Abstract
x% Yb-, 0.5% Er-co-doped Y2O3 transparent ceramics (x = 0, 0.1, 0.5, 1.0, 5.0 and 10) were prepared by the spark plasma sintering, and then we evaluated the effect of Yb doping on optical and upconversion (UC) photoluminescence (PL) properties. Under an excitation at 380 nm, the sharp emission peaks appeared around 550 and 660 nm, and the quantum yield was approximately 2%. The PL decay curves monitoring around 550 nm were fitted by a single exponential decay function. The decay time constants were 0.03–0.11 ms, and the values were typical for the 4f → 4f transitions of Er3+. Regarding the UC PL properties, the sharp UC emission peaks around 550 and 660 nm were observed, and the UC emission intensity of 5.0% Yb-, 0.5% Er-co-doped Y2O3 transparent ceramic sample was the highest among the prepared samples. With increasing x, the chromaticity of UC emission color was tuned from green to red region. Regarding the UC PL decay curves, the rise time became slower as increasing x. This behavior is one of the evidence of energy transfer from Yb3+ to Er3+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phosphors are materials which exhibit a phenomenon of luminescence, and they have been used for a wide variety of devices such as white light-emitting diodes (LEDs) [1, 2], display devices [3, 4], solid-state lasers [5, 6] and radiation measurements [7, 8]. Among the phosphors, upconversion (UC) phosphors have a unique function to convert absorbed two or more low energy photons into high energy photon unlike conventional down-conversion luminescence. In particular, since UC phosphors can convert near-infrared radiation into visible light, they are expected to be applied in various applications including biomedical imaging [9,10,11], color displays [12, 13] and solar cells [14, 15].
Until now, almost all UC phosphors consist of host and dopant components. As the host components, there are many reports on oxide, fluoride and oxyfluoride materials such as Y2O3, NaYF4 and LaOF [16,17,18,19,20]. In particular, Y2O3 has attracted much attention as host components of UC phosphors because of their good thermal and chemical properties and relatively low phonon energy (~ 380 cm−1) [21, 22]. As the dopant components, trivalent lanthanide ions including Tb3+, Ho3+, Er3+, Tm3+ and Yb3+ were often used. In particular, Er-, Yb-co-doped UC phosphors exhibited the intense UC emission because the Yb3+ (2F5/2 → 2F7/2) emission and Er3+ (4I15/2 → 4I11/2) absorption spectra were overlapped, which showed efficient energy transfer from Yb3+ to Er3+ [23,24,25].
In this study, we focus on Yb-, Er-co-doped Y2O3 for the above reasons. So far, almost all Yb-, Er-co-doped Y2O3 have been reported in the forms of nanoparticles [26, 27], film [28, 29], opaque ceramics [30, 31] and single crystal [32]. Recently, Y2O3 transparent ceramics were developed as new material forms and synthesized by the solid-state reaction [33], vacuum sintering [34], hot-isostatic pressing (HIP) [35] and spark plasma sintering (SPS) method [36]. Compared to single crystal, transparent ceramics have some advantages including lower sintering temperature, high mechanical strength and high uniformity of the dopant. However, when Y2O3 transparent ceramics were synthesized, the sintering aids such as La2O3 and ZrO2 were generally used [37, 38]. Lu et al. and Guo et al. reported that the sintering aids affect UC properties [39, 40]. In contrast, Y2O3 transparent ceramics prepared by SPS can be obtained without any sintering aids [41]. Xiaorui et al. reported that Yb-, Er-co-doped Y2O3 transparent ceramics with various Er concentrations showed high UC emission [42]. However, there are no reports on Yb concentration dependence of UC PL properties in Yb-, Er-co-doped Y2O3 transparent ceramics synthesized by SPS.
The aim of this study is to investigate the effect of Yb doping on optical and UC PL properties of x% Yb-, 0.5% Er-co-doped Y2O3 transparent ceramics prepared by SPS (x = 0, 0.1, 0.5, 1.0, 5.0 and 10), and the optimal Yb concentrations are determined in terms of UC PL properties.
2 Experiment
x% Yb-, 0.5% Er-co-doped Y2O3 transparent ceramics with various concentrations of Yb (x = 0, 0.1, 0.5, 1.0, 5.0 and 10) were synthesized by the SPS machine (LabX-100, Sinter Land). As the raw powders, Y2O3 (> 99.99%, Furuuchi Chemical co., Ltd.), Er2O3 (> 99.99%, Furuuchi Chemical co., Ltd.) and Yb2O3 (> 99.99%, Nippon yttrium co., Ltd.) were used. The mixed powder was placed in a graphite die with two graphite punches and set into SPS machine. The SPS machine was operated as shown in Fig. 1. After the SPS sintering process, the samples were heated at 1200 °C for 8 h in the air atmosphere to remove oxygen vacancies. In the obtained samples, the surfaces were polished using a polishing machine (MetaServ 250, Buehler), and the thickness was fixed at 1.0 mm to evaluate optical and UC properties. For the phase identification, X-ray diffraction (XRD) patterns were obtained using X-ray diffractometer (MiniFlex, Rigaku). The density of samples was evaluated using the Archimedes method with pure water. As the optical properties, the in-line transmittance spectrum was obtained using a spectrometer (V670, JASCO). The photoluminescence (PL) excitation and emission spectra and quantum yield (QY) were measured by using Quantaurus-QY (C11347, Hamamatsu Photonics). The chromaticity of UC emission color was confirmed using a luminance colorimeter (BM-5A, Topcon) and plotted in the 1931 Commission International I’Eclairage (CIE) diagram. Under an excitation at 980 nm, UC PL spectra were obtained using original setup [43]. As the excitation source, we used a continuous-wave laser of 980 nm, and the laser power and power density were 200 mW and 11.3 W/cm2, respectively. To evaluate the decay time constant, PL decay curves were obtained using a Quantaurus-τ (C11367, Hamamatsu Photonics).
3 Results and discussion
3.1 Samples
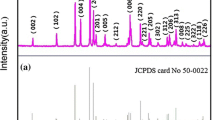
Figure 2 shows a picture of polished Yb-, Er-co-doped Y2O3 transparent ceramic samples. All the samples showed transparency in the visible range, and the mesh patterns on the back of the samples could be seen. The samples look dark, which would be due to the carbon contamination from graphite die during the SPS processing. For the phase identification, the XRD patterns are presented in Fig. 3. All the diffraction peaks were in agreement with standard card patterns of cubic phase Y2O3 (COD: 1009014) without any additional peaks. Thus, all the samples were single phase. The peak positions were constant in all the sample because the ionic radius of Yb3+ (0.86 Å) was similar to that of Y3+ (0.89 Å). Therefore, Yb3+ ions can replace the Y3+ ions without significantly changing of the lattice parameter. The density and relative density of the samples are plotted in Fig. 4. The density of x = 0 sample was 5.01 g/cm3 which was close to the theoretical density of Y2O3 (5.013 g/cm3) [44], and the relative density of all the samples was 99.7–99.9%.With increasing x, the density increased because the atomic mass of Yb was larger than that of Y.
3.2 Optical properties
Figure 5 represents in-line transmittance spectra of Yb-, Er-co-doped Y2O3 transparent ceramic samples. The transmittance of all the samples was ~ 60% in the range of 1000 to 2700 nm. With the wavelength decreased, the transmittance was lowered. It may be attributed to some scattering centers including oxygen vacancies and grain boundary [45]. In fact, the behavior was similar to the previous studies about transmittance of Y2O3 transparent ceramics prepared by HIP and SPS [46,47,48]. In all the samples, some sharp absorption peaks due to the 4f → 4f transitions of Er3+ ions were observed [36, 49]. In addition, Yb-, Er-co-doped Y2O3 samples showed the absorption peaks around 950 nm ascribed to the 4f → 4f transitions of Yb3+ [35, 50, 51]. The intensity of absorption peaks due to Yb3+ increased with increasing x. Figure 6 shows PL excitation and emission spectra of 1.0% Yb-, 0.5% Er-co-doped Y2O3 transparent ceramic sample as a representative. The excitation and emission spectral shapes of all the samples were similar to that of 1.0% Yb-, 0.5% Er-co-doped sample. The multiple sharp excitation peaks appeared from 250 to 500 nm, which were typical for the 4f → 4f transitions of Er3+ ions. Under an excitation at 380 nm, the sharp emission peaks around 550 and 660 nm were observed. The emission peaks around 550 and 660 nm were assigned to the 2H11/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions of Er3+ ions. The PL QYs of all the samples under an excitation at 380 nm are plotted in Fig. 7. The QYs of x = 0 sample was highest among the prepared samples. As the rise of x, the PL QYs decreased. Figure 8 represents PL decay curves of Yb-, Er-co-doped Y2O3 transparent ceramic sample monitoring around 550 nm under an excitation at 380 nm. To achieve an accurate evaluation, we deconvolute the IRF from the decay curves. All the curves were fitted by a single exponential decay function. The obtained decay time constants of all the samples were 0.03–0.11 ms, and the values were typical for the 4f → 4f transitions of Er3+ [52, 53]. When Yb concentrations increased, the decay time constants became faster. This trend was similar to the past report and it was related to the back energy transfer (BET) from Er3+ to Yb3+ ions [54].
3.3 Upconversion (UC) properties
Figure 9 shows UC PL spectra of Yb-, Er-co-doped Y2O3 transparent ceramic sample under an excitation at 980 nm. In all the samples, the sharp UC emission peaks around 550 and 660 nm were observed, and the origin of the peaks around 550 and 660 nm was due to the 2H11/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions of Er3+ ions. The UC emission intensity of the 2H11/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions of Er3+ ions increased with increasing x, and the intensity of 5.0% Yb-, 0.5% Er-co-doped Y2O3 transparent ceramic sample was the highest among the prepared samples. According to the previous reports, the optimal Yb concentration of Yb-, Er-co-doped Y2O3 nanoparticles and ceramics was 8 and 2%, respectively [55, 56]. Therefore, it is suggested that the optimal Yb concentration was different depending on the material forms. In addition, the UC PL intensity ratio (4F9/2 → 4I15/2 / 2H11/2, 4S3/2 → 4I15/2) gradually increased with increasing x unlike PL because there were differences in the luminescence mechanism between PL and UC emission as shown in Fig. 10. The UC luminescence mechanism of Yb-, Er-co-doped Y2O3 can be mainly classified into three different processes: (i) excited-state absorption (ESA), (ii) energy transfer upconversion (ETU) from Er3+ to Er3+ ions and (iii) ETU from Yb3+ to Er3+ ions [57]. In the Er-, Yb-co-doped Y2O3, the dominant processes of the 2H11/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions of Er3+ were ESA and ETU, respectively, because the absorption cross section of Yb3+ ions was much larger than that of Er3+ ions against an excitation of 980 nm [58]. Therefore, it was suggested that the process of (iii) increased as the increasing x, and UC emission ratio was changed.
Figure 11 shows chromaticity coordinates of UC PL for Yb-, Er-co-doped Y2O3 transparent ceramic sample under an excitation at 980 nm. The CIE color coordinates were located around green–yellow region in the x = 0 samples, and the color coordinates were shifted to yellow–red region with increasing x. This trend was confirmed in the past literature [22]. UC PL decay curves of Yb-, Er-co-doped Y2O3 transparent ceramic sample monitoring around 550 nm under an excitation at 980 nm are indicated in Fig. 12. The obtained UC PL decay curves under excitation at and 980 nm were fitted as I(t) = I0 exp(− t/τD) − I1 exp(− t/τR), where I0 and I1 are positive constants, τD and τR are decay and rise time constants, respectively [15, 59]. The obtained decay and rise time constants were 0.51–0.84 and 0.017–0.041 ms, respectively. The decay and rise time constants of UC PL were slower in comparison with PL, and the rise time became slower as increasing x. Since the rise time constant was related to ETU process, it was suggested that the ETU process increased as the increasing x. In contrast, the decay time became faster with increasing x, and this tendency was observed in results of PL.
4 Conclusion
We have evaluated the effect of Yb doping on optical and UC PL properties of Yb-, Er-co-doped Y2O3 transparent ceramics synthesized by SPS. In x = 0 sample, the density was 5.01 g/cm3 which was close to the theoretical density of Y2O3, and the relative density of all the samples was 99.7–99.9%. Regarding the PL and UC PL properties, the sharp emission peaks around 550 and 660 nm were observed under the excitation at 380 and 980 nm. Among the prepared samples, the UC emission intensity of 5.0% Yb-, 0.5% Er-co-doped Y2O3 transparent ceramic sample was the highest, and the chromaticity of UC emission color was shifted from green to red region with increasing x. When the monitoring wavelength was 550 nm, the decay time constants of PL and UC PL emission were 0.03–0.11 and 0.51–0.84 ms, which were the typical values for the 2H11/2, 4S3/2 → 4I15/2 transitions of Er3+. Moreover, the rise time constant of UC PL emission became slower with increasing x. This behavior is one of the evidence of energy transfer from Yb3+ to Er3+.
References
E.F. Schubert, Science 308, 1274 (2005)
K. Bando, K. Sakano, Y. Noguchi, Y. Shimizu, J. Light Vis. Environ. 22, 2 (1998)
D. Chen, W. Xiang, X. Liang, J. Zhong, H. Yu, M. Ding, H. Lu, Z. Ji, J. Eur. Ceram. Soc. 35, 859 (2015)
K. Yoshimura, H. Fukunaga, M. Izumi, M. Masuda, T. Uemura, K. Takahashi, R.-J. Xie, N. Hirosaki, J. Soc. Inf. Disp. 24, 449 (2016)
T. Sanamyan, Laser Phys. Lett. 12, 125804 (2015)
J. Lu, J. Lu, T. Murai, K. Takaichi, T. Uematsu, K. Ueda, H. Yagi, T. Yanagitani, A.A. Kaminskii, Jpn. J. Appl. Phys. 40, L1277 (2001)
C.W.E. van Eijk, Nucl. Instruments Methods Phys. Res. A 460, 1 (2001)
H. Kimura, T. Kato, D. Nakauchi, G. Okada, N. Kawaguchi, T. Yanagida, Nucl. Instruments Methods Phys. Res. A 954, 161226 (2020)
L. Wang, M.S. Draz, W. Wang, G. Liao, Y. Xu, J. Biomed. Nanotechnol. 11, 325 (2015)
G. Kaur, S.B. Rai, Luminescence 28, 143 (2013)
C. Duan, L. Liang, L. Li, R. Zhang, Z.P. Xu, J. Mater. Chem. B 6, 192 (2018)
B. Zhu, B. Qian, Y. Liu, C. Xu, C. Liu, Q. Chen, J. Zhou, X. Liu, J. Qiu, NPG Asia Mater. 9, e394 (2017)
L. Gao, X. Shan, X. Xu, Y. Liu, B. Liu, S. Li, S. Wen, C. Ma, D. Jin, F. Wang, Nanoscale 12, 18595 (2020)
X. Huang, S. Han, W. Huang, X. Liu, Chem. Soc. Rev. 42, 173 (2013)
X. Chen, W. Xu, H. Song, C. Chen, H. Xia, Y. Zhu, D. Zhou, S. Cui, Q. Dai, J. Zhang, A.C.S. Appl, Mater. Interfaces 8, 9071 (2016)
Y. Zhang, L. Zhang, R. Deng, J. Tian, Y. Zong, D. Jin, X. Liu, J. Am. Chem. Soc. 136, 4893 (2014)
Q. Zhao, J. Zhao, M. Tao, C. Wang, X. Zeng, Y. Hu, S. Wang, M. Zeng, W. Zhou, H. Gu, Y. Li, J. Lumin. 214, 116580 (2019)
E. Palo, L. Pihlgren, M. Tuomisto, T. Laihinen, I. Hyppänen, J. Kankare, M. Lastusaari, T. Soukka, H.C. Swart, J. Hölsä, Opt. Mater. 59, 49 (2016)
G.Y. Chen, H.C. Liu, G. Somesfalean, Y.Q. Sheng, H.J. Liang, Z.G. Zhang, Q. Sun, F.P. Wang, Appl. Phys. Lett. 92, 113114 (2008)
S.-I. Yamamoto, K. Ohyama, T. Ban, T. Nonaka, Mater. Res. Express 6, 036202 (2018)
A. Ubaldini, M.M. Carnasciali, J. Alloys Compd. 454, 374 (2008)
L.A. Diaz-Torres, P. Salas, J. Oliva, E. Resendiz-L, C. Rodriguez-Gonzalez, O. Meza, Appl. Phys. A 123, 25 (2017)
J. Zhang, Z. Hao, J. Li, X. Zhang, Y. Luo, G. Pan, Light Sci. Appl. 4, e239 (2015)
T. Nonaka, T. Ban, S.-I. Yamamoto, Trans. Mater. Res. Soc. Jpn. 42, 139 (2017)
B.-C. Hwang, S. Jiang, T. Luo, J. Watson, G. Sorbello, N. Peyghambarian, J. Opt. Soc. Am. B 17, 833 (2000)
R. Tomala, W. Strek, Opt. Mater. 109, 110047 (2020)
Y. Li, Y. Zhang, G. Hong, Y. Yu, J. Rare Earths 26, 450 (2008)
G. Cabello-Guzmán, D. González, C. Caro-Díaz, L. Lillo-Arroyo, F. Valenzuela-Melgarejo, G. Cárdenas Triviño, G.E. Buono-Core, B. Chornik, Y. Huentupil, J. Lumin. 204, 401 (2018)
B. Xu, J. Liu, K. Zou, Appl. Phys. A 125, 100 (2019)
V. Singh, P. Haritha, V. Venkatramu, S.H. Kim, Spectrochim. Acta A 126, 306 (2014)
R. Dey, A. Pandey, V.K. Rai, Sens. Actuators B 190, 512 (2014)
R. Bao, N. An, L. Ye, L.-G. Wang, Opt. Fiber Technol. 52, 101989 (2019)
N. Wang, X. Zhang, H. Jiang, T. Dong, D. Yang, Mater. Chem. Phys. 135, 709 (2012)
G. Zhao, Q. Yang, Q. Liu, S. Lu, J. Alloys Compd. 583, 372 (2014)
J. Wang, J. Ma, J. Zhang, P. Liu, D. Luo, D. Yin, D. Tang, L.B. Kong, Opt. Mater. 71, 117 (2017)
J. Zhang, S. Wang, L. An, M. Liu, L. Chen, J. Lumin. 122–123, 8 (2007)
Q. Yi, S. Zhou, H. Teng, H. Lin, X. Hou, T. Jia, J. Eur. Ceram. Soc. 32, 381 (2012)
K. Ning, J. Wang, J. Ma, Z. Dong, L.B. Kong, D. Tang, Mater. Today Commun. 24, 101185 (2020)
S. Lu, Q. Yang, B. Zhang, H. Zhang, Opt. Mater. 33, 746 (2011)
Y. Guo, X. Wu, Q. Wang, D. Wang, Mater. Sci. Pol. 38, 159 (2020)
H. Furuse, S. Nakasawa, H. Yoshida, K. Morita, T.S. Suzuki, B.-N. Kim, Y. Sakka, K. Hiraga, J. Am. Ceram. Soc. 101, 694 (2018)
X. Hou, S. Zhou, W. Li, Y. Li, H. Lin, H. Teng, T. Jia, J. Am. Ceram. Soc. 93, 2779 (2010)
T. Nonaka, T. Kanamori, K. Ohyama, S.I. Yamamoto, Jpn. J. Appl. Phys. 54, 03CA02 (2015)
A. Camenzind, R. Strobel, S.E. Pratsinis, Chem. Phys. Lett. 415, 193 (2005)
W. Zhang, T. Lu, N. Wei, Y. Wang, B. Ma, F. Li, Z. Lu, J. Qi, J. Alloys Compd. 520, 36 (2012)
W.K. Jung, H.J. Ma, Y. Park, D.K. Kim, Scr. Mater. 137, 1 (2017)
X. Huang, X. Zhang, Z. Hu, Y. Feng, J. Wei, X. Liu, X. Li, H. Chen, L. Wu, H. Pan, J. Li, Opt. Mater. 92, 359 (2019)
Z. Hu, X. Xu, J. Wang, P. Liu, D. Li, X. Wang, J. Zhang, J. Xu, D. Tang, J. Eur. Ceram. Soc. 38, 1981 (2018)
J. Zhou, Y. Chen, R. Lei, H. Wang, S. Xu, J. Lumin. 215, 116671 (2019)
L. Zhang, W. Pan, J. Feng, J. Eur. Ceram. Soc. 35, 2547 (2015)
J. Zhang, L. An, M. Liu, S. Shimai, S. Wang, J. Eur. Ceram. Soc. 29, 305 (2009)
A.N. Meza-Rocha, E.F. Huerta, U. Caldiño, E. Zaleta-Alejandre, S.H. Murrieta, J.M. Hernández, E. Camarillo, Z. Rivera-Álvarez, G.C. Righini, C. Falcony, J. Lumin. 154, 106 (2014)
M. Sun, J. Liu, L. Nie, J. Alloys Compd. 816, 152575 (2020)
V.K. Tikhomirov, V.D. Rodríguez, J. Méndez-Ramos, J. Del-Castillo, D. Kirilenko, G. Van Tendeloo, V.V. Moshchalkov, Sol. Energy Mater. Sol. Cells 100, 209 (2012)
Y. Tian, Y. Fu, M. Xing, X. Luo, J. Nanomater. 2015, 1 (2015)
H. Wang, C. Duan, P.A. Tanner, J. Phys. Chem. C 112, 16651 (2008)
D. Matsuura, Appl. Phys. Lett. 81, 4526 (2002)
J. Liao, L. Nie, S. Liu, B. Liu, H. Wen, J. Mater. Sci. 49, 6081 (2014)
R. Martín-Rodríguez, S. Fischer, A. Ivaturi, B. Froehlich, K.W. Krämer, J.C. Goldschmidt, B.S. Richards, A. Meijerink, Chem. Mater. 25, 1912 (2013)
Acknowledgements
This work was supported by Grant-in-Aid for Scientific Research A (17H01375), Scientific Research B (18H03468) and JSPS Research Fellow (19J22091) from JSPS. The Cooperative Research Project of Research Center for Biomedical Engineering, Iketani Foundation and Nippon Sheet Glass Foundation are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, H., Kato, T., Yamamoto, Si. et al. Influence of Yb doping on optical and upconversion photoluminescence properties of Yb-, Er-co-doped Y2O3 transparent ceramics prepared by SPS. J Mater Sci: Mater Electron 32, 6304–6311 (2021). https://doi.org/10.1007/s10854-021-05346-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05346-2