3727/42/7/075405
D. Peng, H. Zou, C. Xu, X. Wang, X. Yao, J. Alloys Compd. 552, 463 (2013). https://doi.org/10.1016/j.jallcom.2012.10.194
P. Fang, H. Fan, J. Li, L. Chen, F. Liang, J. Alloys Compd. 497, 416 (2010). https://doi.org/10.1016/j.jallcom.2010.03.092
C.W. Ahn, H.J. Lee, S.H. Kang et al., J. Electroceram. 21, 847 (2007). https://doi.org/10.1007/s10832-007-9308-y
B.J. Kennedy, Y. Kubota, B.A. Hunter, A. Ismunandar, K. Kato, Solid State Commun. 126, 653 (2003). https://doi.org/10.1016/s0038-1098(03)00332-6
T. Badapanda, R. Harichandan, T. Bheesma Kumar et al., J. Mater. Sci. Mater. Electron. 27, 7211 (2016). https://doi.org/10.1007/s10854-016-4686-z
J.A. Horn, S.C. Zhang, U. Selvaraj, G.L. Messing, S. Trolier-McKinstry, J. Am. Ceram. Soc. 82, 921 (1999). https://doi.org/10.1111/j.1151-2916.1999.tb01854.x
X. Chou, J. Zhai, H. Jiang, X. Yao, J. Appl. Phys. (2007). https://doi.org/10.1063/1.2799081
M. Reddyprakash, S.K. Rout, A. Satapathy, T.P. Sinha, S.M. Sariful, Ceram. Int. 42, 8798 (2016). https://doi.org/10.1016/j.ceramint.2016.02.122
S.K. Rout, E. Sinha, A. Hussian et al., J. Appl. Phys. (2009). https://doi.org/10.1063/1.3068344
P. Fang, H. Fan, Z. Xi, W. Chen, Solid State Commun. 152, 979 (2012). https://doi.org/10.1016/j.ssc.2012.03.007
J.D. Bobić, M.M. Vijatović Petrović, J. Banys, B.D. Stojanović, Ceram. Int. 39, 8049 (2013). https://doi.org/10.1016/j.ceramint.2013.03.075
K. Uchino, S. Nomura, Ferroelectrics 44, 55 (1982). https://doi.org/10.1080/00150198208260644
C.L. Diao, H.W. Zheng, Y.Z. Gu, W.F. Zhang, L. Fang, Ceram. Int. 40, 5765 (2014). https://doi.org/10.1016/j.ceramint.2013.11.015
Y. Chen, H. Zhou, S. Wang, Q. Chen, Q. Wang, J. Zhu, J. Alloys Compd. (2021). https://doi.org/10.1016/j.jallcom.2020.155500
W. Chaisan, R. Yimnirun, S. Ananta, D.P. Cann, Mater. Sci. Eng. B 132, 300 (2006). https://doi.org/10.1016/j.mseb.2006.04.033
Y. Chen, D. Liang, Q. Wang, J. Zhu, J. Appl. Phys. (2014). https://doi.org/10.1063/1.4893366
K.R. Kendall, C. Navas, J.K. Thomas, H.C. zur Loye, Chem. Mater. 8, 642 (1996). https://doi.org/10.1021/cm9503083
W.L. Liu, H.R. Xia, H. Han, X.Q. Wang, J. Crystal Growth 269, 499 (2004). https://doi.org/10.1016/j.jcrysgro.2004.05.089
J. Zhai, X. Yao, J. Shen, L. Zhang, H. Chen, J. Phys. D Appl. Phys. 37, 748 (2004). https://doi.org/10.1088/0022-3727/37/5/016
W. Cai, C. Fu, J. Gao, X. Deng, G. Chen, Z. Lin, Integr. Ferroelectr. 140, 92 (2012). https://doi.org/10.1080/10584587.2012.741466
S. Kojima, R. Imaizumi, S. Hamazaki, M. Takashige, J. Mol. Struct. 348, 37 (1995). https://doi.org/10.1016/0022-2860(95)08583-H
M. Ganguly, S.K. Rout, T.P. Sinha et al., J. Alloys Compd. 579, 473 (2013). https://doi.org/10.1016/j.jallcom.2013.06.104
Acknowledgement
The authors thank the University Department of Physics, Dr. Shyama Prasad Mukherjee University, Ranchi, for providing the necessary infrastructure to carry out our research.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rana, T., Sharma, J.P., Sonu, B.K. et al. Dielectric phase transition and ferroelectric properties of neodymium-doped BaBi4Ti4O15 layered ceramics. J Mater Sci: Mater Electron 32, 11969–11977 (2021). https://doi.org/10.1007/s10854-021-05826-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05826-5