Abstract
Gd and Si co-doped HfO2 gate dielectric thin films were prepared by atomic layer deposition (ALD), while Gd[N(SiMe3)2]3, Hf[NEtMe]4, and H2O are chosen to be precursors. The Gd and Si were successfully co-doped into HfO2 films using only one doping precursor Gd[N(SiMe3)2]3. The doping concentration can be facilely tuned by controlling ALD recipe. The atomic percentages of Si/(Si + Gd + Hf) and Gd/(Si + Gd + Hf) increase from 11.5 to 28.9% and from 6.8 to 28.4% when changing the ALD cycle ratio of GdxSiyO to HfO2 from 1:9 to 1:1. The band gap and band alignment were investigated by X-ray photoelectron spectroscopy. The results imply that the band gap of Gd/Si co-doped HfO2 films has a positive relation with doping concentration. Moreover, the valence band offset decreases with doping concentration first but then increases, while the change of conduction band offset is opposite. The (1:6)-HfxGdySizO films with 11.6 at.% Gd/(Gd + Hf) exhibit the maximum accumulation capacitance and dielectric constant, which are only slightly smaller than those of the HfO2 films. Compared to HfO2 films, the leakage current density of (1:6)-HfxGdySizO films is decreased by at least one order of magnitude. Therefore, Gd and Si co-doping can improve the electrical properties of HfO2 films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since portable electronic products come into public daily life, the dimension of Si-based metal–oxide–semiconductor field-effect-transistor (MOSFET) devices keeps continuous scaling down following Moore’s law [1]. In the past decades, SiO2-based gate dielectric was employed in the mass production of MOSFETs [2, 3]. However, the physical thickness of this insulator layer has been decreased to its physical limit, leading to power consumption and reliability issues due to the quantum tunneling effect [4, 5]. To solve the above issues, one of the promising ways is replacing the SiO2 by a physically thicker layer with a higher dielectric constant dielectric films [4, 6]. Various metal oxides have been applied as high-k gate dielectrics, including ZrO2 [7], Y2O3 [8], HfO2 [9], Ta2O5 [10], Al2O3 [11], and MgO [12]. Besides inorganic materials, a kind of organic–inorganic hybrid films as high-k dielectrics can also exhibit excellent properties, such as SiO2-TiO2-PVP and BaTiO3-PMMA-PVP, which show great application potential in flexible electronics [13, 14]. Among various high-k gate dielectric films, HfO2 with high dielectric constant has been demonstrated to be the most capable substitute for SiO2 to improve device performance [15,16,17]. Normally, gate dielectric films are expected to remain stay amorphous after a conventional activation annealing (∼1000 °C), concerning that grain boundaries may serve as the paths for dopant diffusion and current leakage. Nevertheless, the crystallization temperature of pure HfO2 is too low (~ 500 °C) [18, 19]. Si doping has been reported to suppress the crystallization of HfO2; however, Si dopant might deteriorate the dielectric constant of HfO2 films [20, 21]. On the other hand, it has been demonstrated that Gd doping can enhance the permittivity of HfO2 films [22, 23]. Therefore, co-doping of Gd and Si might be an effective approach to enhance comprehensive electrical properties of HfO2 films.
Atomic layer deposition (ALD) is one of the promising thin film deposition techniques, which is based on sequential self-limited and complementary surface chemisorption reactions. ALD possesses unique advantages such as large area uniformity, three-dimensional conformality, precise and facile thickness control down to sub-nanometer, low processing temperature, and extremely low damage to the substrate surface [24,25,26]. Therefore, ALD has attracted great attention in the surface engineering and nanostructure fabrication in recent years [27,28,29]. Amorphous gate dielectric films are preferred because grain boundaries can act as diffusion path for impurity and leakage current. Furthermore, smoother surfaces and associated improved interface quality can be achieved using amorphous gate dielectric films [5, 30]. ALD has been demonstrated widely to produce amorphous gate dielectric films with excellent interface; hence, ALD is one of the leading technologies for the high dielectric constant gate insulators deposition [31,32,33]. The use of Si-containing rare-earth (RE) precursors of tris[bis(trimethylsilyl)amino]RE {RE[N(SiMe3)2]3, where RE = La [34,35,36], Pr [37], Lu [38], and Me = CH3} has been reported to prepare RE silicate by ALD, which is a promising way to intentionally incorporate Si in rare-earth oxides. Therefore, Gd and Si co-doped HfO2 (HfxGdySizO) films were deposited by ALD, and Gd[N(SiMe3)2]3 was the only doping precursor in this work. The doping concentration tuning can be achieved by changing the ALD cycle ratio of GdxSiyO to HfO2. The effect of doping concentration of Gd and Si on the band alignment and electrical characteristics of the doped HfO2 films were investigated systematically.
2 Experimental section
Substrates used in this work were B-doped p-type Si (100) with resistivity of 1–10 Ω cm. Prior to ALD dielectric films deposition, they were first degreased in ethanol for 5 min by sonication. Then, the Si surface native oxide was removed with 2% diluted hydrofluoric acid solution for 3 min. Finally, the substrates were blown dry with N2 after rinsing by de-ionized water. The substrates were then transferred into the ALD chamber (Picosun SUNALE™ R200 Advanced, Finland) to deposit the HfxGdySizO films at 300 °C. The HfxGdySizO ALD process was performed by combining HfO2 ALD process, using Hf[NEtMe]4 and H2O, with the GdxSiyO ALD process, using Gd[N(SiMe3)2]3 and H2O. Pure N2 (99.999%) was used as both carrier gas and purge gas. During deposition process, the temperature of Hf[NEtMe]4 and Gd[N(SiMe3)2]3 precursors was set as 120 °C and 180 °C to ensure sufficient vapor pressure, while H2O was kept at room temperature. Pulse time of all precursors was 0.3 s, after which was a 6 s N2 flow step to blow reaction by-products and redundant precursors away. The composition of HfxGdySizO films was tuned via changing the ALD recipe. Eight kinds of HfxGdySizO samples with various compositions were prepared by depositing the alternate layers of GdxSiyO and HfO2 with GdxSiyO as the beginning layer. For example, (1:N)-HfxGdySizO films were using GdxSiyO (1 cycle) + HfO2 (N cycles, N = 1, 2, 3, 4, 5, 6, 7, 9) as one loop. The loops were varied to control the thickness of HfxGdySizO films as ~ 10.7 nm. Pure GdxSiyO and HfO2 films were also prepared for comparison. The related process details are presented in Table 1. The entire sample preparation process was conducted in the clean room.
The chemical composition of HfxGdySizO films were measured by X-ray photoelectron spectroscopy (XPS, Thermo Fisher K-Alpha) with a monochromatic Al Kα source (hν = 1486.6 eV). The excited photoelectrons were collected at a takeoff angle of 90°. The binding energy scale was calibrated using C1s peak at 284.6 eV. In addition, the valence band spectra and the O1s electron energy loss spectra of the HfxGdySizO films were also measured by XPS to achieve the band alignment information. Pt top gate electrodes with an area of 1.54 × 10−4 cm2 were fabricated on the surface of the HfxGdySizO films using a shadow mask by sputtering. Capacitance–voltage (C–V) and leakage current density–voltage (J–V) characteristics tests were conducted by a Keithley 4200 semiconductor characterization system at room temperature in a closed Cascade Summit 11000B-M probe station.
3 Results and discussion
The HfxGdySizO ALD process is illustrated in Fig. 1a, where the composition of HfxGdySizO films can be facilely tuned by controlling the ratio of GdxSiyO to HfO2 ALD cycles. XPS was applied to determine the composition of various HfxGdySizO films, and the typical XPS survey spectrum for (1:6)-HfxGdySizO film is presented in Fig. 1b. The C1s, Hf 4f, Gd 4d, Si 2p, and O1s peaks located at 284.6 eV, 17.5 eV, 143.2 eV, 102.2 eV, and 530.6 eV can be easily discerned, confirming that both Gd and Si from Gd[N(SiMe3)2]3 source can be doped into HfO2 films. Furthermore, the chemical composition of various HfxGdySizO films gained from XPS are summarized in Table 1. Figure 1c plots the relationship between the doping concentration (Gd/(Gd + Si + Hf) at.% and Si/(Gd + Si + Hf) at.%) and ALD cycle ratio of GdxSiyO/(GdxSiyO + HfO2). It can be also seen that the Gd and Si co-doping concentrations can be elaborately tuned over a wide range by the ALD cycle ratio of GdxSiyO and HfO2. As a result, the HfxGdySizO films with Si/(Si + Gd + Hf) at.% between 11.5 and 28.9% and Gd/(Si + Gd + Hf) at.% between 6.8 and 28.4% can be achieved via changing the ALD cycle ratio of GdxSiyO/HfO2 from 1:9 to 1:1. To avoid the effects from the silicon substrate during XPS measurement, the composition of pristine GdxSiyO film on Ge substrate was also detected by XPS, as shown in the inset of Fig. 1c. Si 2p signal can still be detected, and the Si/(Si + Gd) at.% is around 40%, which is in accord with the results of pristine GdxSiyO on Si substrate. Therefore, it can be demonstrated that the Si XPS signals of HfxGdySizO samples attribute to Si dopant in HfxGdySizO films rather than Si substrates.
a Schematic illustration of HfxGdySizO ALD process, b XPS survey spectrum of (1:6)-HfxGdySizO film, c Si/(Hf + Gd + Si), and Gd/(Hf + Gd + Si) atomic percentage versus the ALD cycle ratio of GdxSiyO/(GdxSiyO + HfO2). The inset in b and c are the Si 2p XPS spectra for (1:6)-HfxGdySizO film on Si and pristine GdxSiyO films grown on Ge substrate, respectively
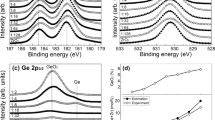
The band offsets at HfxGdySizO/Si interface were also explored by XPS. The valence band offset (VBO, ΔEv) can be determined by Kraut’s method, assuming that the energy difference between the core level and the valence band (VB) edge of the substrate keeps unchanged after dielectrics films deposition [39, 40]. Thus, valence band maximum (VBM) of Si (EVBM(Si)) was chosen as the reference to determine the VBO between the HfxGdySizO films and the Si substrate. Figure 2a shows the XPS valence band spectra of the Si substrate and the HfxGdySizO films deposited on Si substrates. The VBM of the clean Si substrate (EVBM(Si)) has been determined to be 0.52 eV by using the linear extrapolation as shown in Fig. 2a [41, 42]. The VBMs of the (1:N)-HfxGdySizO/Si samples (EVBM(HfxGdySizO)) are determined to be 3.12 eV, 3.09 eV, 2.72 eV, 2.71 eV, 2.77 eV, 2.77 eV, 3.08 eV, and 3.19 eV, respectively. Therefore, the VBOs of (1:N)-HfxGdySizO/Si samples (ΔEv(HfxGdySizO/Si)) are calculated as 2.60 eV, 2.57 eV, 2.20 eV, 2.19 eV, 2.25 eV, 2.25 eV, 2.56 eV, and 2.67 eV using the following formula: ΔEv(HfxGdySizO/Si) = EVBM(HfxGdySizO)-EVBM(Si).
The conduction band offset (CBO, ΔEc) can be achieved by ΔEc(HfxGdySizO/Si) = Eg(HfxGdySizO)-Eg(Si)-ΔEv(HfxGdySizO/Si), subtracting the band gap of the Si substrate and the VBO from the band gap of HfxGdySizO. The band gaps of HfxGdySizO films can be determined by the O1s electron energy loss spectra by using the linear extrapolation method [43, 44], as shown in Fig. 2b. Thus, the band gaps of the (1:N)-HfxGdySizO films (Eg(HfxGdySizO)) can be determined to be 6.13 eV, 6.09 eV, 5.96 eV, 5.92 eV, 5.86 eV, 5.82 eV, 5.73 eV, and 5.61 eV, respectively. Therefore, the CBOs of (1:N)-HfxGdySizO/Si samples (ΔEc(HfxGdySizO/Si)) are estimated to be 2.41 eV, 2.40 eV, 2.64 eV, 2.61 eV, 2.49 eV, 2.45 eV, 2.05 eV, and 1.82 eV, respectively.
According to the band gaps, VBOs, and CBOs results, the schematic band alignment diagram of the (1:N)-HfxGdySizO/Si heterostructures is constructed, as shown in Fig. 3. The band structures indicate that the band gap increases with doping concentration, which may attribute to the existence of Gd–O–Hf bonding and Si–O bonding [23]. In addition, the VBO decreases first and then increases, and the CBO increases first and then decreases along with the Gd/Hf atomic ratio rising. Therefore, the band offsets symmetry of HfxGdySizO/Si heterostructures can be improved by tuning the composition of HfxGdySizO. For example, (1:6)-HfxGdySizO films exhibit both large VBO (2.25 eV) and CBO (2.45 eV) values, beneficial for inhibiting the leakage current by huge barrier heights.
The MOS capacitors with the same physical thickness of (1:N)-HfxGdySizO films on Si substrates were fabricated. The high-frequency (1 MHz) C–V characteristics of these capacitors were measured. The accumulation capacitances of these Pt/(1:N)- HfxGdySizO/Si capacitors are 0.93, 1.01, 1.08, 1.04, 0.98, 0.83, 0.82, and 0.76 μF cm−2, respectively. Considering the Gd/(Gd + Hf) atomic percent of the (1:N)-HfxGdySizO films in Table 1, the accumulation capacitance as a function of the Gd/(Gd + Hf) atomic percentage is plotted in Fig. 4. The results indicate that the accumulation capacitance increases first and then decreases along with doping concentration. Thus, the HfxGdySizO films with Gd/(Gd + Hf) of 11.6 at.% exhibit the maximum accumulation capacitance and dielectric constant. Our results are consistent with previous literature by Adelmann et al. [45], which reported that Gd-doped HfO2 films with the doping concentration of 11.1 at.% exhibit the maximum dielectric constant.
Figure 5a, b compares the high-frequency (1 MHz) C–V and J–V characteristics of (1:6)-HfxGdySizO films and HfO2 films, respectively. The accumulation capacitance densities of (1:6)-HfxGdySizO films and HfO2 films are 1.08 μF cm−2 and 1.17 μF cm−2, which can be extracted from Fig. 5a. The calculated relative dielectric constant of the (1:6)-HfxGdySizO films is 13.3, which is only slightly smaller than that of the HfO2 films (14.4). According to Fig. 5b, it can be observed that the leakage current density of (1:6)-HfxGdySizO films and HfO2 films at gate voltage of − 1 V are 1.22 × 10–4 A cm−2 and 2.62 × 10–3 A cm−2, respectively. Compared to HfO2 films, the leakage current density of (1:6)-HfxGdySizO films is lower with more than an order of magnitude. Therefore, comprehensive electrical properties of HfO2 films can be improved by Gd and Si co-doping, reducing the leakage current density effectively without deteriorating the dielectric constant obviously. Furthermore, the current density of HfxGdySizO/Si heterostructures as a function of Gd/(Gd + Hf) atomic percentage was also provided, as shown in the inset of Fig. 5b. It can be seen that (1:3), (1:5), and (1:6)-HfxGdySizO films all can exhibit a decreased current density due to their both large VBO and CBO. However, the (1:9)-HfxGdySizO film still shows a large current density, which may be ascribed to its small CBO.
4 Conclusion
Gd and Si co-doped HfO2 films (HfxGdySizO) were successfully deposited by ALD in this work. XPS results demonstrate that Gd and Si can be co-doped into HfO2 films using the only doping precursor Gd[N(SiMe3)2]3. By tuning the ALD cycle ratio, the atomic percentages of Si/(Si + Gd + Hf), Gd/(Si + Gd + Hf) and Gd/(Gd + Hf) can be tuned between 11.5 and 28.9, 6.8 and 28.4, and 7.7 and 39.9%, respectively. The band alignment and electrical properties of ALD HfxGdySizO films on Si substrates with different doping concentrations were investigated. With increasing the Gd/Si doping concentration, the band gap increases, the VBO value decreases first and then increases, while the CBO value increases first and then decreases. Thus, (1:6)-HfxGdySizO films achieve the best band offset symmetry with both large VBO (2.25 eV) and CBO (2.45 eV). The (1:6)-HfxGdySizO films with 11.6 at.% Gd/(Gd + Hf) exhibit the maximum accumulation capacitance and dielectric constant which are only slightly smaller than those of the HfO2 films. Compared to HfO2 films, the leakage current density of (1:6)-HfxGdySizO films is decreased by at least one order of magnitude. Above results indicate that co-doping of Si and Gd maybe a promising way to improve comprehensive electrical properties of HfO2 films, effectively reducing the leakage current density while maintaining high dielectric constant.
References
G.E. Moore, Cramming more components onto integrated circuits, Reprinted from Electronics 38(8), April 19, 1965, pp.114 ff; IEEE Solid State Circuits Newsl. 11, 33–35 (2006)
J.-S. Kim, A.M. Tyryshkin, S.A. Lyon, Annealing shallow Si/SiO2 interface traps in electron-beam irradiated high-mobility metal-oxide-silicon transistors. Appl. Phys. Lett. 110, 123505 (2017)
M.L. Green, E.P. Gusev, R. Degraeve, E.L. Garfunkel, Ultrathin (<4 nm) SiO2 and Si-O-N gate dielectric layers for silicon microelectronics: Understanding the processing, structure, and physical and electrical limits. J. Appl. Phys. 90, 2057–2121 (2001)
J. Robertson, R.M. Wallace, High-K materials and metal gates for CMOS applications. Mater. Sci. Eng. R 88, 1–41 (2015)
G.D. Wilk, R.M. Wallace, J.M. Anthony, High-κ gate dielectrics: Current status and materials properties considerations. J. Appl. Phys. 89, 5243–5275 (2001)
D.G. Schlom, S. Guha, S. Datta, Gate Oxides Beyond SiO2. MRS Bull. 33, 1017–1025 (2011)
A. Liu, High-performance fully amorphous bilayer metal-oxide thin film transistors using ultrathin solution-processed ZrOx dielectric. Appl. Phys. Lett. 105, 113509 (2014)
G. Liu, A. Liu, H. Zhu, B. Shin, E. Fortunato, R. Martins, Y. Wang, F. Shan, Low-temperature, nontoxic water-induced metal-oxide thin films and their application in thin-film transistors. Adv. Funct. Mater. 25, 2564–2572 (2015)
X.-F. Li, X.-J. Liu, Y.-Q. Cao, A.-D. Li, H. Li, D. Wu, Improved interfacial and electrical properties of atomic layer deposition HfO2 films on Ge with La2O3 passivation. Appl. Surf. Sci. 264, 783–786 (2013)
L. Pereira, P. Barquinha, E. Fortunato, R. Martins, D. Kang, C.J. Kim, H. Lim, I. Song, Y. Park, High k dielectrics for low temperature electronics. Thin Solid Films 516, 1544–1548 (2008)
P. Ma, L. Du, Y. Wang, R. Jiang, Q. Xin, Y. Li, A. Song, Low voltage operation of IGZO thin film transistors enabled by ultrathin Al2O3 gate dielectric. Appl. Phys. Lett. 112, 023501 (2018)
G. Jiang, A. Liu, G. Liu, C. Zhu, Y. Meng, B. Shin, E. Fortunato, R. Martins, F. Shan, Solution-processed high-k magnesium oxide dielectrics for low-voltage oxide thin-film transistors. Appl. Phys. Lett. 109, 183508 (2016)
H. Najafi-Ashtiani, Performance evaluation of free-silicon organic-inorganic hybrid (SiO2-TiO2-PVP) thin films as a gate dielectric. Appl. Surf. Sci. 455, 373–378 (2018)
H. Najafi-Ashtiani, Low temperature processing of BaTiO3-PMMA-PVP hybrid films as transparent dielectric gate. J. Mater. Sci.-Mater. El. 30, 7087–7094 (2019)
C. Liu, Z. Wang, H. Lu, Y. Zhang, D. Liu, Y.-M. Zhang, Z. Ma, J. Zhao, L. Guo, K. Xiong, Atomic-layer-deposited HfO2/Al2O3 laminated dielectrics for bendable Si nanomembrane based MOS capacitors. Appl. Phys. Lett. 114, 142903 (2019)
A. Kumar, S. Mondal, K.S.R.K. Rao, Tunable band alignment and dielectric constant of solution route fabricated Al/HfO2/Si gate stack for CMOS applications. J. Appl. Phys. 121, 085301 (2017)
G. Venkata Rao, M. Kumar, T.V. Rajesh, D.V. Rama Koti Reddy, D. Anjaneyulu, B. Sainath, S.V. Jagadeesh Chandra, Investigations on the nitride interface engineering at HfO2/Ge stacks for MOS devices. Mater. Today 5, 650–657 (2018)
G. Tian, S. Wu, K. Shu, L. Qin, J. Shao, Influence of deposition conditions on the microstructure of oxides thin films. Appl. Surf. Sci. 253, 8782–8787 (2007)
Y. Yamamoto, K. Kita, K. Kyuno, A. Toriumi, Structural and electrical properties of HfLaOx films for an amorphous high-k gate insulator. Appl. Phys. Lett. 89, 032903 (2006)
R.E. Sah, Silicon nitride, silicon dioxide thin insulating films, and other emerging dielectrics, in VIII: Proceedings of the International Symposium (The Electrochemical Society, City, 2005)
G.D. Wilk, R.M. Wallace, J.M. Anthony, Hafnium and zirconium silicates for advanced gate dielectrics. J. Appl. Phys. 87, 484–492 (2000)
Y. Xiong, H. Tu, J. Du, M. Ji, X. Zhang, L. Wang, Band structure and electrical properties of Gd-doped HfO2 high k gate dielectric. Appl. Phys. Lett. 97, 5243 (2010)
X.J. Liu, A.D. Li, X. Qian, J.Z. Kong, J. Zhou, D. Wu, First-principles study on electronic structure of Gd-doped HfO2 high k gate dielectrics. Integr. Ferroelect. 134, 3–9 (2012)
S.M. George, Atomic layer deposition: an overview. Chem. Rev. 110, 111–131 (2010)
V. Miikkulainen, M. Leskelä, M. Ritala, R.L. Puurunen, Crystallinity of inorganic films grown by atomic layer deposition: overview and general trends. J. Appl. Phys. 113, 021301 (2013)
R.L. Puurunen, Surface chemistry of atomic layer deposition: a case study for the trimethylaluminum/water process. J. Appl. Phys. 97, 121301 (2005)
X. Meng, X.-Q. Yang, X. Sun, Emerging applications of atomic layer deposition for lithium-ion battery Studies. Adv. Mater. 24, 3589–3615 (2012)
X. Meng, X. Wang, D. Geng, C. Ozgitakgun, N. Schneider, J.W. Elam, Atomic layer deposition for nanomaterials synthesis and functionalization in energy technology. Mater. Horiz. 4, 133–154 (2017)
M. Knez, K. Nielsch, L. Niinistö, Synthesis and surface engineering of complex nanostructures by atomic layer deposition. Adv. Mater. 19, 3425–3438 (2007)
P. Barquinha, L. Pereira, G. Goncalves, R. Martins, E. Fortunato, Performance and stability of low temperature transparent thin-film transistors using amorphous multicomponent dielectrics. J. Electrochem. Soc. 156, H824 (2009)
G. Lin, M.-Q. Zhao, M. Jia, J. Zhang, P. Cui, L. Wei, H. Zhao, A.T.C. Johnson, L. Gundlach, Y. Zeng, Performance enhancement of monolayer MoS2 transistors by atomic layer deposition of high-k dielectric assisted by Al2O3 seed layer. J. Phys. D 53, 105103 (2019)
Y.H. Park, M.H. Kim, S.B. Kim, H.J. Jung, K. Chae, Y.H. Ahn, J.-Y. Park, F. Rotermund, S.W. Lee, Enhanced nucleation of high-k dielectrics on graphene by atomic layer deposition. Chem. Mater. 28, 7268–7275 (2016)
Y.-Q. Cao, J. Chen, X.-J. Liu, X. Li, Z.-Y. Cao, Y.-J. Ma, D. Wu, A.-D. Li, HfO2/GeOxNy/Ge gate stacks with sub-nanometer capacitance equivalent thickness and low interface trap density by in situ NH3 plasma pretreatment. Appl. Surf. Sci. 325, 13–19 (2015)
D.H. Triyoso, R.I. Hegde, J. Grant, P. Fejes, P.J. Tobin, Film properties of ALD HfO2 and La2O3 gate dielectrics grown on Si with various pre-deposition treatments. J. Vac. Sci. Technol. B. 22, 2121–2127 (2004)
D.H. Triyoso, R.I. Hegde, J.M. Grant, J.K. Schaeffer, D. Roan, B.E. White Jr., P.J. Tobin, Evaluation of lanthanum based gate dielectrics deposited by atomic layer deposition. J. Vac. Sci. Technol. B. 23, 288–297 (2005)
Y.J. Choi, S.-J. Won, H.-S. Jung, Effects of oxygen source on film properties of atomic-layer-deposited La-silicate film using La[N(SiMe3)2]3. ECS Solid State Lett. 1, N4 (2012)
K. Kukli, M. Ritala, T. Pilvi, T. Sajavaara, M. Leskela, A.C. Jones, H.C. Aspinall, D.C. Gilmer, P.J. Tobin, Evaluation of a praseodymium precursor for atomic layer deposition of oxide dielectric films. Chem. Mater. 16, 5162–5168 (2004)
G. Scarel, C. Wiemer, G. Tallarida, S. Spiga, G. Seguini, E. Bonera, M. Fanciulli, Y. Lebedinskii, A. Zenkevich, G. Pavia, Atomic layer deposition of Lu silicate films using [(Me3Si)2N]3Lu. J. Electrochem. Soc. 153, F271 (2006)
E. Kraut, R. Grant, J. Waldrop, S. Kowalczyk, Precise determination of the valence-band edge in x-ray photoemission spectra: application to measurement of semiconductor interface potentials. Phys. Rev. Lett. 44, 1620 (1980)
Y. Mi, S. Wang, J. Chai, J. Pan, A. Huan, M. Ning, C. Ong, Energy-band alignments at LaAlO3 and Ge interfaces. Appl. Phys. Lett. 89, 2107 (2006)
S.A. Chambers, Y. Liang, Z. Yu, R. Droopad, J. Ramdani, K. Eisenbeiser, Band discontinuities at epitaxial SrTiO3/Si(001) heterojunctions. Appl. Phys. Lett. 77, 1662 (2000)
R. Mahapatra, A.K. Chakraborty, A.B. Horsfall, N.G. Wright, G. Beamson, K.S. Coleman, Energy-band alignment of HfO2/SiO2/SiC gate dielectric stack. Appl. Phys. Lett. 92, 1830 (2008)
S. Miyazaki, Photoemission study of energy-band alignments and gap-state density distributions for high-k gate dielectrics. J. Vac. Sci. Technol. B. 19, 2212–2216 (2001)
H.Y. Yu, M.F. Li, D.L. Kwong, ALD (HfO2)x(Al2O3)1–x high-k gate dielectrics for advanced MOS devices application. Thin Solid Films 462, 110–113 (2004)
C. Adelmann, H. Tielens, D. Dewulf, A. Hardy, D. Pierreux, J. Swerts, E. Rosseel, X. Shi, M. Van Bael, J.A. Kittl, Atomic layer deposition of Gd-doped HfO2 thin films. J. Electrochem. Soc. 157, G105 (2010)
Acknowledgements
This work is supported in part by the Natural Science Foundation of China (51802150, 51721001, and 52073142) and Jiangsu Province (BK20170645 and BK20201252).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, L., Liu, XJ., Cao, YQ. et al. Effect of Gd and Si co-doping on the band alignment and electrical properties of HfO2 dielectric films prepared by atomic layer deposition. J Mater Sci: Mater Electron 32, 4815–4822 (2021). https://doi.org/10.1007/s10854-020-05220-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-05220-7